Petroleum exploitation enriches the sulfonamide resistance gene sul2 in off shore sediments
2021-06-15JingWANGJitiZHOU
Jing WANG, Jiti ZHOU
Key Laboratory of Industrial Ecology and Environmental Engineering(Ministry of Education), School of Environmental Science and Technology, Dalian University of Technology, Dalian 116024, China
Abstract Antibiotic resistance genes (ARGs) have been considered as emerging contaminants in nature owing to their wide distribution and human health risk. Anthropogenic activities can increase the diversity and abundance of ARGs and promote their spread in environment. Off shore environment is aff ected by multiple types of anthropogenic activities, of which excessive accumulation of petroleum substances poses a serious threat. Our previous experimental study has demonstrated that petroleum can increase the abundance of sulfonamide resistance genes (SRGs) in the seawater through horizontal gene transfer. However, the influence of petroleum substances on SRGs in off shore environment, especially adjacent the petroleum exploitation platform, is still unclear. Therefore, the eff ect of off shore oil exploitation on SRGs was investigated in the surface sediments collected from the Liaodong Bay, north China. The genes of sul1 and sul2 were present in all of the collected samples, while the sul3 gene was not detected in any sediments. The absolute abundance of sul2 gene in each sample was higher than sul1 gene. Class 1 integrons enhanced the maintenance and propagation of sul1 gene but not sul2 gene. More importantly, the results indicate that the absolute abundance of sul2 gene present in the off shore sediments that aff ected by petroleum exploitation was significantly higher than those in control. These findings provided direct evidence that off shore oil exploitation can influence the propagation of SRGs and implied that a more comprehensive risk assessment of petroleum substances to public health risks should be conducted.
Keyword: petroleum exploitation; sulfonamide resistance gene; quantitative real-time PCR; off shore sediment; Class 1 integrase
1 INTRODUCTION
Antibiotic resistance genes (ARGs) in environmental settings have been considered as emerging contaminants (Pruden et al., 2006). The increasing emergence and dissemination of ARGs from environmental bacteria to pathogens seriously impairs the effi cacy of antibiotics therapy, thereby representing a major threat to the treatment of clinical infection (Ashbolt et al., 2013). Among natural ecosystems, off shore environment has received special attention in the aspect of ARGs transmission.Noteworthy, a metagenomic analysis of bacterial plasmids from marine sediments found that several sequences shared a high identity with the transposons or plasmids from pathogens (Yang et al., 2013).Another study indicated that five ARGs in a plasmid from a marine microorganism showed significant similarities to sequences from pathogens (Yang et al.,2014). These previous results indicated that off shore sediments could contribute to the acquisition of ARGs by pathogens, thus posing a potential risk to human health.
Beyond the recognized selective pressure of antibiotics, ARGs can also spread and proliferate in natural ecosystems due to other anthropogenic activities (Pruden et al., 2012; Wang et al., 2016). It is well believed that organic contaminants could have the capability to enhance the abundance and persistence of ARGs in anthropogenically impacted ecosystems (Luo et al., 2014; Zhang et al., 2017).Particularly, a study has reported that ARGs in the petroleum-contaminated soils were more abundant than those in the less-contaminated ones (Chen et al.,2017). Petroleum substances are ubiquitous organic contaminants in off shore environment owing to excessive discharge from oil spill, coastal production,and transportation (Simons et al., 2013; Wang et al.,2014). Our previous study demonstrated that petroleum substances in seawater can increased the abundance of ARGs through promoting the horizontal gene transfer (HGT) mediated by class 1 integrons(Wang et al., 2017). However, the influence of petroleum contaminants on ARGs in off shore environment suff ered by petroleum exploitation activity is still unclear.
Liaodong Bay is a hotspot of off shore oil exploitation activity and the production of crude oil from this area is top ranking in north China (Teng et al., 2017). In addition to oil pollution, the off shore oil exploitation can also cause the contamination of heavy metals (Akpoveta and Osakwe, 2014), which have been reported to promote the spread of ARGs(Stepanauskas et al., 2006; McKinney et al., 2010).Notably, the prevalence of class 1 integron has been proposed to serve as a marker of antibiotic resistance(Amos et al., 2015) and anthropogenic pollution levels (Gillings et al., 2015) in natural environments.The 3′-conserved region of class 1 integron contains thesul1gene, which responses for the resistance of sulfonamides (Gillings, 2014). Examination in the degrees of class 1 integron and sulfonamide resistance genes (SRGs) in environments along a gradient of anthropogenic impact can give insights into the propagation and dissemination of ARGs (Gillings et al., 2015). SRGs are widespread in coastal and marine environments, and a previous study found that the abundance of SRGs in Liaodong Bay did not correlate with the concentration of sulfonamides (Lu et al.,2015). In addition, our previous study observed that naphthalene and phenanthrene, typical petroleum substances, could promote the propagation ofsul1gene via the class 1 integron (Wang et al., 2017).Therefore, sediments adjacent to the petroleum exploitation platforms in Liaodong Bay were collected and the abundance of class 1 integrase gene (intI1)and three SRGs (sul1,sul2, andsul3) were measured in the present study. Meanwhile, the correlations between SRGs and the concentrations of total petroleum hydrocarbons (TPHs) and heavy metals were also analyzed.
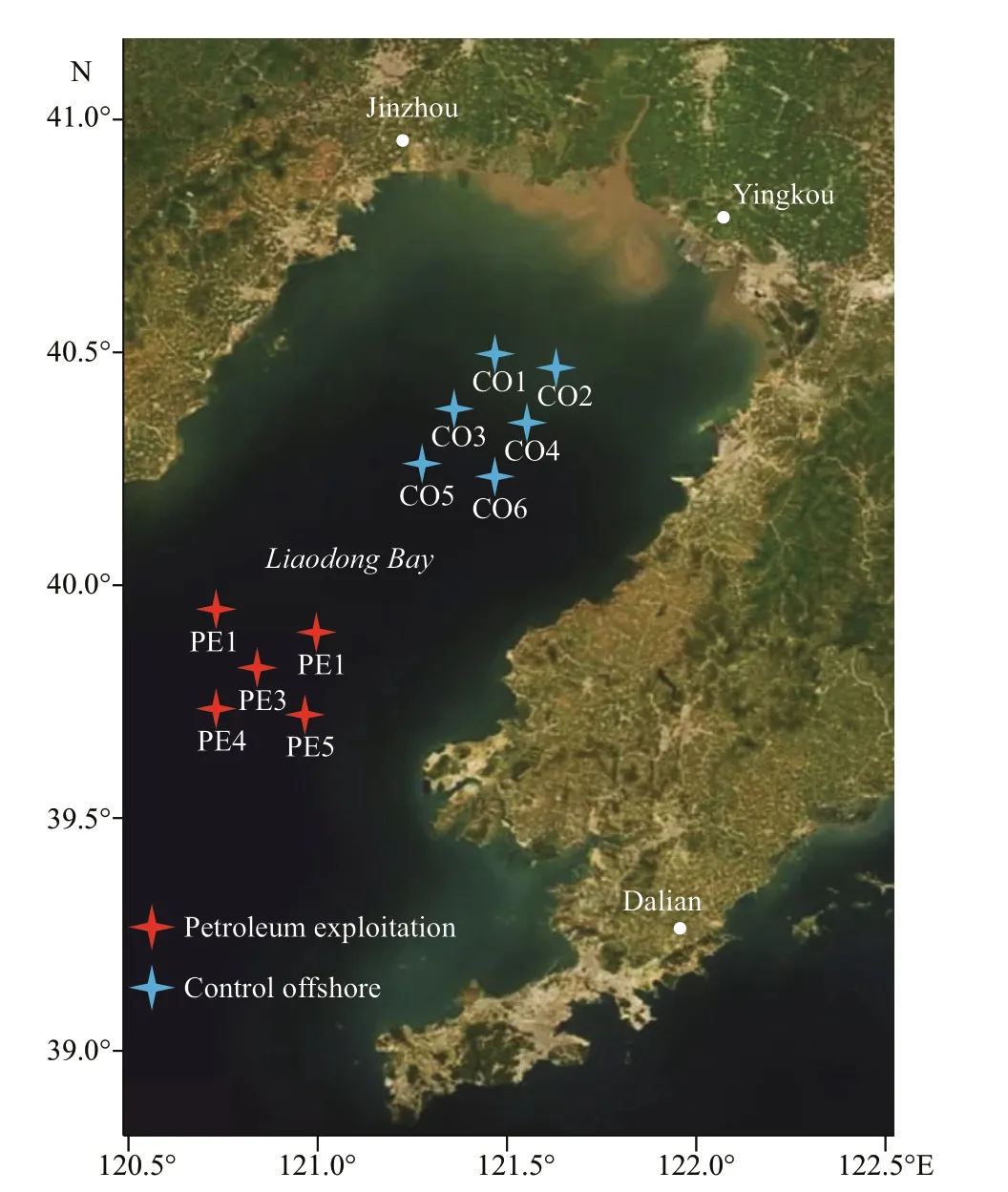
Fig.1 Map of the sampling sites
2 MATERIAL AND METHOD
2.1 Sediment collection and DNA extraction
Surface sediments (~100 g, 0–1 cm) were collected from 11 stations in Liaodong Bay in August 2015(Fig.1). Five sites (PE1–PE5) adjacent to the petroleum exploitation platforms were taken as samples with impacted by petroleum exploitation activity. Six sites (CO1–CO6) located in the off shore area without petroleum exploitation were selected as control. The sediments were aseptically collected with sterile containers, immediately placed on ice,and then transported to the laboratory. Microbial genome DNA was extracted from the sediment samples using the FastDNA SPIN Kit for Soil (MP Biomedicals, CA) according to the manufacturer’s instructions. The concentration and purity of all DNA samples were determined by the NanoPhotometer®Classic Launched (IMPLEN, GRE). All DNA samples were stored at -20 °C for further application.
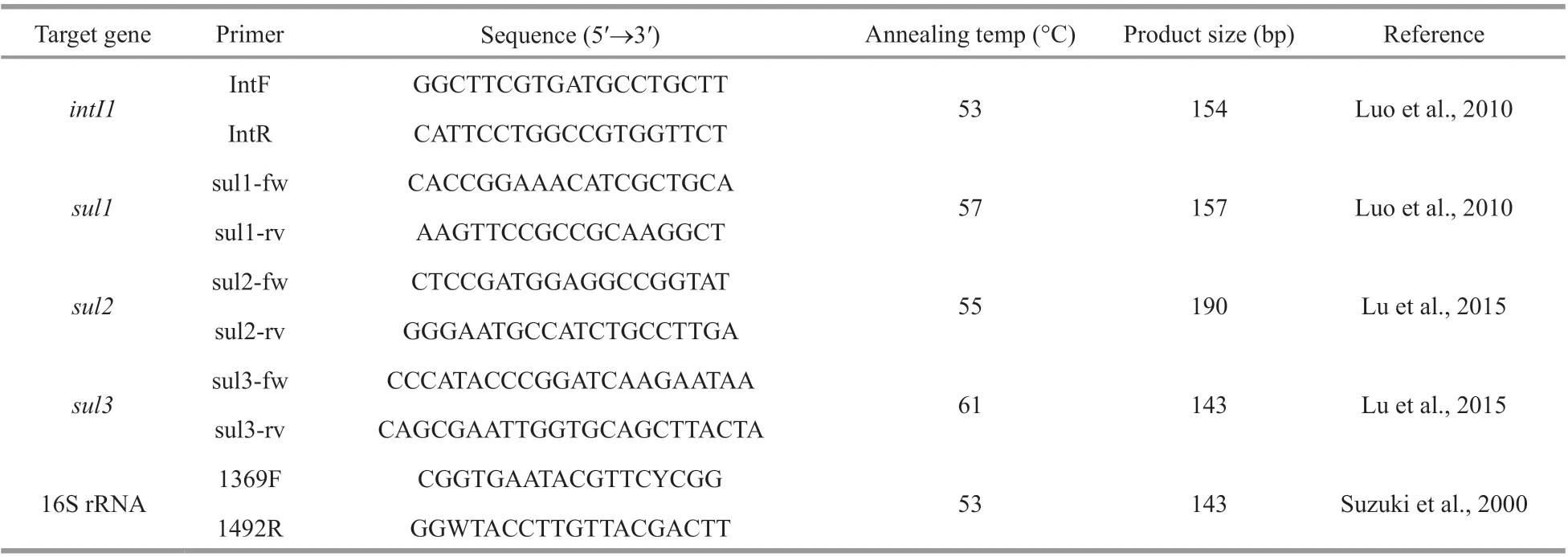
Table 1 The primers for qPCR used in this study
2.2 Sediment characterization
After being transported to the lab, the sediments were dried in a cold lyophilizer for 72 h and sieved through a 2-mm sieve to eliminate coarse rock and plant material. TPH levels in the sediments were determined by assaying for total hydrocarbons. The concentrations of TPHs in each sediment was analyzed by Soxhlet extraction following gas chromatography with a flame ionization detector(Aglient 6890 GC, USA) according to a previous report (Silva et al., 2014). The standard of TPHs was prepared from the mixture of C10–C40 hydrocarbons(No. 46951, DIKMA, China) and the precision of the replicate measurements for the reference material was better than 95%.
In order to analyze the potential impacts of heavy metals on ARGs, five common heavy metals, namely,Cd, Cu, Hg, Pb, and Zn, were selected and measured.The contents of heavy metals in sediment samples were quantified by inductively coupled plasma-mass spectrometry (ICP-MS, Optima, 2000 DV, Perkin Elmer, USA) according to a previous report (Yuan et al., 2004). Quality control was implemented by conducting a parallel analysis of certified reference SOIL GBW-5 (China). In GBW-5, all the heavy metals were detected in the range of standard values of three replicates.
2.3 Quantification of intI1, sul, and 16S rRNA genes
Quantitative real-time PCR (qPCR) analyses were performed on an Agilent Mx3005p qPCR system(Agilent Technologies, USA) using a SYBR Green approach to quantify the bacterial 16S rRNA,intI1,sul1,sul2, andsul3genes. The primers of each gene are shown in Table 1. The qPCR mixture (20 μL)consisted of 10-μL 2×SYBR®Premix DimerEraser(TaKaRa, Dalian, China), 0.2 mmol/L of each primer(Table 1), 1-μL DNA template, and nuclease-free water. To eliminate possible qPCR inhibition, 0.2-μL bovine serum albumin (10 g/L) was added to each qPCR mixture. The qPCR reactions were performed using the following protocol: 94 °C for 2 min followed by 40 cycles of denaturation at 94 °C for 30 s,annealing at the specific temperatures (Table 1) for 30 s and extension at 72 °C for 30 s. A melt curve was analyzed after each qPCR run to ensure the specificity of each reaction, and non-specific amplification for all primers was not occurred. Each reaction was run in triplicates and sterile water was used as the negative control. Standard curves of each gene for the abundance estimation were established according to a previous study (Zhang and Fang, 2006) and the results are listed in Table 2.
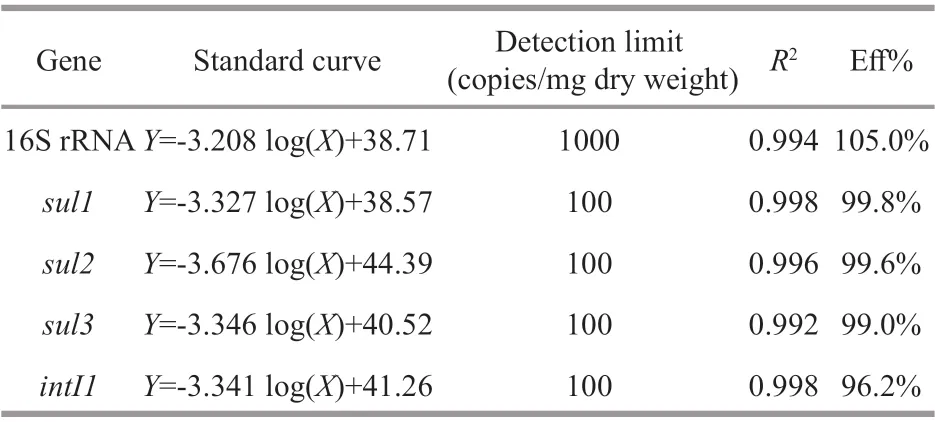
Table 2 qPCR standard curves for 16S rRNAgene,sulfonamide resistance genes and class 1 integrase
2.4 Statistical analyses
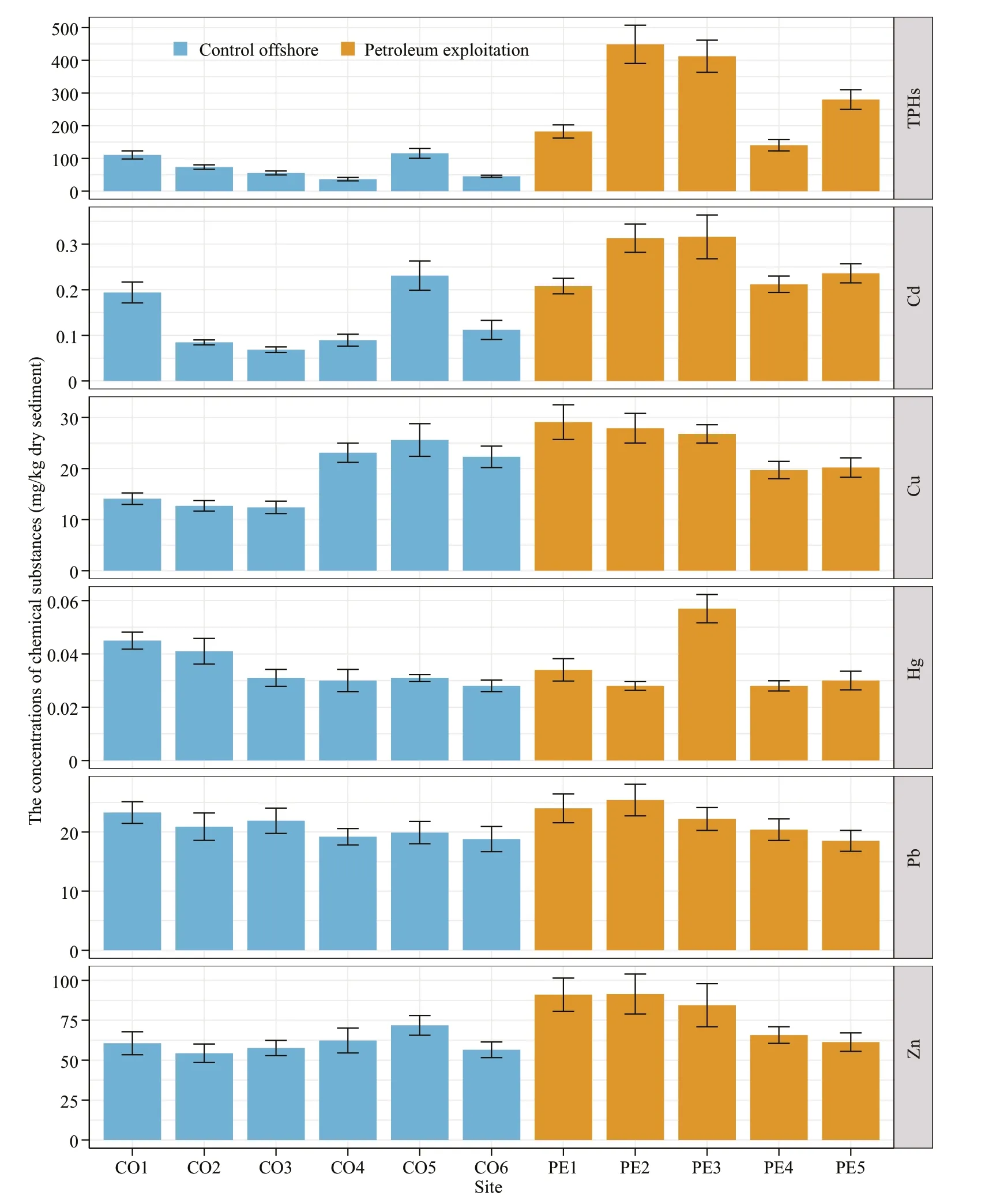
Fig.2 Concentrations of chemical substances in surface sediments of the Liaodong Bay
Barplots for the concentrations of TPHs and heavy metals as well as the absolute abundance of target genes in each sediment were performed by the“ggplot2” package in R. The diff erences in the concentrations of TPHs and the abundance of SRGs between the sediments of PE and CO groups were analyzed witht-test. Correlations based on the Pearson coeffi cient were measured to investigate the linear correspondence among the absolute abundances ofintI1,sul, and 16S rRNA genes in all samples using R and the significance level was chosen atP<0.05.Moreover, the correlations between the abundance of SRGs and the concentrations of TPHs and heavy metals in all samples were analyzed by “psych”package and the result was displayed by “pheatmap”package in R.
3 RESULT AND DISCUSSION
3.1 Sediments characteristics in Liaodong Bay
The chemical characteristics of the surface sediments collected in Liaodong Bay are summarized in Fig.2. The concentrations of TPHs in sediments were 36.6–449.1 mg/kg. Sediments adjacent to the petroleum exploitation platforms had significantly higher TPHs than controls (P<0.05). The concentrations of TPHs in surface sediments from Bohai Bay ranged from 6.3 to 535 mg/kg (Zhou et al.,2014), which contained completely the results of present study. Particularly, the contents of TPHs in the sediments of Laizhou Bay, the south part of Bohai Sea and neighboring Liaodong Bay, were comparable to samples without the impact of petroleum exploitation activities whereas lower than that in the PE sediments collected from Liaodong Bay (Wu et al., 2014). All five heavy metals (Cd, Cu, Hg, Pb, and Zn) were detected in surface sediments from Liaodong Bay. The concentrations of each heavy metal were as follows: Cd, 0.069–0.316 mg/kg; Cu, 12.4-29.1 mg/kg;Hg, 0.025–0.063 mg/kg; Pb, 18.5–25.4 mg/kg; Zn,54.3–91.4 mg/kg. Similar results of heavy metals were found in the sediments of Laizhou Bay (Wu et al., 2014) and the area of Bohai Sea adjacent to Tianjin(Zhou et al., 2014). No significant diff erence in the concentrations of heavy metals was uncovered between the PE and CO samples, suggesting that the heavy metals in Liaodong Bay had no relation with the petroleum exploitation activities.
3.2 The distribution of SRGs in Liaodong Bay
The amount of bacterial biomass in surface sediments of Liaodong Bay was quantified by subjecting 16S rRNA gene (Fig.3). The average bacterial biomass was 9.47×109copies/g dry sediment and no significant diff erence was found in the samples between PE and CO groups (P>0.05). This quantification of 16S rRNA gene copies was substantially lower than those previously reported from Haihe River in China (1011–1012copies/g dry sediment, Luo et al., 2010). Due to the relatively high salinity and low nutrient levels, the bacteria biomass in marine was usually lower than that in inland rivers(Sander and Kalff , 1993). By contrast, these values were considerably higher than those from the mangrove surface sediments having a history of oil contamination (average 4.6×108copies/g dry sediment, Andrade et al., 2012). This phenomenon might be because the oil pollution in the area of this study is not serious and therefore does not significantly inhibit the growth of bacteria.
Four SRGs had been previously identified, namedsul1,sul2,sul3, andsulA, to date,sulAwas only detected in few specific environments (Chen et al.,2019). Therefore, in the present study, the quantities ofsul1,sul2, andsul3were determined in the surface sediments from Liaodong Bay. The genes ofsul1andsul2were present in all of the collected samples,while thesul3gene was not detected in any sediments.Thesul1andsul2genes were also present in all sediments collected from Haihe River, whereassul3andsulAwere not detected (Luo et al., 2010).Moreover,sul1andsul2were also the major SRGs whereassul3was scarcely existed in the water from Daliaohe River and Liaohe Rivers estuaries, which located in the north part of Liaodong Bay (Lu et al.,2015). The abundances ofsul1andsul2genes ranged from 3.39×104to 9.20×105copies/g dry sediment and 5.00×104to 5.17×106copies/g dry sediment,respectively (Fig.3). In addition,sul2gene was more abundant thansul1in the same sediment from Liaodong Bay. Significantly higher abundance ofsul2gene compared tosul1were also detected in the sediments of Haihe River (Luo et al., 2010). Moreover,sul2gene was frequently detected in the sediments from the coastal area of Bohai Bay (Niu et al., 2016;Zhang et al., 2018). These findings suggested that SRGs were widespread and thesul2gene was more prevalent than others in the sediments of Liaodong Bay.
3.3 Correlations between the class 1 integron and SRGs
ARGs can be accumulated in the environment by two diff erent mechanisms: the propagation of ARGscarrying indigenous bacteria and HGT among various bacteria via the mobile gene elements (MGEs) as the vehicle (Reid et al., 2015; Wang et al., 2015;Engelstädter et al., 2016). No significant correlation was observed between the absolute abundances of SRGs and 16S rRNA genes in all sediment samples(P>0.05), indicating that the absolute abundance of SRGs in off shore sediments could have nothing to do with the biomass of bacteria. As the most important MGEs, class 1 integron was quantified by qPCR and the results exhibited that it was presented in all measured samples. The absolute abundances ofintI1gene in sediments of Liaodong Bay ranged from 2.67×104to 3.99×105copies/g dry sediment (Fig.3).The relationship between the absolute abundances ofsul1,sul2, andintI1genes in all sediments were examined and statistically significant correlation was found between thesul1andintI1genes (Fig.4),suggesting that the propagation ofsul1gene could be relate to the class 1 integron. Consistent results were observed in many previous studies involving various environments, including rivers (Luo et al., 2010),estuaries (Lu et al., 2015), wastewater treatment plants (Zhang et al., 2009), and the coastal seawater exposed to petroleum substances (Wang et al., 2017).Apparently, the class 1 integrons can enhanced the maintenance and propagation ofsul1gene in diverse environments.

Fig.3 The absolute abundances of 16S rRNA, sul1, sul2, sul3, and intI1 genes in the sediments from Liaodong Bay
3.4 The influences of petroleum exploitation on the SRGs
In this study, the absolute abundance ofsul2gene in sediments adjacent to the petroleum exploitation platforms were significantly higher than that in control samples (Fig.3,P<0.05). Moreover, correlation analysis was used to reflect the eff ect of sediment features on target genes (Fig.5). Significant correlation was found between the abundance ofsul2gene and the concentrations of TPHs. These results point out that the petroleum exploitation in Liaodong Bay could lead to the propagation ofsul2gene in the surface sediments. The enrichment of petroleum-tolerant bacteria was very common in the petroleum polluted environments (Wang et al., 2014), which often displayed strong resistance to antibiotics (Ben Said et al., 2008; Máthé et al., 2012). Moreover, many genes of the petroleum catabolic pathway have been found to coexist with the ARGs in the same genetic elements(Kweon et al., 2015; Hu et al., 2016). Therefore, the propagation ofsul2gene in the surface sediments adjacent to the petroleum exploitation platforms could due to the selective pressure of petroleum on the petroleum-degrading microorganisms carryingsul2.
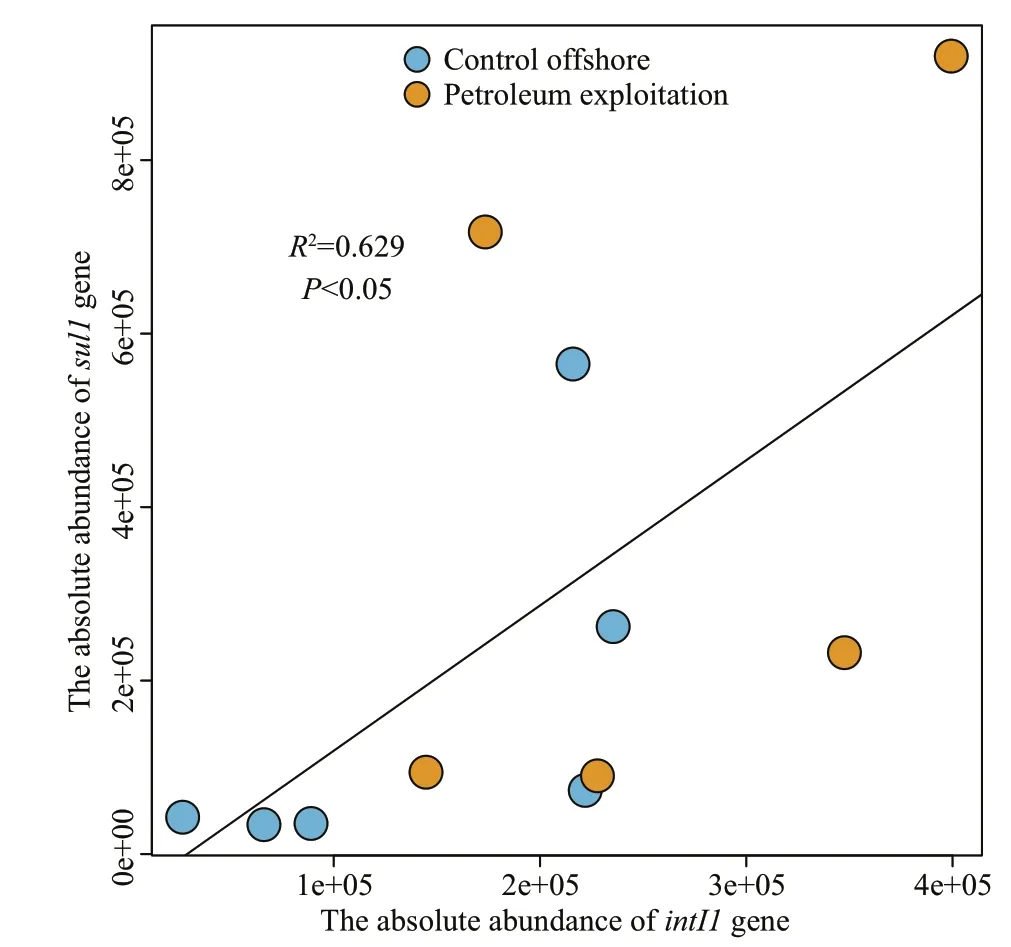
Fig.4 Correlation between the sul1 and intI1 genes in sediments from Liaodong Bay
Antibiotics play an important role in the proliferation of ARGs via mutation and HGT.However, previous studies put forward this theory mainly on the inland aquatic ecosystems such as wastewater treatment plants, dairy lagoons, and rivers(Luo et al., 2010; Storteboom et al., 2010). The concentrations of antibiotics in these environments were much higher than those in off shore, and the selection pressure of antibiotics was relatively weak in off shore environment. Previous study in Liaodong Bay has proved that the no statistically significant correlations of SRGs were observed with the concentrations of sulfonamide and total antibiotics in the Daliaohe River and Liaohe River estuaries (Lu et al., 2015). Additionally, a considerable number of investigations have been indicated that heavy metals contamination in natural environments played a vital role in the propagation and dissemination of antibiotic resistance (Stepanauskas et al., 2006; McKinney et al., 2010). The present of heavy metals will indirectly select the ARGs that locate in the same MGEs with heavy metal resistance genes (Di Cesare et al., 2016).Moreover, some ARGs could share the common structural and functional characteristics with heavy metal resistance genes (Krulwich et al., 2005).However, no significant correlation was obtained between the concentrations of heavy metals and the abundance of target genes in present study. This result mean that heavy metals might not be considered as a major factor that contributes to dissemination of antibiotic resistance in Liaodong Bay.
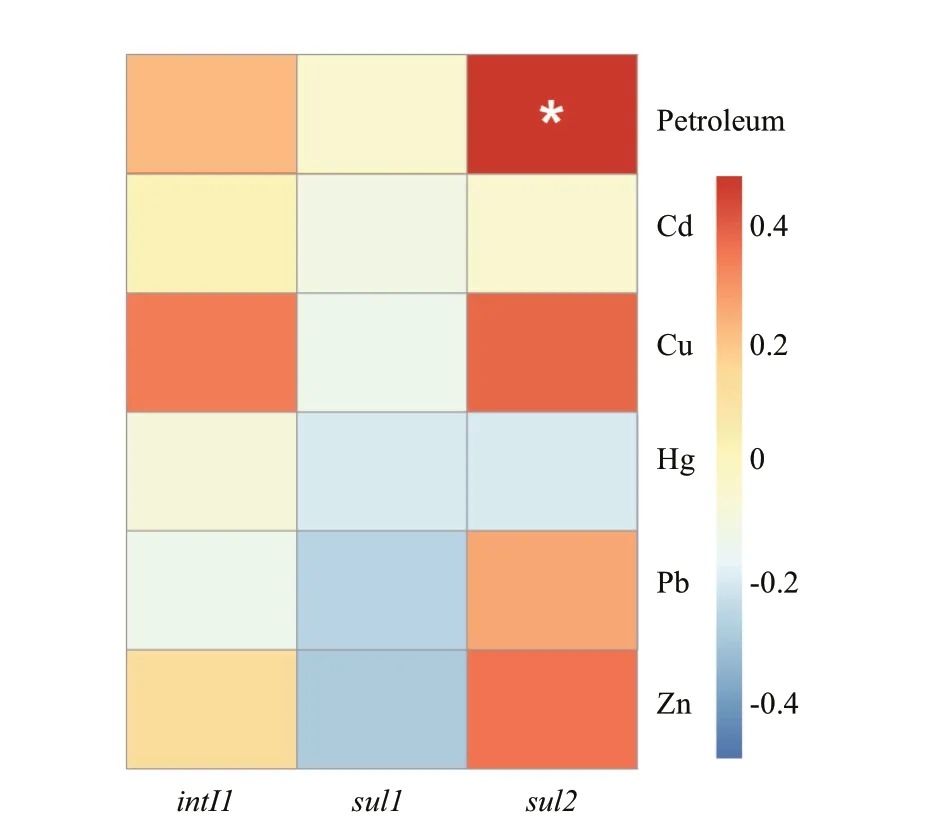
Fig.5 The heatmap shown the correlation between the absolute abundances of class 1 integron and sulfonamide resistance genes with the environmental factors in sediment samples from Liaodong Bay
4 CONCLUSION
By qPCR with the collection of surface sediments,we obtained the distribution of SRGs in the Liaodong Bay. Our results suggest that thesul1andsul2genes are widespread in the Liaodong Bay and thesul2gene is more abundant. HGT mediated by class 1 integrons might contribute to the maintenance and spread ofsul1gene in off shore sediments. Moreover, the absolute abundance ofsul2gene in off shore sediments could be decided by the concentrations of TPHs induced by the activities of off shore oil exploitation.These findings have important implications for understanding the impacts of petroleum contamination induced by off shore oil exploitation on the propagation of SRGs.
5 DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
杂志排行
Journal of Oceanology and Limnology的其它文章
- Steady increase in water clarity in Jiaozhou Bay in the Yellow Sea from 2000 to 2018: Observations from MODIS*
- Phylogenetic diversity and bioactivity of culturable deepsea-derived fungi from Okinawa Trough*
- Allelopathic eff ects of mixotrophic dinoflagellate Akashiwo sanguinea on co-occurring phytoplankton: the significance of nutritional ecology*
- Investigation of the decline of Ulva prolifera in the Subei Shoal and Qingdao based on physiological changes*
- Effi ciency of phosphorus accumulation by plankton,periphyton developed on submerged artificial substrata and metaphyton: in-situ observation in two shallow ponds*
- Mesoscale wind stress-SST coupling induced feedback to the ocean in the western coast of South America*
