Allelopathic eff ects of mixotrophic dinoflagellate Akashiwo sanguinea on co-occurring phytoplankton: the significance of nutritional ecology*
2021-06-15YeyinYANGBozhuHUANGYingzhongTANGNingXU
Yeyin YANG , Bozhu HUANG, Yingzhong TANG , Ning XU
1 Department of Ecology/Key Laboratory of Eutrophication and Red Tide Prevention of Guangdong Higher Education Institutes,Jinan University, Guangzhou 510632, China
2 Guangdong Environmental Monitoring Center, Guangzhou 510308, China
3 Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences,Qingdao 266071, China
A bstract Blooms of Akashiwo sanguinea frequently break out around the world, causing huge economic losses to the aquaculture industry and seriously damaging coastal ecosystems. However, the formation mechanisms of A. sanguinea blooms remain unclear. We investigated the allelopathic eff ects of A. sanguinea on multiple phytoplankton species, explored the mode of allelochemicals action and the way of nutrient factors regulation of the allelopathic activity. Results show that strains of A. sanguinea could inhibit the growth of co-occurring phytoplankton including Scrippsiella trochoidea, Phaeocystis globosa,and Rhodomonas salina, but inhibition of Prorocentrum micans was not obvious. The inhibition rates on phytoplankton were positively correlated with the cell densities of A. sanguinea. The highest inhibition rate of 94% on R. salina was for A. sanguinea CCMA256 culture of 2 000 cells/mL at 72 h. We observed that cells of S. trochoidea, Ph. g lobosa, and R. sali na were lysed when co-cultured with A. sanguinea, with the shortest time for S. trochoidea. Additionally, the growth rates of A. sanguinea were promoted by coculturing with S. trochoidea, Ph. globosa, and R. salina. Four components of A. sanguinea culture were all able to inhibit growth of R. salina: the strongest inhibitory eff ect was found in the sonicated culture,followed by whole-cell culture, filtrates of sonicated culture, and filtrate culture. The crude extract of A.sanguinea culture also lysed cells of R. salina, and the inhibition rates on R. salina increased with the increasing dose of crude extract. It was shown that both nutrient enrichment and nitrogen:phosphorus ratio imbalance enhanced remarkably the allelopathic activity of A. sanguinea. The highest inhibition rate on R.salina of 70% occurred in A. sanguinea JX13 treatment at 2 000 cells/mL under high nutrient condition in 48 h. In JX14 treatment at 2 000 cells/mL for N:P of 10:1, the inhibition rate increased by 1.7 times of that for N:P of 20:1. In addition, the allelopathy of A. sanguinea could not only be a competitive strategy but also a nutrition strategy, playing an important role in formation and/or maintenance of blooms of the mixotrophic dinoflagellate A. sanguinea.
Keyword: Akashiwo sanguinea; harmful algal blooms; mixotrophy; allelopathy; nutrients
1 INTRODUCTION
Over recent decades, harmful algal blooms (HABs)have increased in coastal regions all over the world,and have become a threat to fisheries, environments,public health and economies worldwide (Fleming et al., 2006; Hallegraeff , 2010; Gobler and Sunda, 2012;Chakraborty and Feudel, 2014; Castro et al., 2016).The formation mechanisms of HABs are very complex and related to various aspects of physical chemistry and biology. Studies have shown that degraded water quality from increased nutrient pollution, the imbalance of nitrogen (N) to phosphorus(P) and interactions between marine microalgae (e.g.allelopathy), high irradiance and hydrological factors(water column stability instead of turbulence) may play important roles for the formation and maintenance of HABs (Anderson et al., 2008; Heisler et al., 2008;Tang and Gobler, 2010; Phlips et al., 2011; Driscoll et al., 2013; Accoroni et al., 2015; Ou et al., 2017).
Allelopathic eff ects of harmful algae on the composition of phytoplankton communities and the dynamics of HABs have attracted much attention(Granéli and Hansen, 2006; Tang and Gobler, 2010;Hattenrath-Lehmann and Gobler, 2011). Some harmful algae can produce and release allelochemicals(secondary metabolites) into the ambient environments, which inhibiting or killing target species (competitors or predators), thus causing massive algal proliferation (Driscoll et al., 2013).Allelopathy is considered to be a relevant part of the ecology giving harmful algae growth advantages over other algae (Fistarol et al., 2004). Algal allelochemicals can be released into the environment during growth,and they have a wide range of eff ectiveness and various modes of action on co-occurring algal cells(Legrand et al., 2003; Hakanen et al., 2014). The typical mode of action includes cell lysis, blistering,inhibiting the photosynthetic system, paralyzing cells and growth inhibition. For example,Kareniabrevissecretes lipophilic allelochemicals to fuse with the cell membrane of target algae, damaging membrane integrity and leading to cell dissolution and death(Prince et al., 2008). Further research showed thatK.brevisallelochemicals can reduce photosynthesis of target algae (Poulin et al., 2018). So far, the potential ecological significance of allelopathic eff ects of harmful algae, and the role in development and formation of HABs remains unclear.
The speciesAkashiwosanguineais widespread all over the world, and has been recorded in North America (Horner et al., 1997; Du et al., 2011; Badylak et al., 2014), South America (Kahru et al., 2004),Europe (Gómez and Boicenco, 2004), Asia (Katano et al., 2011) and Australia (Hallegraeff , 1992).Akashiwosanguineais eurythermal and euryhaline (Matsubara et al., 2007) and is famous for forming large,noticeable blooms (Horner et al., 1997) especially in spring or summer. In South Korea,A.sanguineablooms have occurred almost every year for the past 30 years, causing huge economic losses (Park et al.,2013). In China,A.sanguineais also a common harmful alga, reported in the coastal waters of Hong Kong (Hodgkiss and Lu, 2004), Xiamen (Yang et al.,2012) and Yantai (Chen et al., 2015). The outbreak ofA.sanguineablooms is directly or indirectly related to large numbers of deaths of fish and seabirds (Harper and Guillen, 1989; Jessup et al., 2009). Laboratory experiments have shown thatA.sanguineais harmful to abalone larvae (Botes et al., 2003). Our previous study demonstrated that strains ofA.sanguineaisolated from Daya Bay in the South China Sea exhibited toxic eff ects on various marine animals,including fish, prawns and clams, and even caused acute damage to fish epidermis (Xu et al., 2017).However, the influence ofA.sanguineaon cooccurring phytoplankton is not clear.
In this study, the allelopathic eff ects and the action mode ofA.sanguineaon diff erent marine microalgae were investigated. The regulation mechanism of nutrient factors on allelopathy ofA.sanguineawas further explored, to reveal the ecological significance of allelopathy in the formation of the mixotrophic dinoflagellateA.sanguineaand provide a theoretical basis for the prevention and control ofA.sanguineablooms.
2 MATERIAL AND METHOD
2.1 Algal species and culture conditions
Stains ofA.sanguineaJX13 and JX14 were isolated from Daya Bay, South China Sea. StainA.sanguineaCCMA256, provided by Dr. GU Haifeng of the Third Institute of Oceanography State Oceanic Administration, was isolated from Xiamen, Fujian,China. Stains ofA.sanguineaASNP6 and AS2 were provided by Dr. TANG Yingzhong: ASNP6 was isolated from Northport Bay (New York, USA) (Tang and Gobler, 2015), and AS2 was isolated from Chesapeake Bay (Virginia, USA) (Tang and Gobler,2010).
Four target phytoplankton species were used in the study: two species of dinoflagellates (ScrippsiellatrochoideaJX20 andProrocentrummicansJX8), a haptophyte (PhaeocystisglobosaJX4) and a cryptophyte (RhodomonassalinaCCMP1319).Strains JX20, JX4 and JX8 were isolated from the South China Sea and CCMP1319 was donated by Dr.Christopher J. Gobler of Stony Brook University.
All cultures were grown in sterile silicate-free f/2(f/2-Si) culture medium (Guillard, 1975) which made with 0.22-μm filtered and autoclaved seawater. The salinity range of sterile f/2-Si culture medium was 30±1. Cultures were placed in an incubator (GXZ intelligent light incubator, China) (23±1 °C) with a 12-h:12-h light:dark cycle. The light source in an incubator was provided by fluorescent lamps and the light intensity reached ~100 μmol quanta/(m2·s). In mid-exponential phase growth, all cultures were used for experiments.
2.2 Co-culture experiments of A. sanguinea and other phytoplankton
2.2.1 Allelopathic eff ects ofA.sanguineaon coculturing phytoplankton
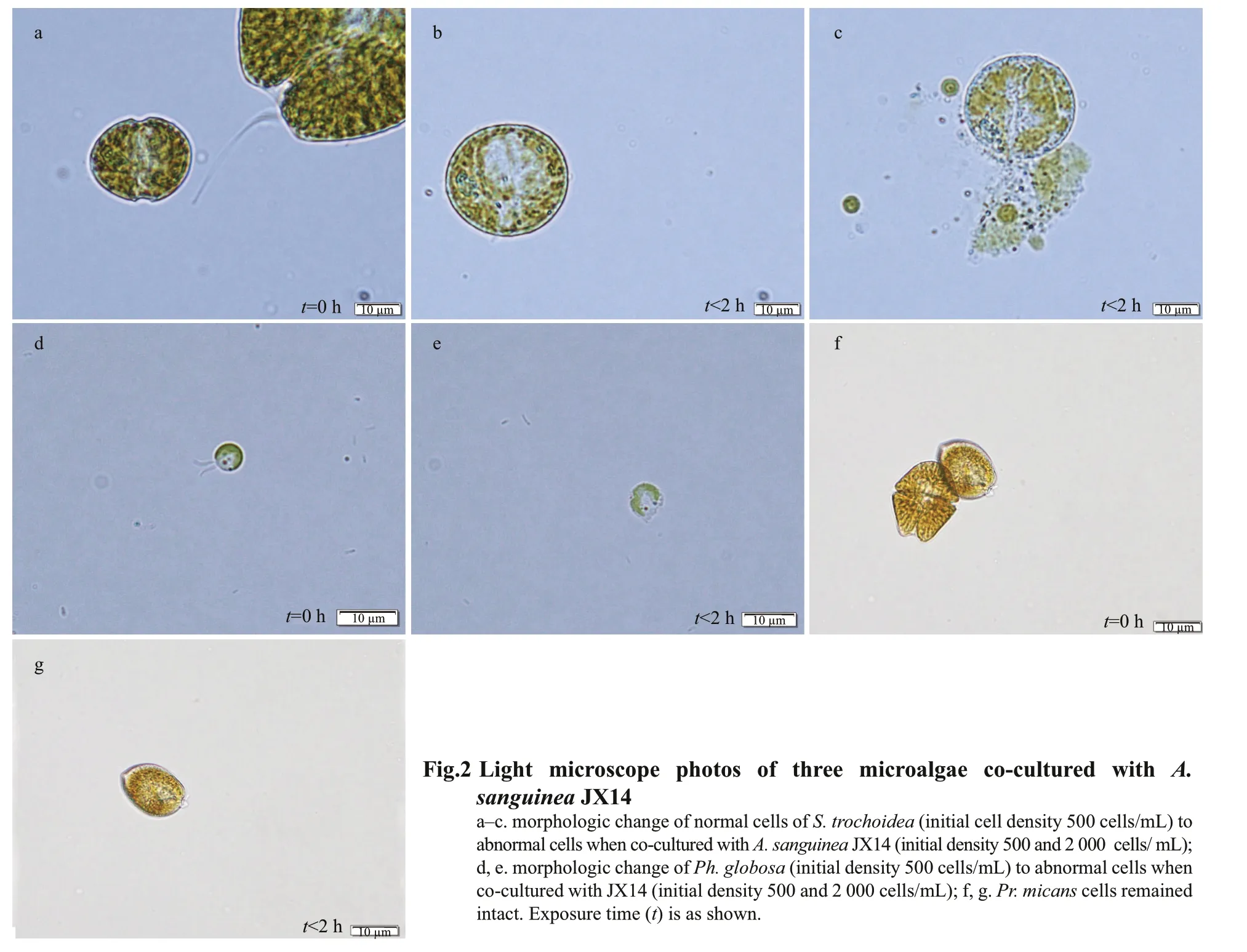
AkashiwosanguineaJX14 in mid-exponential growth phase (4 days after inoculation) was corespectively cultured with three species of microalgae from diff erent categories (S.trochoideaJX20,Pr.micansJX8 andPh.globosaJX4), in six-well culture plates for 48 h. In order to provide adequate nutrition during the experiment, the f/2-Si medium was added at the beginning. The initial cell density ofA.sanguineaJX14 was 500 or 2 000 cells/mL and the initial cell density of each target alga was 500 cells/mL.Monocultures ofA.sanguineaand target algae under the same conditions were used as controls. All treatments and controls had three replicates.
At 0–6, 12, 24, and 48 h, observing and photographing morphological changes and behaviors of cells were under an inverted microscope (Olympus BX53, Japan) with a mounted digital insight camera(DP27). At 48 h, 1 mL of culture was fixed with Lugol’s solution (final concentration of 2%) for counting under a microscope (Olympus CX41,Japan). Before and after the experiments, pH in cultures was determined by pH meter (PHB-3, China).After the experiments, culture filtrate in all treatments was obtained by filtration through a 0.22-μm polycarbonate filter and then N and P concentrations were determined by AutoAnalyzer 3 (Bran+Luebbe,Germany).
2.2.2 Allelopathic eff ects of multiple strains ofA.sanguineaonR.salina
To determine whether the allelopathic eff ects displayed by strain JX14 were a strain-specific feature, experiments were conducted with five strains ofA.sanguinea: JX13, JX14, CCMA256, ASNP6 and AS2. In the following experiments,R.salinaCCMP1319 was used as a model target alga.
In mid-exponential growth phase, CCMP1319 and strains ofA.sanguineainoculated into six-well culture plates for 72 h. The f/2-Si medium was added as described in Section 2.2.1. The initial cell densities in the experiment were 500 and 2 000 cells/mL forA.sanguineaand 500 cells/mL for CCMP1319.Monocultures of five strains ofA.sanguineaand target alga were used as controls under the same conditions. All treatments and controls had three replicates.
Observing and photographing morphological changes and behaviors of cells were under an inverted microscope (Olympus BX53) every 24 h. At 72 h,1 mL of culture was fixed with Lugol’s solution (final concentration of 2%) for counting under a microscope(Olympus CX41). The pH, N, and P concentrations were determined as described in Section 2.2.1.
2.2.3 Eff ects ofR.salinaonA.sanguineagrowth
To understand the eff ects ofR.salinaon the growth ofA.sanguinea, experiments were conducted with 3 strains ofA.sanguinea: JX13, JX14 and AS2.
In mid-exponential growth phase, strains ofA.sanguineawere added into 6-well culture plates for co-culture withR.salinafor 72 h under the same conditions used for maintaining cultures. f/2-Si medium was added at the beginning of the experiment to ensure suffi cient nutrition in the experiment. The initial cell densities ofR.salinain the experiment were 1 000, 2 000, and 5 000 cells/mL, respectively,3 strains ofA.sanguineawas 1 000 cells/mL.Monocultures of 3 strains ofA.sanguineaand tested alga under the same conditions were used as controls.All treatments and controls were in triplicate.
One milliliter culture was fixed with Lugol’s solution (final concentration of 2%) for counting under the microscope (Olympus CX41) at each 24 h.The pH, N, and P concentrations were determined as described in Section 2.2.1.
2.3 Mechanisms of allelopathic eff ects in A. sanguinea
2.3.1 Allelopathic eff ects of diff erent fractions ofA.sanguineaculture onR.salina
To compare whether diff erent fractions ofA.sanguineaJX14 culture displayed diff erent inhibition on target alga growth, experiments were conducted with four components of JX14 culture:sonicated culture, filtrates of sonicated culture, wholecell culture and culture filtrate.
Four components of JX14 culture obtained in midexponential growth phase. We obtained culture filtrate after filtrating 50-mL cultures ofA.sanguineaJX14 with a 0.22-μm polycarbonate filter. We obtained a sonicated culture by treating 100-mL cultures ofA.sanguineaJX14 with a sonicator (10 min, 5 s/5 s,60%) (JYD-900L, China), and ensured that all cells were broken by examination under a microscope. The filtrates of sonicated culture were obtained after filtrating 50 mL of the sonicated cultures with a 0.22-μm polycarbonate filter.
TheR.salinawas inoculated into four components ofA.sanguineaJX14 culture in 10-mL culture tubes for 72 h. The f/2-Si medium was added as described in Section 2.2.1. The initial cell densities for JX14 andR.salinawere both 500 cells/mL. Monoculture ofR.salinawith the same cell density was used as the control. All treatments and control had three triplicates. Every 24 h, 1 mL of culture was fixed with Lugol’s solution (final concentration of 2%) for counting under the microscope (OLYMPUS CX41).
2.3.2 Eff ects of crude extract ofA.sanguineaJX14 culture onR.salinagrowth
The crude extract of JX14 was prepared as follows:in mid-exponential growth phase, 500-mL cultures of JX14 (cell density: 3 000 cells/mL) were collected and centrifuged at 1 000 r/min at 4 °C (10 min) and then JX14 cells were re-suspended in 25 mL of chloroform:methanol:water (13:7:5) solution. The suspension was treated with a sonicator (10 min,5 s/5 s, 60%) (JYD-900L) and checked under a microscope to ensure all algal cells were broken. The broken-cell culture was transferred to a separating funnel until complete stratified. The lower solution obtained from the separating funnel was transferred to a rotary evaporator (temperature 60 °C) and evaporated under vacuum to dryness. Crude extract was re-dissolved in 10 mL of methanol (i.e. 1/50 of culture of JX14) and stored at -18 °C for later use.
To explore the eff ects of crude extract of JX14 on the growth of AS2 and CCMP1319, experiments were conducted withA.sanguineaAS2 andR.salinaCCMP1319. The AS2 and CCMP1319 in midexponential growth phase were inoculated into sixwell culture plates and 2, 6, or 10 μL of crude extract respectively were added to the co-culture system with a final volume of 10 mL, which was maintained for 48 h. The f/2-Si medium was added as described in Section 2.2.1. The initial cell densities of AS2 and CCMP1319 were both 500 cells/mL. The controls were set as follows: monoculture of CCMP1319, coculture of AS2 and CCMP1319 without methanol and extract, co-culture of AS2 and CCMP1319 respectively with 2, 6, and 10 μL of methanol. All treatments and controls had three triplicates.
During the experiment, morphological changes and behaviors of cells were observed and photographed at 0–6, 24, and 48 h under a microscope (OLYMPUS BX53). At 48 h, 1 mL of cultures was taken out and fixed with Lugol’s solution (final concentration of 2%) for counting under a microscope (OLYMPUS CX41).
2.4 Eff ects of nutrients on allelopathic activity of A. sanguinea
To explore the eff ects of nutrients on allelopathic activities ofA.sanguinea, strains JX13 and JX14 ofA.sanguineawere cultured under diff erent conditions.(1) The JX13 and JX14 were grown in cultures of three diff erent nutrient levels (all μg/L): middle(180 N and 20 P), high (448 N and 50 P) and low(12 N and 1.3 P); (2) the JX13 and JX14 were grown at three N:P ratios (all μg/L): 10:1 (226 N and 50 P),20:1 (448 N and 50 P), and 30:1 (672 N and 50 P).Nitrate and phosphate were used as N and P sources,respectively.
Before the experiment, JX13 and JX14 were precultured for three cycles under the above six nutrient conditions. Cultures of JX13 and JX14 were treated with a sonicator (10 min, 5 s/5 s, 60%) (JYD-900L)and we ensured that all cells were broken under a microscope. The sonicated culture was filtered to obtain filtrate using a 0.22-μm polycarbonate membrane.
TheR.salinaCCMP1319 was inoculated into sonicated filtrates of JX13 and JX14, in 10-mL culture tubes for 48 h. The f/2-Si medium was added as described in Section 2.2.1. The initial cell densities of JX13 and JX14 were 500 and 2 000 cells/mL, and that for CCMP1319 was 500 cells/mL. Monoculture of CCMP1319 with the same cell density was used as the control. All treatments and control had three replicates. At 48 h, 1 mL of samples was fixed with Lugol’s solution (final concentration of 2%) for counting under the microscope (Olympus CX41).
2.5 Statistics
Experimental data were analyzed by one-way analysis of variance (ANOVA). This statistical analysis was performed by SPSS version 17.0.Treatments diff erences were considered to be statistically significant atP<0.05 and extremely significant atP<0.01.
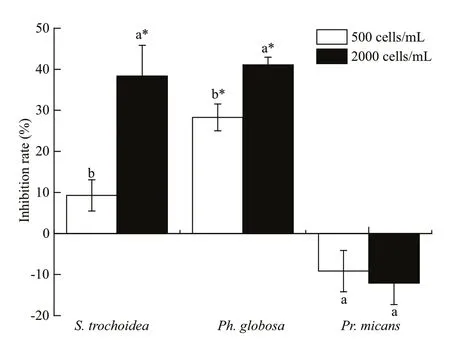
Fig.1 Inhibition rates of A. sanguinea on three marine microalgae
Inhibition rate=(1−Ntreatment/Ncontrol)×100%(Williamson and Richardson, 1988),
whereNtreatmentandNcontrolare numbers of target algal cells in treatments and controls, respectively.
Specif ic growth rate (μ)=ln(Nt/N0)/T,
whereNtis the number of algal cells at timet,N0is the number of algal cells at the beginning of the experiment andTis the time of co-culturing.
3 RESULT
3.1 Co-culture experiments of A. sanguinea and other phytoplankton
3.1.1 Allelopathic eff ects ofA.sanguineaon coculturing phytoplankton
The results showed thatA.sanguineaJX14 signif icantly reduced the cell densities of two target species, the dinof lagellateS.trochoideaand the prymnesiophyteP.globosa, compared to their respective controls during co-culturing experiments(P<0.05, Fig.1). For JX14 treatments of 2 000 cells/mL,the cell densities ofS.trochoideaandPh.globosawere signif icantly lower than controls (P<0.05;Fig.1), and the inhibition rates on both species were around 40%. It is noteworthy that the JX14 growth rate increased when co-cultured withS.trochoideaandPh.globosain all treatments compared with its monoculture. However, JX14 had no signif icant negative eff ect on growth ofPr.micans(P>0.05). At the end of experiments, the f inal nitrate concentration in culture medium was 4.096–7.158 mg/L, f inal phosphate concentration was 0.430 3–1.073 mg/L,and pH was 7.6–8.5.
We did observe a series of morphological changes of the target algae when co-cultured with JX14. For example, 30 min after co-culturing with JX14, cells ofS.trochoideaandPh.globosagradually lost motility,and cell morphology showed a series of signif icant changes, losing typical surface characteristics,swelling into spheres and f inally cell membrane breakage occurred (i.e. cell contents released)(Fig.2a–e). However,Pr.micansmorphology did not change and the cells remained intact (Fig.2f & g).
3.1.2 Allelopathic eff ects of multiple strains ofA.sanguineaonR.salina
F our strains ofA.sanguineasignif icantly inhibited the growth ofR.salina, but not for strain AS2 (Fig.3).In the treatments of 2 000 cells/mL for fourA.sanguineastrains (JX13, JX14, CCMA256, and ASNP6), theR.salinacell densities signif icantly diff ered to those of the monoculture (P<0.01); the inhibition rates onR.salinasignif icantly diff ered amongA.sanguineastrains (P<0.05; Fig.3). The growth-inhibiting eff ects of strains JX13, JX14,CCMA256, and ASNP6 onR.salinawere densitydependent. Specif ically, the inhibition rates of JX13,JX14, and CCMA256 for 2 000 cells/mL treatments onR.salinasignif icantly diff ered from those at 500 cells/mL in 72 h (P<0.05; Fig.3). In all treatments,CCMA256 (initial cell density 2 000 cells/mL)showed the highest inhibition rate of around 90%. At the end of experiments, nitrate concentration in culture medium was 1.008–4.824 mg/L, phosphate concentration was 0.092–0.647 mg/L, and pH was 7.8–8.8.
3.1.3 Eff ects ofR.salinaonA.sanguineagrowth
The presence ofR.salinahad signif icant implications for growth ofA.sanguinea. When JX13 or JX14 were co-cultured withR.salina, their growth rates signif icantly increased compared with monocultures, but AS2 treatment did not show the same trend (Fig.4). That is, the presence ofR.salinamay have promoted growth ofA.sanguinea.
The cell densities ofA.sanguineaJX13 in all treatments were signif icantly higher compared with the monoculture at 24 and 72 h (P<0.05; Fig.4a), with a similar trend at 48 h. The specific growth rates of JX13 co-cultured withR.salinawere significantly higher than for monoculture at 24, 48, and 72 h(P<0.05; Fig.4b). WhenR.salinacell density was higher, the cell densities and the specific growth rate of JX13 were higher at 24, 48, and 72 h. The cell densities and specific growth rate of JX13 increased by around 40% compared with control whenR.salinacell density was 5 000 cells/mL at 72 h.
The cell densities and the specific growth rates of JX14 in all treatments were significantly higher compared with monoculture at 24 and 72 h (P<0.05;Fig.4c & d). WhenR.salinacell density was 5 000 cells/mL at 72 h, the cell densities and specific growth rate of JX14 were increased by around 30%compared with control.
Although the cell density and growth rate of JX13 and JX14 were significantly promoted in coculturing treatments withR.salina, there were no significant diff erences between AS2 co-cultured withR.salinaand AS2 monoculture (P>0.05; Fig.4e& f).
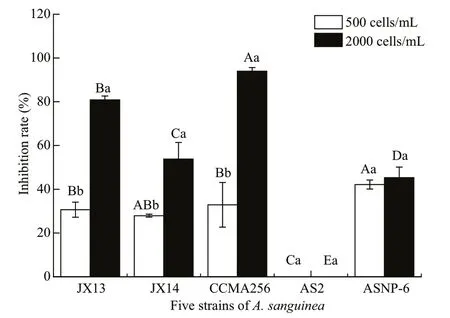
Fig.3 Inhibitory eff ects of five strains of A. sanguine a on R. salina
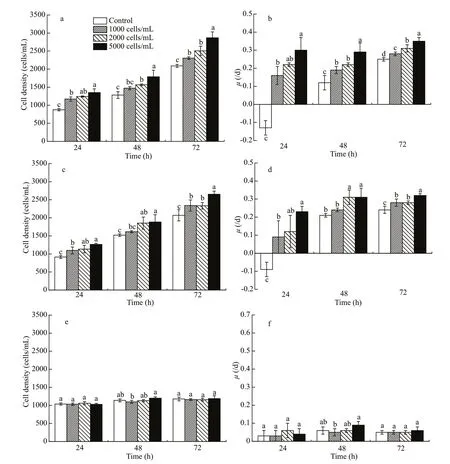
Fig.4 Cell density and growth rate of diff erent strains of A. sanguine a co-cultured with R. salina
3.2 Mechanisms of allelopathy in A. sanguinea
3.2.1 Allelopathic eff ects of four components ofA.sanguineacultures onR.salina
F our culture fractions ofA.sanguineaJX14 (i.e.whole-cell culture, culture filtrate, sonicated culture,and filtrates of sonicated culture) all had significant inhibitory eff ects onR.salinagrowth (P<0.05; Fig.5);however, the eff ects of four culture fractions onR.salinawere diff erent. After incubation for 72 h,sonicated JX14 culture showed the highest inhibitory rate onR.salina(32%), followed by whole-cell culture (28%), filtrates of sonicated culture (17%),with the lowest inhibition rate for culture filtrate (8%).The inhibition rate of sonicated culture significantly diff ered to that for other components at 24, 48, and 72 h (P<0.05; Fig.5). Sonicated culture and filtrates of sonicated culture show the highest inhibitory rate at 48 h, and then decreased with time. Whole-cell culture exhibited stronger inhibition ofR.salinawith prolonged incubation time. Filtrates of sonicated culture show stronger inhibitory eff ects than wholecell culture at 24 and 48 h. However, the inhibition rate of culture filtrate on R. salina was significantly lower at 24, 48, and 72 h than for all other treatments(P<0.05; Fig.5).
3.2.2 Allelopathic eff ects of extracts ofA.sanguineaonR.salinagrowth
The results revealed thatR.salinagrowth was significantly inhibited by allA.sanguine aJX14 crude extract treatments compared with all monoculture and co-culture controls after 48 h (P<0.01; Fig.6a). There was no significant diff erence between co-culture control and methanol controls, with both showing inhibition rates of around 0%. The inhibition rates significantly diff ered among the three dosages (2, 6,and 10 μL) of crude extract treatments (P<0.05;Fig.6a). When adding 10 μL of crude extract of JX14(equivalent to 1 500 cells) into co-culture of AS2 andR.salina, the highest inhibition rate was 23% at 48 h.
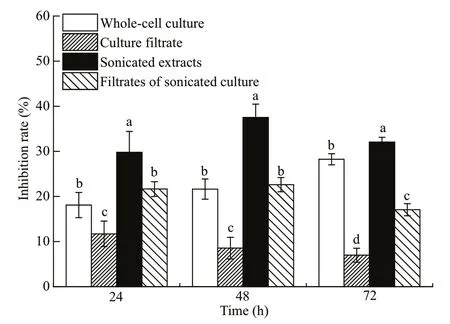
Fig.5 Inhibitory eff ects of four diff erent components of A. sanguine a JX14 on R. salina
The growth rate ofA.sanguineaAS2 was around 0.1/d and did not significantly diff er among all treatments (Fig.6b).
3.3 Eff ects of nutrients on allelopathic activity in A. sanguinea
N utrient level could change the allelopathic ability ofA.sanguinea. The inhibition rates onR.salinawere higher if JX13 and JX14 were cultured under high nutrient conditions (Fig.7a & b). In all treatments,the inhibition rates of JX13 or JX14 onR.salinasignificantly diff ered among the low, medium, and high nutrient conditions (P<0.05; Fig.7a & b). The highest inhibition rate onR.salinaof 70% was forA.sanguineaJX13 treatment with 2 000 cells/mL under high nutrient conditions at 48 h.
Diff erent N:P ratios also exhibited remarkable influence onA.sanguineaallelopathic ability (Fig.7c& d). WhenA.sanguineaJX13 and JX14 were 2 000 cells/mL, treatments with N:P of 10:1 and 30:1 had significantly higher inhibition rates than for N:P of 20:1 (P<0.05; Fig.7c & d). TheA.sanguineaJX13 and JX14 at 2 000 cells/mL treatments had higher inhibition rates onR.salinathan treatments with 500 cells/mL. In JX14 treatment at 2 000 cells/mL for N:P of 10:1, the inhibition rate increased by 1.7 times compared with those for N:P of 20:1.
4 DISCUSSION
4.1 Characteristics of allelopathy in A. sanguinea
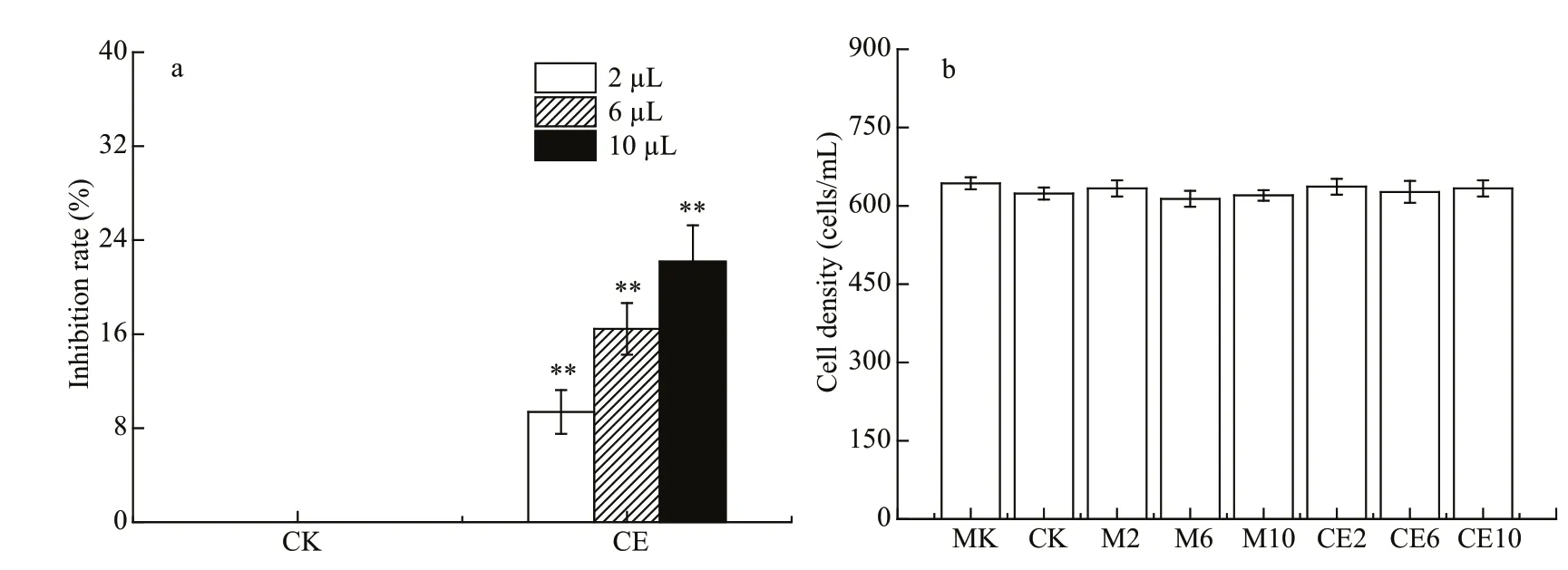
Fig.6 Eff ects of crude extract of A. sanguine a JX14 on growth of R. salina and A. sanguine a AS2
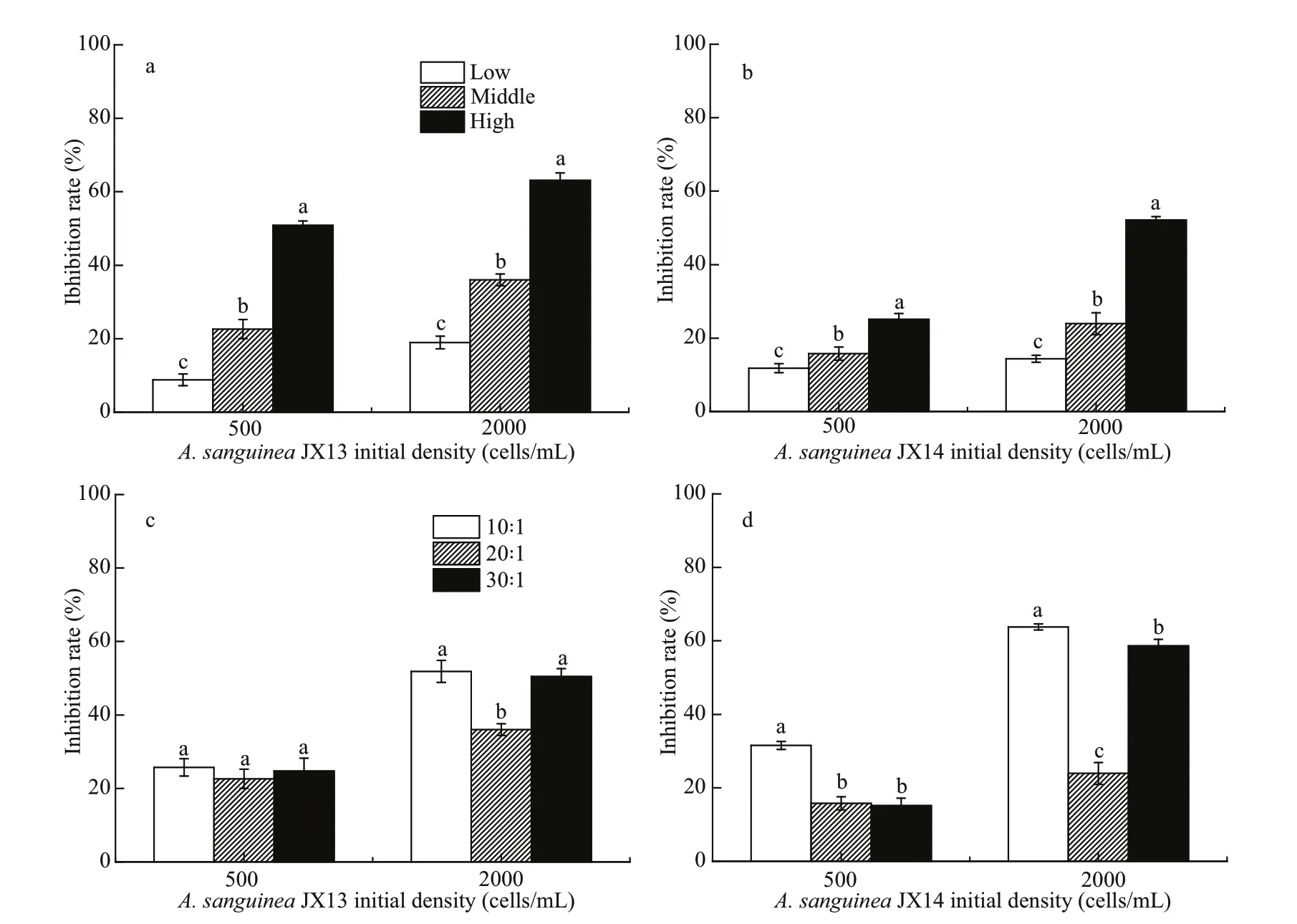
Fig.7 Inhibitory eff ect of A. sanguine a JX13 and JX14 on R. salina at diff erent nutrient concentrations and nutrient ratios
It was demonstrated that strains ofA.sanguineahad allelopathic eff ects on multiple co-occurring phytoplankton species. In the co-culture experiment,A.sanguineaJX14 showed significant inhibitory eff ects on three target species:Ph.globosa,S.trochoideaandR.salina(Figs.1 & 3). For example,the highest inhibition rate was 94% whenA.sanguineaCCMA256 was co-cultured withR.salinaat 72 h(Fig.3). In Section 3.2.1, cell-free treatments (culture filtrate, sonicated culture, and filtrates of sonicated culture) ofA.sanguinea JX14 had inhibitory eff ects onR.salina, suggesting that the inhibitory eff ect did not require direct cell contact. The inhibitory eff ect decreased with time, indicating that the concentration of allelopathic substances decreased (i.e. by consumption or degradation). However, the inhibitory rate of whole-cell culture treatment gradually increased with time, indicating that the complete living cells could continuously release allelopathic substances. The results in Section 3.2.2 showed thatR.salinagrowth was significantly inhibited when allelopathic extracts ofA.sanguinea JX14 were added, and the inhibition rate was positively correlated with the dose of allelopathic extracts. Thus, the allelochemicals could stabilize in the environment and inhibit competitors. These results showed thatA.sanguineaproduced and released allelochemicals—secondary metabolites that inhibit the growth of target species.
The allelopathic eff ects ofA.sanguineasignificantly diff ered among strains isolated from diff erent areas. The results in Section 3.1.2 showed that fourA.sanguineastrains, JX13, JX14, CCMA256,and ASNP6, exhibited significant inhibitory eff ects onR.salina. At the same density (2 000 cells/mL),the Chinese strains (JX13, JX14, and CCMA256)showed stronger toxicity and inhibitory eff ects onR.salinathan the American strains (AS2 and ASNP6).The two American strains exhibited contrasting diff erences in the inhibition rates onR.salina. Even at high cell density, AS2 did not inhibitR.salina, but ASNP6 had an inhibition rate exceeding 40%. There were also some diff erences among Chinese strains,among which the inhibitory eff ects of Xiamen strain CCMA256 was the strongest and the inhibitory eff ect of JX13 was greater than that of JX14, although these two strains were both from samples taken in Daya Bay in the South China Sea. It was previously found thatAlexandriumtamarenseisolated from British waters included both virulent and non-virulent strains(Higman et al., 2001). The variation inA.sanguineatoxicity among strains may be related to their genetic diversity, but this requires more evidence.
The response of phytoplankton toA.sanguineawas species-specific. Cells of three phytoplankton species (Ph.globosa,S.trochoidea, andR.salina),when co-cultured withA.sanguinea, were lysed within 6 h, but no inhibitory eff ects were observed onPr.micans. Thus, allelopathy was not always eff ective. Our previous study also showed thatPseudo-nitzschiaspp. had significant allelopathic inhibitory eff ects onA.sanguinea,R.salinaandChattonellamarina, but speciesProrocentrumminimumandPh.globosawere not aff ected by the presence ofPseudo-nitzschiapungens(Xu et al.,2015). The dinoflagellateK.brevisalso can interfere with the growth of half of phytoplankton tested by allelopathic substances, which was regard as a species-specific allelopathic strategy (Kubanek et al.,2005).
4.2 Mechanisms of the allelopathy in A. sanguinea
The results in Section 3.2.1 further showed the possible allelopathy mechanisms inA.sanguinea.Cell-free filtrate of A. sanguinea JX14 significantly inhibited the growth of target algaR.salina, indicating that JX14 could secrete allelopathic substances to the ambient environment and so aff ect competitors. The allelopathic eff ects of sonicated culture of JX14 were significantly higher than other culture components,with an inhibition rate of around 40% at 48 h,indicating that the allelopathic substances were mostly stored in living cells. Our experiment showed that the allelopathic eff ects of cell-free culture treatments (culture filtrate, sonicated culture, and filtrate of sonicated culture) of JX14 onR.salinagradually weakened over time. However, allelopathic eff ects of whole-cell culture increased over time,demonstrating thatA.sanguineaallelochemicals could be continuously secreted out of the cells. It is reasonable that the allelopathic eff ects would decrease with time without replenishment ofliving cells.Granéli and Johansson (2003) also found thatPrymnesiumparvumculture filtrate had significant inhibitory eff ects onThalassiosiraweissflogii,Rhodomonascf.balticandPr.minimumwithin 36 h,but the growth of the tested algae resumed after 36 h.Our experiments supported thatA.sanguineacells can produce and secret allelochemicals and display allelopathic eff ects on co-existing phytoplankton.Nevertheless, we did not discuss the possible role of cell contact in theA.sanguineaallelopathy. It is clear thatA.sanguineawould directly contact phytoplankton in nature, and further experimental evidence is needed to determine whether direct cellular contact aff ects the strength of allelopathy.
The results in Section 3.2.2 demonstrated very interesting results: in the co-culture experiment(Section 3.1.2), AS2 had no inhibitory eff ect onR.salina(Fig.3); however, once the JX14 crude extract was added, AS2 showed a significant inhibitory eff ect with dosage response. The experimental results further confirmed that allelopathic substances stored in JX14 cells and released into the environment could inhibit the growth of co-existing phytoplankton. It is worth noting that the growth rate of AS2 did not change in the experiment with the addition of JX14 crude extract (Fig.6).
Numerous studies have indicated that increasing HABs are related to eutrophication or excess nutrient loading (Anderson et al., 2008; Heisler et al., 2008;Glibert et al., 2010). The N and P are lost to aquatic environments by multiple pathways, such as fertilizer use for agriculture, animal excretion, industry sewage or atmospheric deposition (Galloway et al., 2002;Glibert et al., 2014, 2018). The results in Section 3.3 showed how nutrients regulated allelopathy inA.sanguinea. We found that high nutrient levels enhanced the inhibition rate onR.salinabyA.sanguinea. It was showed that, whenA.sanguineaJX13 was cultured at high nutrient conditions, the highest inhibition rate onR.salina(70%) was for the highest cell density treatment at 48 h (Fig.7a),indicating thatA.sanguineacells under high nutrient conditions produced more allelochemicals. Similarly,it was reported that, as ambient nitrate concentration increased, an increase in cellular toxin occurred concurrently, andAlexandriumtamarense(ATKR-020415) seemed to utilize excess N for toxin production (Leong et al., 2004).
Additionally, the N:P can also regulate the inhibition rate ofA.sanguineaonR.salina. In our study, the inhibition rates ofA.sanguineawere higher for lower (10:1) or higher (30:1) N: P compared to those around the Redfield ratio (16:1). According to Granéli and Johansson (2003), nutrient restriction can activate the secondary metabolic activity ofPrymnesiumparvum, which raises the allelopathic eff ect on tested algae under nutrient restriction above that under nutrient suffi ciency.
It is noteworthy that our study showed thatA.sanguineaJX14 under N limitation (N:P=10:1)exhibited significantly more potent allelopathic eff ects onR.salinathan under P limitation (30:1).A.sanguineais considered a mixotrophic dinoflagellate,and when environmental nutrients are imbalanced or limited, mixotrophic dinoflagellate turn from primary producers into consumers that feed on other microorganisms to obtain more nutrients (Bockstahler and Coats, 1993). Since imbalanced nutrients favored stronger allelopathy byA.sanguinea, there may be some relationship between allelopathic eff ects and the nutrition strategy ofA.sanguinea.
4.3 Potential ecological implications of the allelopathic eff ects exhibited in A. sanguinea
In regard to studies of allelopathy in microalgae,many reports have focused on inhibitory eff ects on growth of target species (Matsuoka et al., 2000; Adolf et al., 2006; Kim et al., 2016), but rarely mentioned the eff ects of target species on the growth of donor species. In this study, we not only found that the growth rates of target species,Ph.globosa,S.trochoideaandR.salina, were significantly decreased in the co-culture experiments, but also observed that the target phytoplankton were lysed and the growth of donor speciesA.sanguineawas promoted at the same time. For example, in Section 3.1.3, cell density and the specific growth rate ofA.sanguineaJX13 increased by around 40% whenR.salinacell density was 5 000 cells/mL at 72 h. The cell densities of JX13 and JX14 were positively correlated withR.salinacell density at the end of the experiment. This suggested thatA.sanguineaJX13 and JX14 could absorb and utilize the organic nutrients released byR.salinaand so promote their own growth. Hansen and Hjorth (2002) found that growth ofChrysochromulinaericinawas aff ected by prey concentration under suitable illumination. The ingestion rates ofProrocentrumdonghaienseandPr.micanson cyanobacteriaSynechococcussp. increased with increasing prey concentration (Jeong et al.,2005b). It is interesting to note that strain AS2 had no inhibitory eff ect onR.salina; even following addition of allelopathic extracts of JX14 into the co-culture system of AS2 andR.salina, althoughR.salinacells were lysed, the growth of AS2 was not promoted(Fig.6). In other words, JX13 and JX14 seemed able to promote their own growth via allelopathy, during which they produced allelochemicals lysing the cooccurring phytoplankton and exploited the resulting organic nutrients. However, strain AS2 had little (if any) allelopathic eff ect and also had poor heterotrophic capability. Thus, allelopathy should play an important role in the nutrition strategy ofA.sanguinea, which may help it exploit organic nutrients to outcompete co-occurring phytoplankton.
Many dinoflagellate species have been shown to be of mixotrophic type (Jeong et al., 2005a; Jang et al.,2017; Ok et al., 2017), which cannot survive only by photosynthetic autotrophy, and need diff erent kinds of organic nutrients in their growth process, i.e. a heterotrophic pattern. Mixotrophic dinoflagellates play a variety of roles in the marine food web, both as predators, preying on a variety of prey such as bacteria, algae, heterotrophic protozoa and metazoa,and as prey of a variety of predators (Jeong et al.,2016; Lim et al., 2018). Mixotrophic dinoflagellates displayed a growth advantage when feeding mixotrophically compared to strict autotrophic growth (Jeong et al., 2004; Glibert et al., 2009; Flynn et al., 2013).
For example, one study demonstrated the benefits of mixotrophy in the dinoflagellateCochlodiniumpolykrikoides. When growing as a mixotroph, with cryptophytes as prey, the maximum specific growth rate ofC.polykrikoideswas double that when growing as a phototroph (Jeong et al., 2004). In this study,whenA.sanguineaJX13 (initial cell density 1 000 cells/mL) was co-cultured withR.salina(initial cell density 5 000 cells/mL) for 72 h, the cell density of JX13 increased by 50% compared with monoculture(Fig.4a). Obviously, mixotrophic capacity is an important nutrient strategy for dinoflagellates, making them more competitive in conditions ofinorganic nutrient deficiency. Mixotrophy is also regarded as a major mode of nutrition by HABs in eutrophic waters and may contribute to the maintenance of blooms(Burkholder et al., 2008).
It has been reported that organisms with mixotrophic capacity, such asKarlodiniumveneficumandPr.parvum, could release toxins (or allelochemicals) that participate in the capture of prey(Skovgaard and Hansen, 2003; Adolf et al., 2007;Berge et al., 2012). Toxicity and allelopathic eff ects are diff erent representations for diff erent targets:toxins deter predators and allelochemicals inhibit the growth of co-existing algae (Granéli and Salomon,2010). In our study,A.sanguineaused allelochemicals to kill or dissolve competitors (S.trochoidea,Ph.globosa, andR.salina), which inhibited their growth while also promoting its own growth. Remmel and Hambright (2012) suggested thatPrymnesiumtoxins were used to assist micropredation, first attacking gill cells, then releasing toxins to dissolve cell membranes and finally thePrymnesiumabsorbed the contents of the dissolved cells. Thus, mixotrophic species may have advantages in their ability to exploit nutrients acquired from prey, such as bacterial and algal competitors and even from their predators, and toxins or allelochemicals may participate in the process of mixotrophy. Collectively, allelopathy ofA.sanguineamay be related to nutrient strategy, which may play an important role in the formation and persistence ofits blooms.
5 CONCLUSION
It was demonstrated that livingA.sanguineacells could produce and release allelopathic substances continuously, lysing co-existing phytoplankton(R.salina,S.trochoideaandPh.globosa) cells and inhibiting the growth of the target species. Diff erent strains ofA.sanguineadisplayed diff erent allelopathic eff ects onR.salina, and the responses of diff erent phytoplankton toA.sanguineawere species-specific.Allelopathic substances can exist in the environment stably, but may be consumed or degraded with time in the absence oflivingA.sanguineacells. Both nutrients and N:P regulated allelopathy inA.sanguinea. High nutrient level and lower (10:1) or higher (30:1) N:P enhanced the inhibition rate ofA.sanguineaonR.salina.
Results show that growth of toxicA.sanguineaJX13 and JX14 was promoted by the co-occurring phytoplanktonR.salina, but the growth of non-toxicA.sanguineaAS2 was not promoted, suggesting that allelopathy may play an important role in the nutrition strategy ofA.sanguinea.
6 DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
杂志排行
Journal of Oceanology and Limnology的其它文章
- Steady increase in water clarity in Jiaozhou Bay in the Yellow Sea from 2000 to 2018: Observations from MODIS*
- Phylogenetic diversity and bioactivity of culturable deepsea-derived fungi from Okinawa Trough*
- Investigation of the decline of Ulva prolifera in the Subei Shoal and Qingdao based on physiological changes*
- Effi ciency of phosphorus accumulation by plankton,periphyton developed on submerged artificial substrata and metaphyton: in-situ observation in two shallow ponds*
- Petroleum exploitation enriches the sulfonamide resistance gene sul2 in off shore sediments
- Mesoscale wind stress-SST coupling induced feedback to the ocean in the western coast of South America*
