Stability of phenolic compounds and drying characteristics of apple peel as affected by three drying treatments
2021-06-05QinchengJinfengBiJinyongYiXinyeWuXunLiYunyunZho
Qincheng M Jinfeng Bi*, Jinyong Yi*, Xinye Wu Xun Li Yunyun Zho
a Key Laboratory of Agro-products Processing, Ministry of Agriculture and Rural Affairs, Institute of Food Science and Technology, Chinese Academy of Agricultural Sciences, Beijing 100193, China
b School of Food Science and Technology, Dalian Polytechnic University, Dalian 116034, China
ABSTRACT
To realize high-value utilization of discarded apple peel, this study investigated the effects of three selected commercial drying methods on drying kinetics, microstructure, color, phenolic stability and antioxidant capacity of apple peel. Apple peel was dehydrated by hot air drying (AD) at 75, 65 and 55 °C, heat pump drying (HP) at 65, 55 and 45 °C, and vacuum freeze-drying (FD), respectively. The results showed that HP was superior to AD and FD in terms of drying rate. In addition, HP at 65 °C provided high-quality dried apple peel due to less browning and brighter appearance, the highest retention of total phenolics (29.35 mg/g) and the strongest antioxidant activities, with the highest ABTS and FRAP value of 127.15 and 219.57 μmol TE/g,respectively. The content of the six major individual phenolics, i.e. caffeic acid, (-)-epicatechin, hyperoside,rutin, phlorizin and quercitrin, were found to be the highest in HP dried samples. Interestingly, the content of rutin was even increased after HP compared to the fresh sample. Considering drying efficiency, organoleptic quality and phenolic stability of the products, HP at 65 °C is suggested for drying of apple peel.
Keywords:
Apple peel
Heat pump drying
Phenolic compounds
Antioxidant capacity
Color
1. Introduction
Apple is known to be one of the most widely planted fruits in the world [1], and the largest planting area and peak annual output are owned by China. Apple has been processed into many kinds of products, including juice, puree, chips, vinegar, etc. It is worthy to note that over 3 million tons of apples were annually processed in China (source from National Bureau of Statistics of China), which produced large quantities of discarded pomace and peel, thus in arising a concern of resource utilization. The strong antioxidant capability of apple peel has been proved in previous studies, for instance, the major contributor of antioxidant capacity in apple peel was phenolics, whose contents were about two to seven-fold higher than pulp fraction [2]. Meanwhile, the characteristic flavonoids(e.g. quercetin glycosides) that were absent in pulp conferred high antiradical activity to apple peel [3]. Although apple pomace has been used in industrial production of fermented feed and pectin [4], the peel has not realized industrialization as an antioxidant additive in food or health care products. There fore, it was possible for apple peel to be processed as an ingredient of functional foods if processing will not cause large nutritional losses and easy to handle directly in apple processing factory.
Apple peel with moisture content of 80% is difficult to store due to the spoilage leaded by microbial decomposition. Drying is one of the extensively used methods to extend storage life of raw materials through removing water to inhibit spoilage as well as reduce the activity of enzymes [1]. Hot air drying is one of the most common drying methods with advantages of simple operation and low cost,but it has a negative impact on the color and quality of dried products[5]. Heat pump dryer can control the air humidity and recycle both the latent and sensible heat from the exhaust air passing, which exhibits its feature of energy-saving [6]. Compared with the thermal drying methods, vacuum freeze-drying obtains the highest quality of product by sublimating water under low temperature and anoxic environment,but it may not be suitable for low value-added materials like apple peel,because of expensive cost and time-consuming drying procedure [5].Mphahlele et al. and Dorta et al. [7,8] have found that vacuum freezedrying retained the highest contents of bioactive compounds for pomegranate peel and mango peel. While some researchers have reported the better quality of the dried by-products obtained by means of controlling the temperature of hot air or combining hot air drying with microwave and ultrasound, such as tomato peel [9] and pitted olive pomace [10]. Perera and Rahman [11] claimed that compared with hot air drying, heat pump drying was more energy-efficiency and kept higher retention of thermo sensitive substances like phenolics [12],however, it has been largely unexploited in fruit and vegetable processing by-products. Some people found that heat pump may be preferred in terms of drying performance and product qualities when they studied the drying of grape pomace [13,14].
The key step to increase the value of apple peel is to find a fast,convenient, energy-efficient drying method. We speculated that heat pump drying might be an effective procedure for apple peel drying based on drying rate and overall qualities. The present study concerned the selection of the most suitable drying technology for industrial production of apple peel among hot air drying, heat pump drying and freeze drying by investigating the influence of different drying methods on drying efficiency, color, nutritional attribution and antioxidant capacity of apple peel.
2. Materials and Methods
2.1 Sample preparation
Fuji apples from Shandong province were bought from the local market in Beijing. Apples were selected in the light of uniformed epidermis color (striped red) and consistent mature degree and then lightly flushed and manually peeled with a peeler knife to obtain the peels with 3 mm thinness. The peels were packed into zip lock bags to avoid browning before drying operation.
2.2 Drying experiments
2.2.1 Hot air drying (AD)
The apple peel was dried by a hot air dryer (DHG-9023A, Yiheng Technology Co., Ltd, Shanghai, China) at 75 °C (AD75), 65 °C(AD65) and 55 °C (AD55), respectively, with constant air velocity of 2.11 m/s and fluctuant relative humidity of (25 ± 5.0)%.
2.2.2 Heat pump drying (HP)
The apple peel was dried by a heat pump dryer with a closed-loop dehumidification system (Zhengxu New Energy Technology Co., Ltd,Dongguan, China) at 65 °C (HP65), 55 °C (HP55) and 45 °C (HP45),respectively. In fact, the maximum temperature of the heat pump dryer was limited in 65 °C according to the equipment specification.The drying experiments were conducted at 12% relative humidity with constant air velocity of 2.5 m/s.
2.2.3 Freeze drying (FD)
The apple peel was firstly frozen at -80 °C for 12 h, and then dried by a vacuum freeze dryer (Alpha1-4Lplus, Marin Christ,Osterode, Germany). The FD experiment was performed at vacuum pressure of 0.01 kPa, condenser temperature of -55 °C and shelf temperature of 20 °C [9].
During the initial drying period, the samples dried by AD and HP were weighed every 3 min and every 15 min thereafter until the moisture content of the sample reaching 0.06 g/g (dry basic, d.b.),at which point the equilibrium moisture content was obtained [15].Then, the samples were crushed by a grinder for 5 times of 10 s with interval of 20 s to prevent nutritional losses caused by overheating.There were three batches of powder were collected for each sample.
2.3 Drying kinetics
The moisture ratio (MR) of apple peel was calculated by the following Eq. (1) [16]:

where Mtis moisture content (g/g, d.b.) at t time; M0and Meare initial and equilibrium moisture contents (g/g, d.b.), respectively.
The drying rate (DR) was calculated according to the following Eq. (2) [17]:

where Mt+dtand Mtare moisture contents (g/g d.b.) at t + dt and t time, respectively.
2.4 Specific energy consumption
The specific energy consumption of different drying methods was calculated by the following Eq. (3):

where E is the specific energy consumption (kW·h/kg); W0and W1are the watt hour meter readings (kW·h) at the beginning and the end of drying, respectively; m is the mass of moisture loss (kg) in apple peel.
2.5 Microstructure
The cross-section microstructure of dried apple peel was observed by scanning electron microscopy (S-570, Hitachi Ltd., Japan). The sample was fixed on a copper stub with the cross-section upward, and then plated by gold though ion sputtering apparatus. The scanning images were observed at magnification of 110 ×.
2.6 Color
The appearance of dried apple peel was detected by using a DigiEye (V270, HunterLab Co., Ltd, Reston, USA), which is composed of an illumination cabinet, a computer and a camera(D3500, Nikon, Tokyo, Japan). The color measurement of dried sample included four steps, (i) extract the image of the sample from the background, (ii) predefine the RGB range values of the sample as a reference, (iii) adjust the predefined range values until the correct separation of the sample from background, (iv) record the values of color parameter at this time. The overall color difference (ΔE) of dried sample was given in the Eq. (4) [18]:
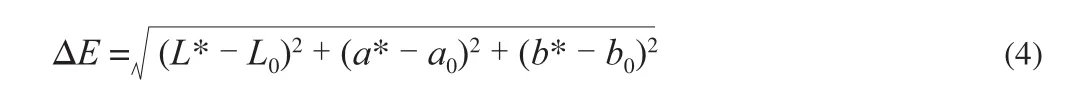
where L*, a* and b* indicate the lightness, the red-green value and the yellow-blue value, respectively, while L0, a0and b0are those of the fresh sample.
2.7 Titratable acid (TA)
TA content was determined by a potentiometric titrimeter (907 Titrando, Switzerland Wantong China Co., Ltd, Beijing, China), and calculated as the following Eq. (5) [19]:

where c (mol/L) and V1(mL) are the concentration and the volume of NaOH solution, respectively, V0is the volume of the sample solution (mL), m is the mass of the sample (g), k is the conversion coefficient, 0.067 g/mmol (malic acid).
2.8 Total sugar
Total sugar content was determined through phenol-sulfuric acid method [19]. Apple peel powder of 0.3 g was mixed up with 20 mL distilled water and 2.0 mL HCl (6.0 mol/L) and then heated in water bath at 96 °C for 2 h. After cooling down for 20 min, 2.0 mL 6.0 mol/L NaOH was added into the mixture and then centrifuged at 10 000 × g for 15 min. Finally, the supernatant was diluted with distilled water to 50 mL. The absorbance of above supernatant was measured at 490 nm using an ultraviolet visible spectrophotometer(UV1800, Shimadzu, Japan).
2.9 Ascorbic acid (VC)
The VC content was determined as described by Liu et al. [20]with some modifications. Apple peel powder of 1.0 g was mixed with Oxalic acid-EDTA solution to 25 mL, and then centrifuged at 10 000 × g for 10 min to obtain the supernatant. Next, mixed 5.0 mL supernatant with 1.5 mL 3% metaphosphoric acid-acetic acid, 2.0 mL 5% sulfuric acid and 2.0 mL 5% molybdate-ammonium, and followed by adding distilled water to 25 mL. Then the above mixture was heated in a water bath at 30 °C for 20 min and kept in room temperature at 24 °C for 1 h. Finally, the VC content was measured with the ultraviolet visible spectrophotometer at 700 nm.
2.10 Total dietary fiber (TDF)
The TDF content was determined using AOAC [21] with a few modifications. Apple peel powder (1.0 g) was successively hydrolyzed with α-amylase (50 μL) at 95 °C for 35 min, protease(100 μL) at 60 °C for 30 min and amyloglucosidase (100 μL) at 60 °C for 30 min in a beaker. The enzymatic hydrolysate was mixed with 95% ethanol of 4 times volume and precipitated at room temperature for 1 h. The digested sample was filtrated using a dietary fiber filtration instrument (CFS6-GDE, Velp Scientific Co., Ltd., Italy). The residue was washed by 78% ethanol, 95% ethanol and acetone for two times, respectively, and then dried at 105 °C overnight and weighed.The protein content of the residue was detected by using an automatic Kjeldahl apparatus (Kjetec, Foss, Hillerod, Denmark), the ash content was detected by dried at 525 °C for 5 h in a muffle furnace (SX2-4-10N, Yiheng Experimental Equipment Co., Ltd, Shanghai, China).The measurement of TDF was conducted in duplicate and calculated as the following Eq. (6) [21]:

where mR, mP, mA, mBand m are the mass (g) of residue, protein,ash, blank sample and average sample, respectively.
2.11 Total phenolics
Apple peel powder of 0.3 g was mixed with 80% (V/V) methanol of 30 mL, carried out ultrasonic wave for 90 min in the dark, and then centrifuged at 10 000 × g for 15 min. The supernatant was collected and kept in a refrigerator at 4 °C to avoid light before qualitative and quantitative analysis for phenolics. The total phenolics content (TPc) was measured using the Folin-Ciocalteu method reported by Jeong et al. [22].Results were expressed as mg gallic acid equivalents per 100 g(mg GAE/100 g) of the dry sample.
2.12 Identification of phenolic compounds
Identification of phenolic compounds was performed using HPLC as described by Ran et al. [23] with some modifications.The previous obtained supernatant was filtered through a 0.45 μm micropore membrane before analysis by HPLC system (Waters-1525,Waters, USA), which was composed of a photo-diode array (PDA)detector and a C18column (250 mm × 4.6 mm) with thermostatically controlled at 35 °C. The mobile phase was consisted of 2% (V/V)acetic acid in water (eluent A) and methyl alcohol (eluent B). The monitor wavelength was 280 nm. Standard curve was made by the following phenolic standards: gallic acid, protocatechuic acid,neochlorogenic, (+)-catechin, chlorogenic acid, caffeic acid, ferulic acid, (-)-epicatechin, hyperoside, rutin, phlorizin and quercitrin.
2.13 Antioxidant capacity
Antioxidant capacity was evaluated by the experiments of DPPH and ABTS free radical scavenging abilities and ferric reducing ability of plasma (FRAP), as described by Si et al. [24]. Briefly, in the measurement of DPPH value,five times diluted supernatants of 2.0 mL were added into 0.1 mmol/L DPPH solution of 4.0 mL and kept in the dark for 30 min, then the absorbance of the mixture at 517 nm was measured by ultraviolet visible spectrophotometer. In the measurement of ABTS value, 2.45 mmol/L K2S2O8solution and 7 mmol/L ABTS were mixed (1:1, V/V) to obtain ABTS+solution and then kept in the dark for 14 h at room temperature. The ABTS+solution was mixed with 80% methanol to achieve an absorbance of 0.70 ± 0.02 at 734 nm. Ten times diluted supernatants of 0.4 mL were reacted with 3.6 mL ABTS+solution for 1 min, then the absorbance of the mixture was measured at 734 nm. As for the measurement of FRAP value, the mixture of five-times diluted supernatants of 0.2 mL and FRAP solution of 6.0 mL was placed in water bath at 37 °C for 30 min, then the absorbance of the mixture at 593 nm was measured.A standard curve was obtained using different concentrations of Trolox solution. Results were expressed as μmol Trolox equivalents per gram (μmol TE/g) of the dry sample.
2.14 Statistical analysis
All experiments were repeated in triplicate. The results were reported as mean ± standard deviation and the data recorded in dry basis (d.b.). Statistical analyses were performed using Excel,OriginPro and SPSS software. Significant differences between means were calculated by ANOVA and Duncan’s test at a significance level of P ≤ 0.05.
3. Results and Discussion
3.1 Drying kinetics
As shown in Fig. 1a and 1b, the MRs of dried apple peel were decreased continuously as the drying exposure time of AD and HP increases. The moisture content of apple peel treated by AD was reduced to 0.06 g/g d.b. in 45 min for AD75, 75 min for AD65 and 90 min for AD55, respectively. As for HP, the drying time was 65 min for HP65, 80 min for HP55 and 135 min for HP45, respectively, while the FD took approximately 40 h. Besides, Fig. 1c and 1d shows that the drying time of HP at 65 °C and 55 °C was about 13% and 11% shorter than that of AD at the same drying temperatures, respectively.It might be due to the air velocity of HP was higher than that of AD, which was consistent with the observation of Taeri et al. [14].Meanwhile, the more obvious humidity difference between the dry air, the sample could accelerate the evaporation of material moisture during HP processes [25].
Moreover, the variations of DRs versus moisture content (d.b.)are shown in Fig. 1e and 1f. It can be seen that DRs decreased continuously with decreasing moisture content, which was because the content of free water was higher in the initial stage of drying,after that the internal diffusion mechanism dominated due to the remaining surface-bound water and the shrinkage of apple peel [26].In both drying methods, DRs increased with the increasing of drying temperature, this finding was in agreement with the studies on garlic slices and jujube slices [26,27]. As shown in Fig. 1g and 1h, the initial drying rates were 0.202 and 0.151 g/g·min for the samples of AD65 and AD55, respectively, and 0.210 and 0.199 g/g·min at HP65 and HP55, respectively, which indicated that HP at lower temperature could achieve similar dehydration rate as AD at a higher temperature. The result was consistent with the study of Ong, Law,and Hii [28], who considered that the lower air humidity resulted by water vapor condensation during HP contributed to improve driving force for dehydration on material surface, hence increasing the drying rate. When the moisture content dropped to about 0.43 g/g·min, the drying rate of AD65 was resemble to that of HP65. Jangam and Mujumdar [29] explained that drying rate was rarely enhanced by the dehumidified drying air in late drying period.

Fig. 1 The drying curves of apple peel during AD and HP processes at different temperatures. a-d. The moisture ratio of apple peel during drying processes. e-h.The drying rates of apple peel during drying processes.
Besides, specific energy consumption was an important parameter to evaluate the drying process, which was of great significance in practical production. The specific energy consumption cost of FD (25.75 kW·h/kg) was much higher than that of AD (4.88-10.24 kW·h/kg) and HP (3.00-5.42 kW·h/kg). With the increase of drying temperature and the shortening of drying time, the specific energy consumption decreased. The lower values of specific energy consumption of HP were due to the fact that the heat released by water vapor condensation could be recycled in the HP dryer. To sum up, HP featured with higher drying efficiency for drying of apple peel as compared with AD.
3.2 Appearance and color parameters
The appearance of dried apple peel subjected with different drying methods is shown in Fig. 2. FD sample was bright and light without shrinkage, which could be as a standard form. Compared with FD sample, AD and HP samples dried at different temperatures generated substantial shrinkage. The AD sample appearance presented dark red color with obvious browning and exhibited darker as the drying temperature increases, while HP sample showed a relative bright appearance that was similar to the FD one. Notably, the darkest color was observed in HP45 sample among all the HP samples, which possibly due to the excessive exposure time, resulting in degradation of pigments or aggravation of non-enzymatic browning [30].
The drying conditions significantly influenced the color parameters of the dried samples as the obviously reduction ofL*anda* values contrasted with the increase ofΔEvalue were observed in Table 1, which considered as the aggravation of browning degree [31].Compared with the fresh sample, theL* value (82.88) of FD sample was increased slightly, thea* andb* values were decreased, which contributed to the lowest ΔEvalue (21.00). Less browning of apple peel caused by the low temperature and vacuum conditions during FD, meanwhile, the presence of sponge-like tissue voids led to the light appearance. As for AD sample, theL* anda* values decreased,and ΔEvalues increased with the increasing of drying temperature.No significant difference was exhibited in terms ofL* anda* values among HP samples dried at 65 and 55 °C as well as FD sample.The research of Chin and Law [32] has reported that HP obviously improved the color change ofGanoderma tsugaeas compared with AD. It could be explained that enzymatic and non-enzymatic browning were likely to happen when apple peel exposed to heat and oxygen during drying, while circulating low humidity air and short effective drying time throughout HP process could reduce the extent of the non-enzymatic browning effectively, contributing to maintain the original color of the sample [32,33]. The color degradation of AD75 and HP45 samples could be ascribed to the high temperature and excessive heat time during drying process, respectively, which were also stated by José A et al. [34].
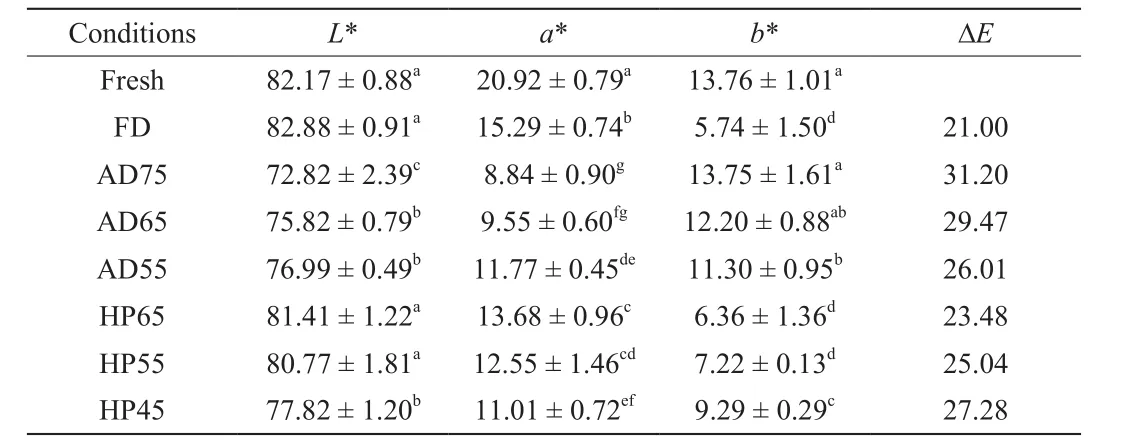
Table 1Color parameters of apple peel dried by FD, AD and HP.
3.3 Microstructure
The effects of different drying methods on the cross-section microstructure of the apple peel are displayed in Fig. 3. FD sample exhibited porous structure with thin pore wall, demonstrating that FD played a positive role in maintaining the porous cellular structure of plant tissue. As described by Chen et al. [35], the porous microstructure of FD sample was formed by ice sublimation in the vacuum environment without cellular shrinkage and collapse from external forces. The AD and HP samples were observed thicker pore walls and much more dense structures than those of FD sample,which was ascribed to the shrinkage and collapse of cells during the evaporation of water. In addition, a thin wax layer was observed on apple peel surface under scanning electron microscopy.

Fig. 2 The appearance of apple peel dried by FD, AD and HP.
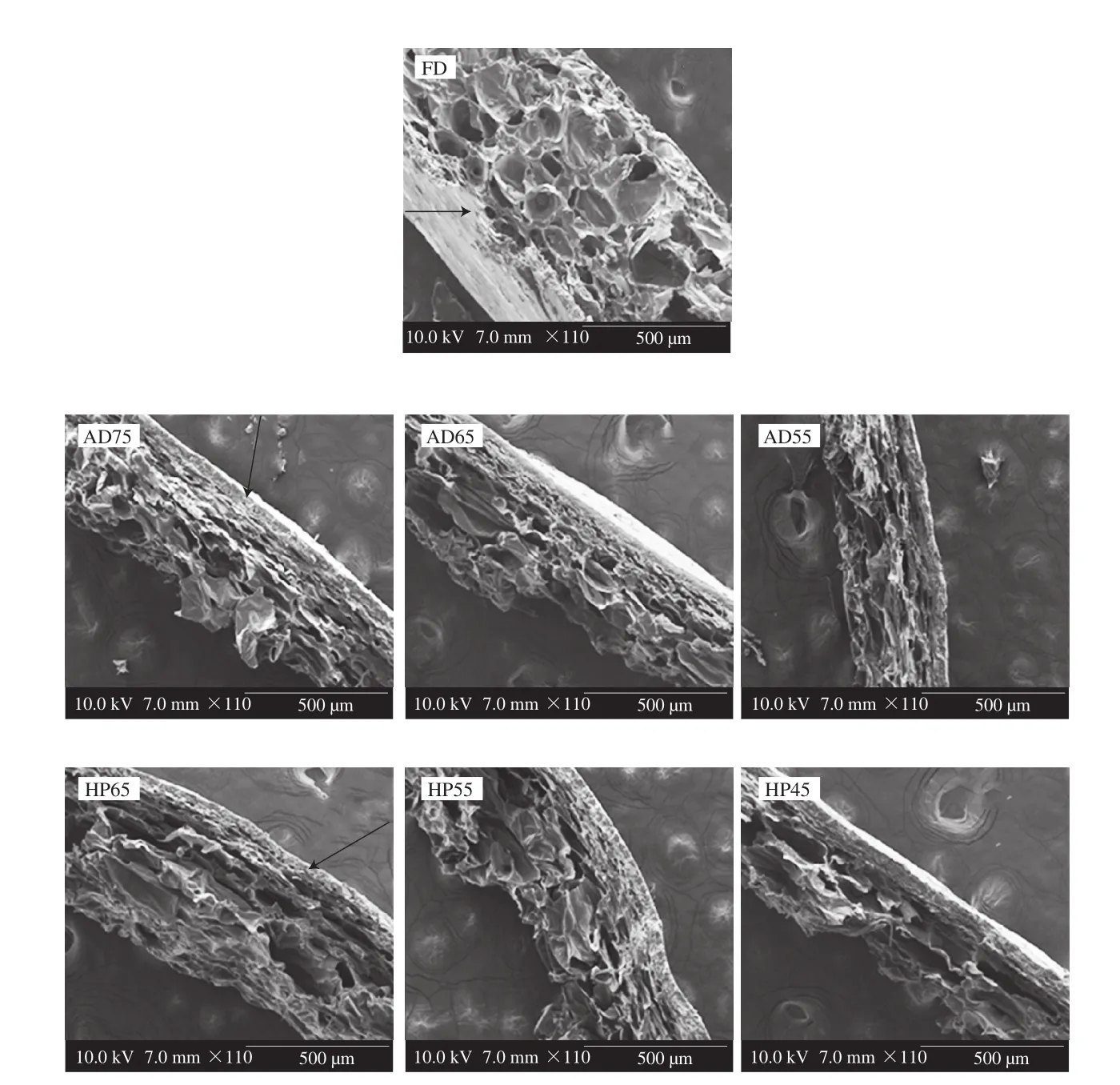
Fig. 3 The microstructure images of cross-section of apple peel dried by FD, AD and HP. The images were captured by using scanning electronic microscope under × 110 magnification.
3.4 Total sugar, titratable acid, ascorbic acid, and total dietary fiber
The evaluation of basic nutritional ingredients of dried apple peel in this study was done, as shown in Table 2. FD was considered as the superior drying method to maximally keep original appearance and nutrients of fresh materials [5], thus, the retention of nutrients for FD sample was used as control. The content of total sugar and titratable acid could affect the flavor and taste of products which added dried apple peel used as dietary supplement. The highest and the lowest total sugar contents were found in AD75 sample (506.68 mg/g) and HP65 sample (431.68 mg/g), respectively, and the highest (28.67%)and lowest (19.28%) contents of titratable acid were observed in FD sample and HP45 sample, respectively. The retention content of VC is usually selected as one of evaluation indexes of drying method because of its unstable characteristic under drying process [36].The highest VC content was obtained in HP sample (1.30 mg/g),followed by FD sample (1.03 mg/g). Notably, no difference among the VC contents subjected to HP at different drying temperatures was found, while the content of VC was significantly affected by drying temperatures for AD. In details, when compared with VC content of FD sample, 8% and 4% reduction were observed in AD samples at 65 and 55 °C conditions, whereas the value increased 26% of HP samples at the same temperatures. Some studies reported that long drying time and high temperature gave rise to the oxidation and degradation of VC, further leading to the lower content of VC in final products [37].Apple peel provided a good source of TDF, whose contents of different dried apple peel ranged from 34.22 g/100 g to 38.71 g/100 g and no significant difference was existed among these treatments.This result was consistent with the study by Liu et al. [38], who also indicated that different drying methods could change the ratio of insoluble to soluble dietary fiber in orange peels, but will not affect the content of TDF.

Table 2Basic nutritional components of apple peel dried by FD, AD and HP.
3.5 Total content of phenolics and identification of individual phenolic compounds
Phenolic compounds played an important role in antioxidant property of apple peel rather than VC [39]. As shown in Fig. 4, the highest TPc was observed in HP65 sample (2 939.45 mg GAE/100 g),followed by FD sample (2 740.24 mg GAE/100 g), and compared with the fresh sample (4 094.25 mg GAE/100 g), the losses of TPc were 28.21% and 33.08%, respectively. Similarly, Harbourne et al.[40] also found that the lower TPc of willow bark treated by FD than some thermal drying methods. This might be attributed to the increased activity of enzymes upon thawing caused degradation of some phenolic compounds [41], or the relative complete porous microstructure of FD sample affected the release and dissolution of phenolic compounds. Besides, AD75 sample exhibited the lowest TPc(2 498.11 mg GAE/100 g), and Liu et al. [42] explained that some of phenolic compounds were more vulnerable to be degraded at high temperature and oxygen environment. It also might be attributed to the combination of phenolic with polysaccharide or proteins at high temperature that failed to be detected using the same methods.

Fig. 4 The total phenolic contents of apple peel dried by FD, AD and HP.Bars represent mean ± standard deviation (n = 3). Different letters indicate significant difference (P < 0.05).
In order to further explore the influence of different drying methods on the retention of individual phenolic substances, the phenolic compounds were identified and quantified by HPLC,as showed in Table 3. Seven dominant phenolic substances were detected from the dried apple peel, they were chlorogenic acid, caffeic acid, (-)-epicatechin, hyperoside, rutin, phlorizin and quercitrin. The contents of these individual phenolic substances were significantly decreased after drying operation, except for rutin. Rutin was the most abundant flavonoid in fresh Fuji apple peel with content of 2793.12 μg/g, and its contents increased in AD65 and AD55 samples and all HP samples. The highest and the lowest contents of rutin were found in HP55 sample (3 422.32 μg/g) and AD75 sample (1 976.63 μg/g),respectively. The increase of phenolics might be attributed to a release of bound phenolics from the plant tissue caused by drying or grinding [43]. Moreover, rutin was also protected by other phenolics such as chlorogenic acid which was more easily to be decomposed in heat condition, thus caused rutin to escape from degradation. The highest content of chlorogenic acid was observed in FD sample(1 023.91 μg/g), and the content was obviously affected by drying temperature during AD and HP, which due to that theO-diphenolic hydroxyl groups of chlorogenic acid molecules were vulnerable to be oxidative decomposed at high temperature. As for caffeic acid and hyperoside, there was no significant difference in the retention among different drying methods. Rizzi and Boekley [44] reported that the degradation of caffeic acid occurred at 80 °C, while the temperatures in this study were not high enough to show this degradation. In addition, the highest retentions of phlorizin and quercitrin of HP65 sample were 1.25 and 1.69 times higher than the minimum retentions in FD sample, respectively. Severe degradation was found in these phenolic substances of all the AD samples, which could be illustrated that the relatively independent and efficient operation mode of HP with the characteristics of dehumidification played a remarkable role for the phenolic preservation during drying.
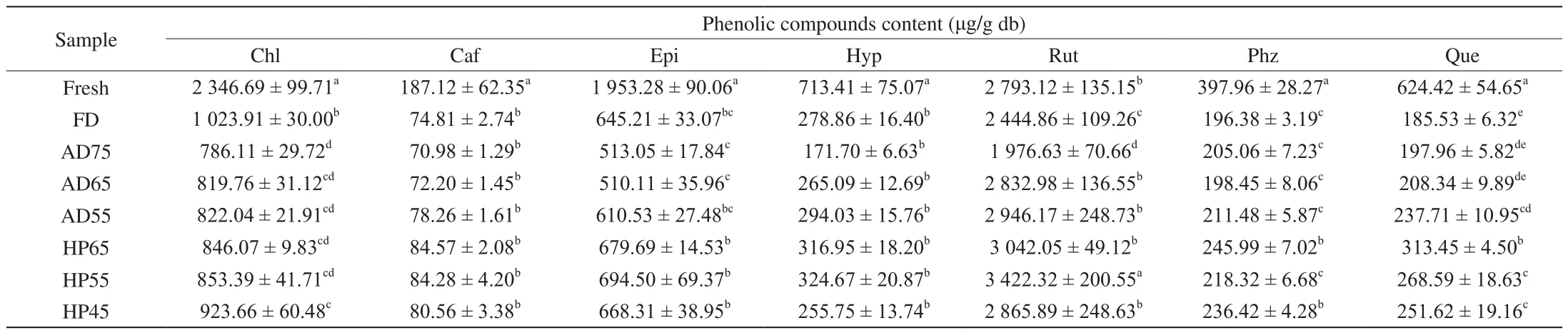
Table 3The contents of individual phenolic substances of apple peel dried by FD, AD and HP.
3.7 Antioxidant capacity
The antioxidant activities of dried apple peel produced by different drying methods are shown in Table 4. The lowest two DPPH radical scavenging abilities were found in FD sample (37.24 μmol TE/g)and HP45 sample (46.14 μmol TE/g), respectively, and there was no difference in DPPH radical scavenging ability among other dried samples. For ABTS and FRAP assays, the highest radical scavenging ability and reducing ability were found in HP65 sample, with the values of 127.15 and 219.57 μmol TE/g, respectively. Compared to the fresh sample, about 77.91% of ABTS radical scavenging ability and 72.54% of FRAP were retained in HP65. Besides, AD75 sample showed the weakest ABTS radical scavenging ability (84.70 μmol TE/g)and FRAP (174.98 μmol TE/g), and this might be ascribed to the antioxidant degradation caused by high drying temperature.

Table 4Antioxidant activities (DPPH, ABTS and FRAP) of apple peel dried by FD,AD and HP.
Based on the evaluation from the DPPH, ABTS and FRAP assays,it was found that drying process resulted in antioxidants degradation of apple peel, which was in agreement with the reports of Tomaino et al. [45]. As drying temperature reduced, significantly increase in antioxidant activity of AD sample was noted, which was ascribed to the thermal degradation of phenolic compounds [46]. Generally, the antioxidant capacity of the dried samples was positively correlated with the contents of phenolic compounds. Thus, HP samples performed better abilities in maintaining the antioxidant capacity was resulted from its higher phenolic compounds content. However, it was also found that antioxidant ability of HP samples treated at different temperatures had no positive correlation with phenolic content, which might be because the free radical scavenging ability of the peel after drying was not only related to the content of total phenolic, but also might be related to the non-heat-sensitive antioxidant component in the peel. In contrast, FD had no advantage of keeping antioxidants in dried apple peel as compared with HP. Similar results were also found by Si et al. [24] and Wojdyło et al. [47], it might be caused by the polyphenol oxidase lost activity in high temperature,meanwhile, high temperature promoted cell wall rupture and released hydrolase that might be inactivated, thus in preventing the antioxidant activity of from being destroyed [48]. In addition,Shewale, Rajoriya, and Hebbar [49] have reported that drying at low humidity air resulted in better retention of ascorbic acid and phenolic, especially for heat sensitive fruits and vegetables.Although FD was considered as a proper method for processing better dried products, HP technology was more superior to retain the higher antioxidant potential in this work due to the lower humidity and less air replacement during drying in the HP dryer with the closed-loop dehumidification system [11].
4. Conclusion
Apple peel obtained as by-product in apple processing industries could be stored and utilized by dehydration process. This research evaluated the effects of the three commercialized drying methods on the changes of overall quality, including physical properties,nutritional bioactive components and antioxidant activity of apple peel. Data from this research suggested that the apple peel dried by HP at 65 °C showed relatively high drying rate, exhibited high retention of VC and phenolic, and displayed high antioxidant capacity. In addition, the extent of browning of the apple peel was limited by HP at 65 °C, indicating a brighter appearance. Moreover, HP was in favor of preserving these most abundant individual phenolic substances of apple peel, as compared with AD and even FD, and thus maintaining high antioxidant activity. Considering drying efficiency and overall qualities of products, HP at 65 °C is an effective approach to obtain high quality dried apple peel with superior color and abundant antioxidants.
Acknowledgement
This work was supported by the National Key R & D Program of China (2016YFD0400700, 2016YFD0400704) and the Central Public-interest Scientific Institution Basal Research Fund (No.S2019RCCG01).
Conflict of Interest
The authors declare that there is no conflict of interests regarding the publication of this article.
杂志排行
食品科学与人类健康(英文)的其它文章
- Bioactive compounds and probiotics-a ray of hope in COVID-19 management
- Approaches to evaluate nutrition of minerals in food
- The role of glutamine in supporting gut health and neuropsychiatric factors
- Anti-hyperglycemic effects of dihydromyricetin in streptozotocin-induced diabetic rats
- Aroma profile of two commercial truffle species from Yunnan and Sichuan, China:inter- and intraspecific variability and shared key compounds
- Development of water-soluble zein colloid particles and in situ antibacterial evaluation by multiple headspace extraction gas chromatography
