Development of water-soluble zein colloid particles and in situ antibacterial evaluation by multiple headspace extraction gas chromatography
2021-06-05YanYinFuzhenZhouYechongYinYonghongPeng
Yan Yin*, Fuzhen Zhou, Yechong Yin, Yonghong Peng*
a School of Life Science, Huizhou University, Huizhou 516007, China;
b Research and Development Center of Food Proteins, School of Food Science and Engineering, South China University of Technology, Guangzhou 510640, China;
c School of Life Science, South China Normal University, Guangzhou 510640, China
ABSTRACT
In this work, water-soluble zein/sodium caseinate (SC) nanoparticles were prepared as a delivery system of antimicrobials through anti-solvent procedure. Two model antimicrobials (nisin and thymol) were coencapsulated into zein particles by different interaction. Release properties indicate that zein colloid particles can be used not only in neutral food but also in acidic foods. It is worth mention that the release of thymol was delayed simply by adding nisin. To investigate the antimicrobial properties of zein/SC particles,Escherichia coli and Bacillus subtilis, Listeria monocytogenes were selected as gram-negative and grampositive indicator bacteria. CO2 produced by incubated germs was detected by headspace gas chromatography.Long-term effective antimicrobial activities of zein particles in Luria broth was attained by burst release of nisin and slowly sustained release of thymol. Synergistic antimicromibial properties against L. monocytogenes of thymol and nisin loaded by zein/SC particles in milk were also observed. The antimicrobial property of zein/SC particles could enhance microbial safety of food, giving this delivery system promising applications in food industry.
Keywords:
Zein/SC nanoparticles
Antimicrobial
Thymol
Nisin
Delivery system
1. Introduction
Although processing technology has been rapidly developed,foodborne diseases stem from the recontamination of ready-toeat (RTE) foods by pathogenic and spoilage microorganisms remains [1-3]. Commercial antibacterial agents are often used for food microbiological safety assurance to control food quality. Thereinto natural antimicrobials becomes more and more prevalent because of the development of environmental awareness. However, many defects arise when natural antimicrobials directly mix into foods, e.g. unpleasant odor,low stability and short duration. Developing long-term effective antimicrobial delivery systems is a feasible operation.
Nisin, as a well-studied antimicrobial, has been widely applied in foods against gram-positive bacteria, including Listeria monocytogenes and Bacillus subtilis. Unfortunately, the activity of nisin is easily affected by the environmental factors [4]. Thymol extracted from the thyme plant is GRAS. Thymol exhibits effective inhibition against spoilage bacteria and food borne pathogens, e.g.,Salmonella typhimurium, Escherichia coli, and L. monocytogenes [5,6].Nevertheless, the above-mentioned defects of direct mixing seriously limited the application in food industry. Encapsulating antimicrobials in colloidal particles is a valid solution. The particle as carrier of antimicrobials in food matrixes is conducive to maintain the longterm effectiveness of antimicrobials via the sustained release strategy.Besides, synergistic antimicrobial effect between free nisin and thymol was observed in previous studies [7-10]. Neither burst release nor initial low followed by sustained release of antibacterial agents seems to result in an ideal antibacterial effect, yet combining the both may have an unexpected effect.
Zein with generally recognized-as-safe (GRAS) status has been used in foods for a long time [11]. Zein is insoluble in water but soluble in 40%-95% ethanol aqueous solution [12]. Zein is capable of self-assembly to form various nano-/micro-structures due to its amphiphilicity [13,14]. In recent decades, zein-based functional colloidal structures have attracted wide interest, especially in delivery of bioactive agents [15]. Many methods have been developed to prepare zein-based colloid particles, including antisolvent process, spray drying and flash nanoprecipitation [16].Usually, plain zein colloidal particles possess poor colloidal stability and limited redispersibility in water. Therefore, a series of stabilizers were introduced into zein-based carrier systems, including lecithin[17], sodium caseinate [3,18,19], β-lactoglobulin [20], pectin [21]. In previous reports, antimicrobial substances like lysozyme [22], thymol [3],nisin [23], silver [24] were encapsulated in zein delivery system by spray drying. Anyway, zein-based nanoparticle is a potential delivery system for antimicrobials.
In this work, sodium caseinate, as a stabilizer to improve redispersibility of zein particles, was used to fabricate an appropriate colloidal delivery system for simultaneous encapsulation of thymol and nisin. Particle size, surface potential, microstructure, antibacterial properties and the release dynamics of antimicrobial nanoparticles were evaluated. Headspace gas chromatography technique for accurately monitoring bacterial growth is employed to characterize the antibacterial properties of antimicrobial zein colloid particles.E. coli and B. subtilis, L. monocytogenes were selected as gramnegative and gram-positive indicator bacterial. Synergistic effect is figured out between antibacterial substances and antibacterial range of complex zein/SC particles. The practical use of antimicrobial system is assessed in foods, e.g. milk.
As CO2released during metabolic activities, the molar fraction of CO2indirectly indicate bacterial metabolic activity under certain circumstances. Gardini et al. [25] considered that the bacterial number increased with CO2percentage in equilibrium in the head space of sealed vials. Subsequently, Chai et al. [26] confirmed that the mass of CO2measured in the head space vials was nearly produced by bacterial growth, when the results of the plate counting method was positively correlated with that of the headspace gas chromatography.Compared to the conventional plate counting method, headspace gas chromatography technique is more time-saving, accurate and more imporsonal. CO2is chosen as a marker whose peak area was determinated by multiple headspace extraction gas chromatography reflecting the bacteriostatic efficacy of antibacterial zein/SC nanoparticles.
2. Materials and Methods
2.1 Materials
Zein (food grade), thymol (95% purity) and nisin (analytical grade) were obtained from Sigma-Aldrich, Inc. (Shanghai, China).E. coli ATCC 25922, B. subtilis ATCC 6633, L. monocytogenes ATCC 19115 and Micrococcus luteus ATCC 10240 strain were obtained from Huankai Biotechnology Co., Ltd, Guangdong, China.All other chemicals selected were of analytical grade.
2.2 Particle synthesis
An antisolvent procedure was used to prepare zein particles [27].Precisely, zein (1.0 g) with or without thymol (0.1 g) and/or nisin(0.02 g) were dissolved in 40 mL of ethanol/water binary solvent(80:20, V/V). Then aqueous solution of sodium caseinate (1.0%, m/V)was poured into zein solutions under continuous stirring(1 000 r/min) for 10 min. A RV 10 digital rotary evaporator (Ika-Works Inc., Germany) was employed to ethanol in dispersions. The dispersions were then subjected to centrifugation (4 000 r/min, 10 min).The final dispersions were then freeze-dried to obtain powder samples.Nanoparticles were denominated as control (zein/SC particles), N (nisin loaded zein/SC particles), T (thymol loaded zein/SC particles), NT(nisin and thymol loaded zein/SC particles), respectively.
2.3 Analysis of particle size and surface potential
The size and zeta-potential of colloidal particles were measured by dynamic light scattering (DLS) using a Zetasizer Nano (Malvern Instruments Ltd., UK). The freeze-dried colloidal particles were also re-dispersed in the deionized water to measure the particles size.
2.4 Morphology observation
Topographic structures of zein/SC particles were analyzed by a Zeiss Merlin Field emission scanning electron microscope (Zeiss,Oberkochen, German). Representative SEM images were reported.
2.5 Determination of encapsulated thymol
An aliquot (20 mg) of lyophilized particles were mixed with 2 mL of ethyl acetate followed by centrifugation at 12 000 r/min for 5 min. The absorbance was compared to standard curves at 275 nm for the thymol.Encapsulation efficiency of thymol was calculated according to Equ (1).

Where E is encapsulation efficiency of thymol, meis the quality of encapsulated thymol, mtis the total quality of thymol.
2.6 Determination of encapsulated nisin
Aliquots (100 mg) of N or NT powers were dissolved in 5 mL of milli-Q water at 4 °C for 12 h, which was centrifuged at 12 000 g for 20 min. Encapsulation efficiency of nisin was calculated according to Equ (1).
2.7 Release of nisin from the nanoparticles determined by agar diffusion assay
Agar diffusion techniques are usually used for quantification of nisin from 1960s [28]. It is confirmed that nisin can effectively inhibit the growth of Micrococcus luteus (ATCC 10240) in a dose-dependent manner [29]. Micrococcus luteus was employed as the indicator bacterium to determinate the activity of nisin using the Oxford plate assay [30]. The bacteria suspension (1.0 × 105CFU/mL) was homogeneously smeared on the MH plate. 100 μL of nisin dispersion was added. Inhibition zone diameters were recorded to obtain a standard curve. 5 mL of milli-Q water (pH 7.0 or pH 2.0), which contained 100 mg of N or NT powers, was added into a dialysis bagwith the molar weight cut-off of 7-8 KDa in 45 mL of 0.02% Tween 20 at 25 °C. The release of nisin was calculated according to Equ (2).

Ris release ratio of nisin,mris the quality of released nisin at r moment,meis the quality of total encapsulated nisin.
2.8 Release of thymol from the nanoparticles
The dialysis bags were incubated in 45 mL of 0.02% Tween 20 at 25 °C, where 0.5 g of thymol-loaded zein/SC nanoparticles (T or NT)powers were dissolved in 5 mL of milli-Q water (pH 7.0 or pH 2.0).The absorbance at 275 nm was recorded. The release of thymol was calculated according to Equ (2).
2.9 Antimicrobial properties determined by headspace gas chromatography
The culture was mixed by 100 mL of sterilized dispersion of luria broth, 500 μL of bacterial (E. coli,B. subtilis,L. monocytogenes,1 × 105CFU/mL) culture and antibacterial zein/sodium caseinate nanoparticles (500 mg control, 520 mg N, 550 mg T, 570 mg NT).5 mL of the culture sealed into a 22 mL headspace sample vial was incubated at 37 °C, 160 r/min. The headspace vials were periodically sampled to determine concentration of CO2by a series 580 gas chromatography (GOW-MAC Instrument Co, US) [26].
2.10 Bacterial inhibition assay in milk
The culture was mixed by 100 mL of sterilized milk, 500 μL of bacterial (L. monocytogenes, 1 × 105CFU/mL) culture and antibacterial zein/SC nanoparticles (750 mg control, 780 mg N, 825 mg T,855 mg NT). Then, 5 mL of the culture sealed was incubated at 37 °C,160 r/min. Finally, the headspace CO2was detected using the same conditions as mentioned above.
2.11 Statistics
An analysis of variance (ANOVA) of the data was performed using the SPSS 13.0 statistical analysis system, and a least significant difference (LSD) with a confidence interval of 95% or 99% was used to compare the means.
3. Results and Discussion
3.1 Particle size and zeta potential of antimicrobial particles
The mean particle size and surface potential of zein/SC particles,with or without nisin and/or thymol, before or after freeze-drying were evaluated, as summarized in Table 1. The mean particle size and zeta potential of zein/SC particles were (205.3 ± 11.4) nm and(-42.9 ± 0.28) mV, respectively. Interestingly, the addition of nisin and/or thymol did not show significant effect on the particle size and zeta potential. The particle size and zeta potential of zein/SC particles in this study were almost the same with that reported by Li et al. [27]and Zhang et al. [31], regardless of the different concentrations of zein. The negative zeta potentials (about -40 mV) of nanoparticles,which was opposite to the charge of plain zein particles (35 mV),confirmed a layer of SC coated on zein particles [19,31] accounting for the excellent redispersibility of lyophilized zein/SC particles. The size of re-dispersed zein/SC colloidal particles ((211 ± 12.3) nm)confirmed no aggregate formed during freeze-dried process.

Table 1Encapsulation properties of thymol and nisin loaded zein particles.
3.2 Encapsulation performance of antimicrobial-loaded nanoparticles
As shown in Table 1, encapsulation efficiency of thymol in zein/SC nanoparticles were as high as 96%, whether nisin added or not.Hydrophobic thymol molecule tended to migrate toward the core of zein/SC nanoparticles during ethanol evaporation procedure contributing to high encapsulation efficiency [27]. Unfortunately,the embedding rate of nisin was quite low (7%). It was announced that hydrophobic drugs possessed higher loading and encapsulation efficiency than hydrophilic drugs due to intrinsic hydrophobic property of zein [32,33]. The addition of thymol slightly increased encapsulation percent of nisin (9%), which was extremely lower than the data reported by Xiao et al. [23] (> 30%) because of different drying technology and glycerol inclusion. Besides, nisin may just be simply captured into zein/SC particles’ network resulting in low encapsulation efficiency and burst releases of nisin, which may be beneficial to short-term bacteriostatic effect of zein particles.Interestingly, the encapsulation efficiency of thymol in our research was higher than other reports. Thymol encapsulation efficiency of zein/SC/chitosan hydrochloride nanoparticles was in the range of 66%-63% [31], which prepared using liquid-liquid dispersion method. Zein/SC nano-capsules [34] were fabricated via preencapsulation followed by spray-drying, whose encapsulation ratio of thymol was about 25%. Compared to previous studies, antisolvent method followed by freeze drying process was more suitable for the preparation of thymol-loaded antimicrobial zein/SC particles, while spray drying process usually resulted in higherE% of nisin. The encapsulation efficiency of nisin was in range from 31% to 84% in zein particles by spray drying, while that of thymol was lower than 8% [23].Amphiphilic zein was capable of self-assembly to form unique nanostructures with hydrophobic pockets and hydrophilic surface during solvent evaporation [35,36]. Hydrophobic thymol molecule may tend to migrate to the core of zein/SC nanoparticles when the particle dispersion suffered solvent evaporation [3]. Solvent polarity increased in the colloid dispersion as ethanol evaporation, while thymol migrated toward the interior of zein nanoparticles resulting in high encapsulation efficiency and sustained releases. However, it is a totally opposite process for nisin. Hydrophilic nisin molecule may migrate to the hydrophilic surface of zein nanoparticles or the SC coating layer even aqueous solution surrounding zein/SC particles during solvent evaporation and freeze-drying process.
3.3 Morphological characterization of antimicrobial particles
The morphology and microstructure of zein/SC nanoparticles with or without antimicrobials were observed by SEM. As shown in Fig. 1, both plain and antimicrobial-loaded zein/SC nanoparticles were spherical shape with uniform and smooth surfaces. Free thymol crystals were not observed from SEM images, which was consistent with the results of high encapsulation efficiency of thymol. The average particle size estimated from SEM were about 195 nm,which was a bit smaller than that determined by DLS technique.The differences was possibly due to the evaporation of water(hydrodynamic diameter vs. powder diameter), while the results were similar to those of zein nanoparticles [31] characterized by TEM and DLS technique. The size of thymol- and nisin-loaded zein nanoparticles via spray drying [23] was measured by scanning electron microscopy(SEM). Compared with results in this paper, the surface morphology of freeze-dried nanoparticles was smoother than that obtained by spray drying. Obviously, drying technology play a crucial role in the formation and performance of the particles, the addition of SC may also contribute to the difference of surface morphology as well as other properties, e.g.,particle size, water solubility.
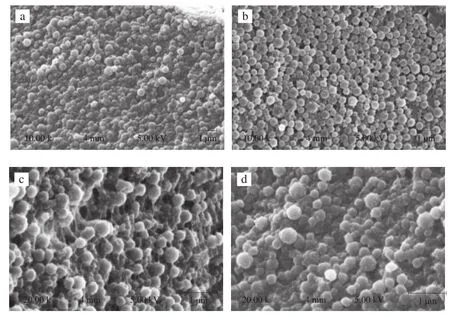
Fig. 1 SEM images of control (zein/SC particles, a/c) and NT (nisin and thymol loaded zein/SC particles, b/d), Panel c/d are the amplified images of a/b,scale bar 1 μm.
3.4 Release properties of antimicrobial zein/SC nanoparticles
Fig. 2 showed thein vitrorelease profiles of thymol and nisin from zein/SC nanoparticles in deionized water at pH 7.0 and pH 2.0.An initial rapid release phase (0-13 h) followed by subsequently slower release (13-50 h) were observed in release curve of thymol whatever nisin was co-encapsulated or not. Finally, a balanced release period was achieved after 50 h. It has little effect on the trend of thymol release when nisin were co-encapsulated with thymol into zein/SC nanoparticles. Compared to T, cumulative release of thymol was slightly decreased in T/NT, which means the release of thymol was delayed simply by adding nisin. Addition of nisin in zein/SC particles might strengthen hydrophobic interaction or hydrogen bonding between zein and thymol, which may make it difficult for thymol migrating from zein hydrophobic inner core to aqueous solution to reduce the release rate under test conditions. Moreover, pH value also influenced thymol release. It is important to be controllable for release of thymol. The sustained release of thymol will be crucial to high antimicrobial activity of zein/SC particles. Most of nisin was explosively released in the early stage. Encapsulation properties (e.g.,location, interaction between zein and antimicrobial), hydrophobicity and pH condition may lead to the disparate release behavior of nisin and thymol. The stronger binding affinity of thymol with zein than nisin is more beneficial to its sustained release [37]. When released from the nanoparticles, antimicrobials must overcome the resistance from nanoparticles structure before dispersing in aqueous solution.Hydrophobic thymol is difficult to dissolve in water, while it is much easier for nisin. Thus the force for thymol was weak to diffuse from zein/SC particles into aqueous solution. Water-soluble nisin was more likely to migrate out of the hydrophobic core, when water diffused across into interior of particles. The original nanostructure of zein/SC particles changed, which caused abrupt release. However, the trend of release profiles of thymol or nisin were similar under different pH conditions (pH 7.0 and pH 2.0).

Fig. 2 Release kinetics of nisin and thymol from antimicrobial zein/SC nanoparticles in different pH environment: (A) pH 7.0, (B) pH 2.0; T/T:Release of thymol from thymol-loaded zein/SC particles; N/N: Release of nisin from nisin-loaded zein/SC particles; T/NT: Release of thymol from nisin and thymol-loaded zein/SC particles; N/NT: Release of nisin from nisin and thymol-loaded zein/SC particles.
3.5 Antimicrobial properties
Antimicrobial properties in luria broth medium (pH 6.5) were determinated. The peak area of CO2determined by headspace gas chromatography is employed to reflect growth of microorganism because of the indication of bacterial metabolic activity. The results of antimicrobial properties against E. coli are shown in Fig. 3. Zein/SC particles and N (nisin loaded Zein/SC particles) had little inhibitory effect on the growth of E. coli, which may because nisin was a natural antimicrobial sensitive to gram-positive bacteria (including B. subtilis, L.monocytogenes) and insensitive to gram-negative bacteria (e.g., E. coli).Obviously, T (thymol-loaded zein/SC particles) and NT (thymol and nisin-loaded zein/SC particles) significantly deactivated E. coli, while the growth of E. coli was fully inhibited during the testing time (about 27 h).

Fig. 3 Evolution of CO2 produced by E. coli incubated with different samples. E. coli, control, N, T, NT were the samples treated by free from antimicrobials (without particles), zein/SC particles, nisin loaded zein/SC particles, thymol loaded zein/SC particles, nisin and thymol loaded zein/SC particles, respectively.
The antimicrobial properties against B. subtilis shown in Fig. 4 had the same trends as that against E. coli. In brief, little antibacterial effect of control (zein/SC particles) against B. subtilis was observed.N was less effective against B. subtilis than T and NT. N suppressed the proliferation of B. subtilis in first 3 h. But N was inactive against B. subtilis, the same as the control after 7.5 h. B. subtilis is sensitive to nisin. N had insufficient nisin to inhibit the growth of pathogenic bacteria for the long term, ascribed to limited encapsulation and burst release. T and NT exhibited excellent antimicrobial activities against E. coli and B. subtilis because of high encapsulation and sustained release of thymol, which contributed to enhance efficacy and applicability of antimicrobial zein/SC nanoparticles in realistic foods.

Fig. 4 Evolution of CO2 produced by B. subtilis incubated with different samples. B. subtilis, control, N, T, NT were the samples treated by free from antimicrobials (without particles), zein/SC particles, nisin loaded zein/SC particles, thymol loaded zein/SC particles, nisin and thymol loaded zein/SC particles, respectively.
The effects of different samples on the growth of L.monocytogenes was shown in Fig. 5A. The control showed little antimicrobial activity against L. monocytogenes. N and NT were more effective than T, which was very different to the results obtained from experiments against E. coli and B. subtilis. NT obviously suppressed the growth of L. monocytogenes in the first 11 h. Thymol and nisin had a synergistic effect in inhibiting L. monocytogenes, which was coincided with previous studies [7-10]. In this work, E. coli and B.subtilis, L. monocytogenes were selected as gram-negative and grampositive indicator bacterial strains, repectively. Zein/SC antibacterial nanoparticles fabricated by anti-solvent procedure possessed excellent antibacterial properties under experimental conditions, indicating that zein/SC antibacterial nanoparticles could be applied in a wide range of food systems to effectively inhibit the growth of pathogen germs including E. coli and B. subtilis, L. monocytogenes.

Fig. 5 Evolution of CO2 produced by L. monocytogenes incubated with different samples, A: in luria broth, B: in milk. L. monocytogenes, control, N,T, NT were the samples treated by free from antimicrobials (without particles),zein/SC particles, nisin loaded zein/SC particles, thymol loaded zein/SC particles, nisin and thymol loaded zein/SC particle, respectively.
3.6 Antibacterial Properties against L. monocytogenes in Milk
Fig. 5B showed antibacterial properties against L. monocytogenes of zein/SC antibacterial nanoparticles in milk. NT in Fig. 5B displayed less valid antibacterial properties than that in Fig. 5A had partially lost, which may result from the binding between milk components and thymol/nisin via electrostatic and hydrophobic interactions. Similar results were observed by Xiao et al. [23], who fabricated nisin and thymol loaded zein capsules by spray-drying technique and determined bacteria population via the plate count.The multiple carbon sources in milk for L. monocytogenes may make a difficult measure of bacteria growth rate through headspace gas chromatography since the differences in carbon dioxide production.Zein/SC particles used as a delivery system for antimicrobials not only benefit to cover up the smell of antibacterials with undesirable flavors, but also promote sustained release to prolong antibacterial effect of preserved food. In general, zein/SC antibacterial particles can be applied in diverse foods including milk to inhibit pathogenic bacteria and improve the safety of food.
4. Conclusions
In this paper, an economical and practical zein/SC particulate antimicrobial delivery system was prepared to inhibit E. coli, B.subtilis and L. monocytogenes effectively, whoes antibacterial activity was greatly enhanced by the combination of thymol and nisin due to synergistic effect. However, in order to have a better antibacterial performance of zein/SC nanoparticles, limited encapsulation and burst release of nisin must be overcome. Release properties in aqueous solution clearly demonstrated that complex zein/SC antimicrobial nanoparticles with good redispersibility could be used not only in neutral food but also in acidic food. Synergistic antimicrobial properties aganist L. monocytogenes of zein/SC antimicrobial nanoparticles in milk displayed the ability of protein-based particles to enhance microbial safety in food industry.
Conflict of Interest
The authors declare that they do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Acknowledgments
This work was partially supported by the Scientific Research Project of Education Department of Guangdong Province(2017KQNCX188), and the Project of Science and Technology of Huizhou City (2017ZX045). We also appreciate Special Funds for the Cultivation of Guangdong College Students’ Scientific and Technological Innovation (“Climbing Program” Special Funds) (pdjhb0486).
杂志排行
食品科学与人类健康(英文)的其它文章
- Bioactive compounds and probiotics-a ray of hope in COVID-19 management
- Approaches to evaluate nutrition of minerals in food
- The role of glutamine in supporting gut health and neuropsychiatric factors
- Anti-hyperglycemic effects of dihydromyricetin in streptozotocin-induced diabetic rats
- Aroma profile of two commercial truffle species from Yunnan and Sichuan, China:inter- and intraspecific variability and shared key compounds
- Stability of phenolic compounds and drying characteristics of apple peel as affected by three drying treatments
