Aroma profile of two commercial truffle species from Yunnan and Sichuan, China:inter- and intraspecific variability and shared key compounds
2021-06-05BinLuJesPerezMorenoFengmingZhngAndreRinldiFuqingYu
Bin Lu, Jesús Perez-Moreno, Fengming Zhng,c, Andre C. Rinldi*, Fuqing Yu,*
a Yunnan Key Laboratory for Fungal Diversity and Green Development, Kunming Institute of Botany, Chinese Academy of Science, Kunming, China
b Colegio de Postgraduados, Campus Montecillo. Texcoco, México
c Key Laboratory for Forest Resources Conservation and Utilisation in the Southwest Mountains of China, Ministry of Education, Southwest Forestry University, Kunming, China
d Department of Biomedical Sciences, University of Cagliari, I-09042 Monserrato (CA), Italy
ABSTRACT
Aroma is central to the worldwide success of truffles as gourmet food and the high prices paid for these edible mushrooms. In this study, volatile organic compounds (VOCs) from fruiting bodies of two Chinese truffles of commercial relevance, Tuber indicum and Tuber pseudohimalayense, were analyzed using headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography-mass spectrometry (GC-MS).We aimed to characterize the aroma profile and determine whether it would be influenced by provenance and stage of maturation. We thus collected and analyzed young, middle mature and mature fruiting bodies of each species from different locations in Yunnan and Sichuan provinces, located in southwestern China. Overall,76 VOCs were identified, belonging to different chemical classes, i.e. alcohols and phenols, aldehydes and ketones, benzenes and methoxy compounds, hydrocarbons and amines. A large number of volatiles identified in T. indicum and T. pseudohimalayense are reported here for the first time for these truffles. While more than 50% of identified VOCs were produced by both truffle species, considerable differences were present in the aroma profiles of fruiting bodies collected at various maturation stages, revealing a dynamic pattern in the biosynthesis of VOCs. Furthermore, truffles of different provenance had distinct proportions of volatile constituents, suggesting that, besides genetic factors, edaphic and microclimatic conditions influence the synthesis of VOCs in a complex manner.
Keywords:
Edible mushrooms
HS-SPME
Tuber
Volatile organic compounds
1. Introduction
Truffles - hypogeous fungi belonging to the family Tuberaceae(Ascomycota, Pezizales) - are considered one of the most valuable gourmet foods in the world. Tuber melanosporum Vittad., the Périgord black truffle, and T. magnatum Pico, the delectable white Piedmont truffle, are the most prized amongst truffle species. The white truffle, in particular, can fetch surprisingly elevated prices, and the species’ industry turnover is estimated at some 495 million euros in Italy alone [1]. The global truffle market is growing rapidly in value, being expected to reach a CAGR (Compound Annual Growth Rate) of more than 19% during the period 2019-2023, according to the latest market research report [2]. Looking at the long-term, it is expected that within 20 years, the annual global trade in truffles will reach the staggering figure of 5.3 billion euros [1].
While just a couple of species of truffles hit the headlines -usually for the amount of money they are paid at special auctions -the variety of these prized mushrooms is much higher. Currently, the number of Tuber species worldwide is estimated to range between 180 and 220 [3], of which about 30 are commercially traded. The genus seems to have a prevalently Northern Hemisphere distribution,with the geographic origin being predicted to be either Europe or Asia, although much remains to be known on the diversification of Tuber, especially in Australasia [3,4]. Clearly, the evolution of the genus has been significantly driven by the interaction with plant hosts that support the ectotrophic mode of nutrition ofTuberspecies [5,6],and with a variety of animals (insects and mammals) that accomplish spore dispersion [7]. The ecology ofTuber, however, might be even more complex than so far envisaged, as revealed by recent findings that hyphae of selected species can colonize the roots of nonectomycorrhizal plants as endophytes [8].
It is well known that what we call ‘taste’ is actually a mix of stimuli that our brain elaborates in a complex manner, somehow fusing food’s taste, sight, touch and smell in a single sensation [9].Unquestionably, truffle aroma plays a pivotal role in qualifying the organoleptic properties of these mushrooms. As one might expect,however, volatiles released byTuberhave a broader purpose than pleasing our nose, making our mouth water. Indeed, the chemical ecology of these bioactive compounds is multifaceted, regulating the interactions of truffles with soil microorganisms, plants, and animals,including those responsible for spore dispersal [10,11]. Intriguingly,the bacterial community associated withTuberis directly involved in the production of at least some of the volatiles typical of truffle aroma, such as the sulphur-containing thiophene derivatives [12,13].
China is currently an important arena for truffle studies. Forty species are present in the country, and new ones are being described[14]. Consumption of truffles is particularly important in the contiguous Sichuan and Yunnan provinces, located in southwestern China, and so are the relevant cultivation efforts [15,16]. The present work reports on the identification of volatiles from two commercial species ofTuberfrom Yunnan and Sichuan, namelyT. indicumCooke & Massee [MycoBank #188017] andT. pseudohimalayenseG. Moreno, Manjón, J. Díez & García-Mont. [MycoBank #437558](=T. pseudoexcavatumY. Wang, G. Moreno, L.J. Riousset, Manjón &G. Riousset) (Fig. 1). The variability of aroma composition depending on truffle maturity stage and provenance was also investigated, in an attempt to determine volatile profiles and how these might correlate with product commercialization and consumption. While most of previous studies on the aroma composition of Chinese truffles were aimed at the identification of volatile organic compounds (VOCs),comparison of aroma constituents on geographical/maturation basis is pivotal in order to eventually test authenticity and traceability.
2. Materials and Methods
2.1 Study sites and fungal material
Fresh specimens ofT. indicumandT. pseudohimalayensewere purchased from local markets (such as Yongping County and Yongren County, Yunnan Province, and Yumen Town and Huidong County,Sichuan Province) during the maturation period of fruit bodies from October to December, 2018. Truffle samples were brought to the laboratory, stored at -20 °C until use, and identified by ITS sequence analysis. Samples used for VOCs analysis were from two provenances per truffle species as follows:Tuber indicumfrom Yongping and Yongren both in Yunnan province; andT. pseudohimalayensefrom Yumen and Huidong in Sichuan province (Fig. 2).

Fig. 1 Mature specimens of T. indicum (A) and T. pseudohimalayense (B) collected in Yunnan and Sichuan, respectively.

Fig. 2 Map of Yunnan and Sichuan provinces located in southwestern China, with indication of collection locations for T. indicum and T. pseudohimalayense examined in this study.
2.2 Chemicals
A divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) Stable Flex fiber of 50/30 μm was purchased from Supelco,USA and used for headspace solid-phase microextraction (HS-SPME).
2.3 HS-SPME
For every SPME analysis, A PTFE silicon septum was used to close the vial and make it airtight. The vial was heated until the temperature was 55 °C, and the equilibrium was reached after 6 min.The fiber was then exposed to the headspace of the sample for 20 min. Once the extraction time ended, the fiber was removed from the vial and placed in the injection port of the gas chromatogram. A desorption time of 6 min with the injection temperature of 270 °C was adequate to desorb most of the analytes from the fiber in all conditions. After desorption from the fiber, the extract was directly transferred to the analytical column. The fibers were cleaned daily to prevent contamination.
2.4 Gas chromatography-mass spectrometry (GC-MS) analysis
The analyses of volatile compounds were carried out using gas chromatography-mass spectrometry (Agilent Technologie, Agilent 7890A along with Agilent 5975C, USA). Chromatographic separation was performed on a DB-5 capillary column (50 m × 0.25 mm ID,0.25 μm film thickness; Agilent Technologie, USA). The following chromatographic program was used: the oven temperature was held for 1 min at 30 °C, then increased to 100 °C at a rate of 2 °C/min(held for 5 min), then increased to 170 °C at a rate of 5 °C/min (held for 15 min). The carrier gas (He) flow was constant at 1 mL/min.The injection port was operated in the splitless mode at 270 °C. The operating conditions for the MS system were as follows: the ion source temperature was 230 °C, electron ionization mode at 70 eV.Full scan mode with a scan range of m/z 30-400 was used for data acquisition. Each component was subjected to NIST11 library search and data were analyzed by using MSD ChemStation software (Agilent,version G1701EA E.02.02.1431). For each analyte, its relative mass fraction was calculated by peak area normalization method.
2.5 Evaluation of main volatile aroma components
The relative odor activity value (ROAV) was used to evaluate the strong contribution of each volatile substance to the overall flavor of the sample [17-19]. The ROAVstan= 100 is defined as the component that contributes the most to the flavor of the sample, and the ROAV of other volatile components, if present, is less than 100. The calculation formula was as the following:

in which, CAand TArepresent the relative content of each volatile compound percentage and odor threshold (μg/kg), respectively, and Cstanand Tstanrepresent the relative concentration of the component at which it makes the greatest contribution to the overall flavor and the corresponding odor threshold (μg/kg), respectively. Relevant available thresholds are from literature references. It is generally believed that aromatic compounds with high ROAV are most likely to be the main contributors to the overall aroma.
2.6 Statistical analysis
Data were reported as mean ± SD. There were three replicates for each treatment and P-values for differences between different treatments within the same species were calculated using the Student’s t-test (P ≤ 0.05).
3. Results
HS-SPME coupled with GC-MS is a popular approach for the analysis of volatile compounds in truffles species [10,20-24]. Our HS-SPME/GC-MS analysis of T. indicum and T. pseudohimalayense aromatic profiles revealed a large variety of compounds. Overall, 76 VOCs were identified, belonging to different chemical classes, i.e.alcohols and phenols, aldehydes and ketones, benzenes and methoxy compounds, hydrocarbons and amines (Tables 1 and 2, Fig. 3). When single truffle species are considered, 66 and 46 VOCs were detected in T. indicum and T. pseudohimalayense, respectively (Tables 1 and 2, Fig. 4), with 54 (T. indicum) and 40 (T. pseudohimalayense)substances being reported here for the first time for these particular truffle species. More than 50% of identified VOCs (40 over 76) were produced by both truffle species.

Fig. 3 Venn diagram showing the sets of specific and shared VOCs detected in T. indicum and T. pseudohimalayense from two different locations each.TiS1 = T. indicum from Yongping, Yunnan; TiS2 = T. indicum from Yongren,Yunnan; TpS1 = T. pseudohimalayense from Yumen, Sichuan; TpS2 =T. pseudohimalayense from Huidong, Sichuan.

Fig. 4 Venn diagrams of the VOC's produced by T. indicum (TiS1 + TiS2, a)and T. pseudohimalayense (TpS1 + TpS2, b) at different stages of maturation.See Fig. 3 for abbreviations of truffle names and provenances.
Looking closely at the chemistry of identified VOCs and their relative abundance, when T. indicum is considered, the alcohols 1-octen-3-ol and phenylethyl alcohol and the aromatic 1-methoxy-3-methyl-benzene (3-methyanisole) were quantitatively important components of the aroma profile, at any stage of maturation (Table 1).1-butanol, 2-methyl-, 3-octanol, 3,4-dimethoxytoluene, and 2,3-dimethoxytoluene were also relatively abundant, although their relative quantity varied significantly with maturation stages and provenance (Table 1). A comparable, but not identical aroma profile was recorded in the case of T. pseudohimalayense (Table 2). 1-octen-3-ol, phenylethyl alcohol, 3-octanol and above all 1-methoxy-3-methyl-benzene gave a relatively important contribution also to this truffle aroma; on the other hand, several volatiles such as (Z)-2-octen-1-ol, 1-octen-3-one, and 3-octanone were in average more concentrated in the aroma of T. pseudohimalayense than in T. indicum(Table 2). In different samples collected at the same location, there are significant changes in the content of some chemical components at different stages of maturity. Relevant examples include 2-methyl-1-butanol, 3-octanol, 3-octanone, 1-methoxy-3-methyl-benzene,1-octen-3-one, (E)-2-octenal, 1,4-dimethoxy-2-methyl-benzene,1,3-dimethoxy-benzene. Among these, 1-methoxy-3-methyl-benzene,1,4-dimethoxy-2-methyl-benzene, and 1,3-dimethoxy-benzene displayed the largest statistical variability (Table 1 and Table 2).

Table 1Volatile compounds present in T. indicum at different maturation stages.
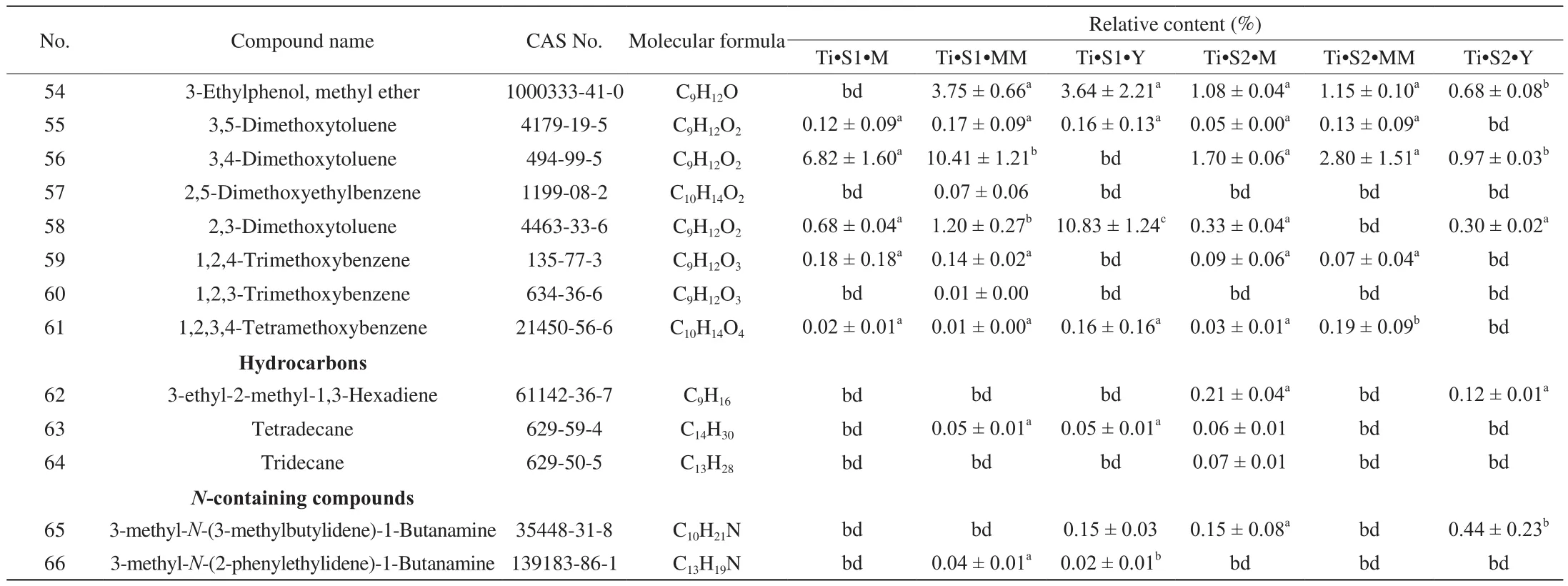
Table 1 (Continued)

Table 2Volatile compounds present in T. pseudohimalayense at different maturation stages.
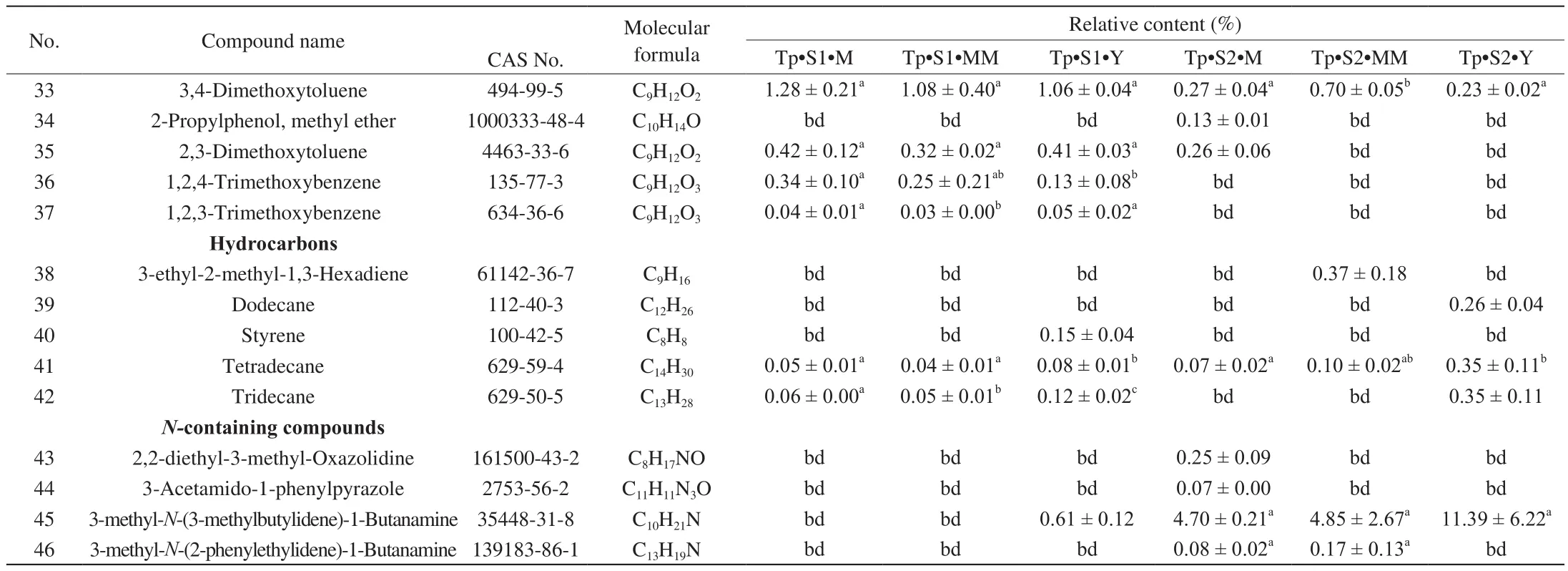
Table 2 (Continued)
In general, only a small proportion of volatile compounds contribute significantly to the overall flavor of any truffles, and some other ingredients may only assist the overall aroma. The contribution of volatile compounds to the aroma of truffles is determined by their content and odor threshold. Some compounds with low content but low odor threshold may also play an important role in the overall flavor of truffles. The ROAV values of the aroma components of truffles at different maturity stages according to this test are shown in Table 3 and Table 4 forT. indicumandT. pseudohimalayense,respectively. According to Table 3, the key aroma compounds(ROAV ≥ 1) at different maturation stages inT. indicumwere 1-octen-3-ol, 3-octanone, 1-methoxy-3-methyl-benzene, 1-octen-3-one; in addition, (E)-2-octenal, phenethyl alcohol, 1-octanol,nonanal, 3-octanol, 2-undecone, and hexanal play an important role in modifying the overall aroma (0.1 ≤ ROAV < 1). As shown in Table 4,the key aroma compounds (ROAV ≥ 1) at different maturation stages inT. pseudohimalayenseare 1-octen-3-ol, 1-octen-3-one,3-octanone, phenylacetaldehyde, 1-methoxy-3-methyl-benzene, (E)-2-octenal; furthermore, 1-octanol, 3-octanol, phenethyl alcohol,2-undecone, hexanal, and nonanal, play an important role in overall aroma modification (0.1 ≤ ROAV < 1).
The compound 1-octen-3-ol, also known as mushroom alcohol,is an extremely potent olfactory attractant for many insect species[25]; it has been isolated from a number of mushrooms and from the essential oils of aromatic plants, and has also been identified in mature fruiting bodies ofT. borchii[26,27]. Anisole homologues were reported for the first time in black truffles thanks to the study ofT. melanosporumaroma, with 3-methyanisole being described as characterized by “an earthy and flowery note, with an undertone of chocolate,” while 1,3-dimethoxybenzene (resorcinol dimethyl ether), also present (although in low amount) in bothT. indicumandT. pseudohimalayense, is reported to possess “a sweet and earthy odor, with a powerful hazelnut note” [28].
A specific aim of our research was to investigate the influence exerted by two key factors, i.e. truffle provenance and maturation stage, on the composition of aroma in two of the most important wild Chinese truffles. To this end, we selected two different Yunnan or Sichuan locations for bothT. indicumandT. pseudohimalayense,respectively (Fig. 2), and in each location collected young, middle mature and mature fruiting bodies of the given truffle. Looking at the resulting Venn diagrams shown in Fig. 4, it is clear that a large number of VOCs in both species are shared among all stages of maturations. However, each single stage has its own VOCs profile,and some other volatiles are shared among any pairs of maturation stages (with the single exception of the young-mature pair inT. pseudohimalayense) (Fig. 4). These findings reveal a dynamic pattern in the biosynthesis of VOCs, with some compounds that most likely play their role only in a specific moment in the part of the fungal life cycle that culminates with the production of fruiting bodies.When looking at the VOCs of truffles originating from different locations, other interesting data emerge. While for bothT. indicumandT. pseudohimalayensethere was little or no variation in the total number of VOCs detected in fruiting bodies of different provenance,the composition and distribution of VOCs varied significantly among populations, particularly in the case ofT. pseudohimalayense(Table 1, Table 2, Fig. 5 and Fig. 6). For example,N-containing compounds 3-methyl-N-(3-methylbutylidene)-1-butanamine and 3-methyl-N-(2-phenylethylidene)-1-butanamine were present almost exclusively inT. pseudohimalayensefrom a single origin (S2 in Table 2 and Fig. 6). This indicates that aroma maturation is a complex process,likely influenced by the genotype of the fungus and of symbiotic bacteria (and possibly also of host plant) but also by a number of edaphic factors and microclimatic conditions that our limited survey cannot disclose at this stage.

Fig. 5 Different composition of aroma from fruit bodies of T. indicum (a,b) and T. pseudohimalayense (c,d) collected in two distinct locations at three stages of maturation (see Fig. 6 for further details). Abbreviations of the truffle species and provenances are described in the legend of Fig. 3.
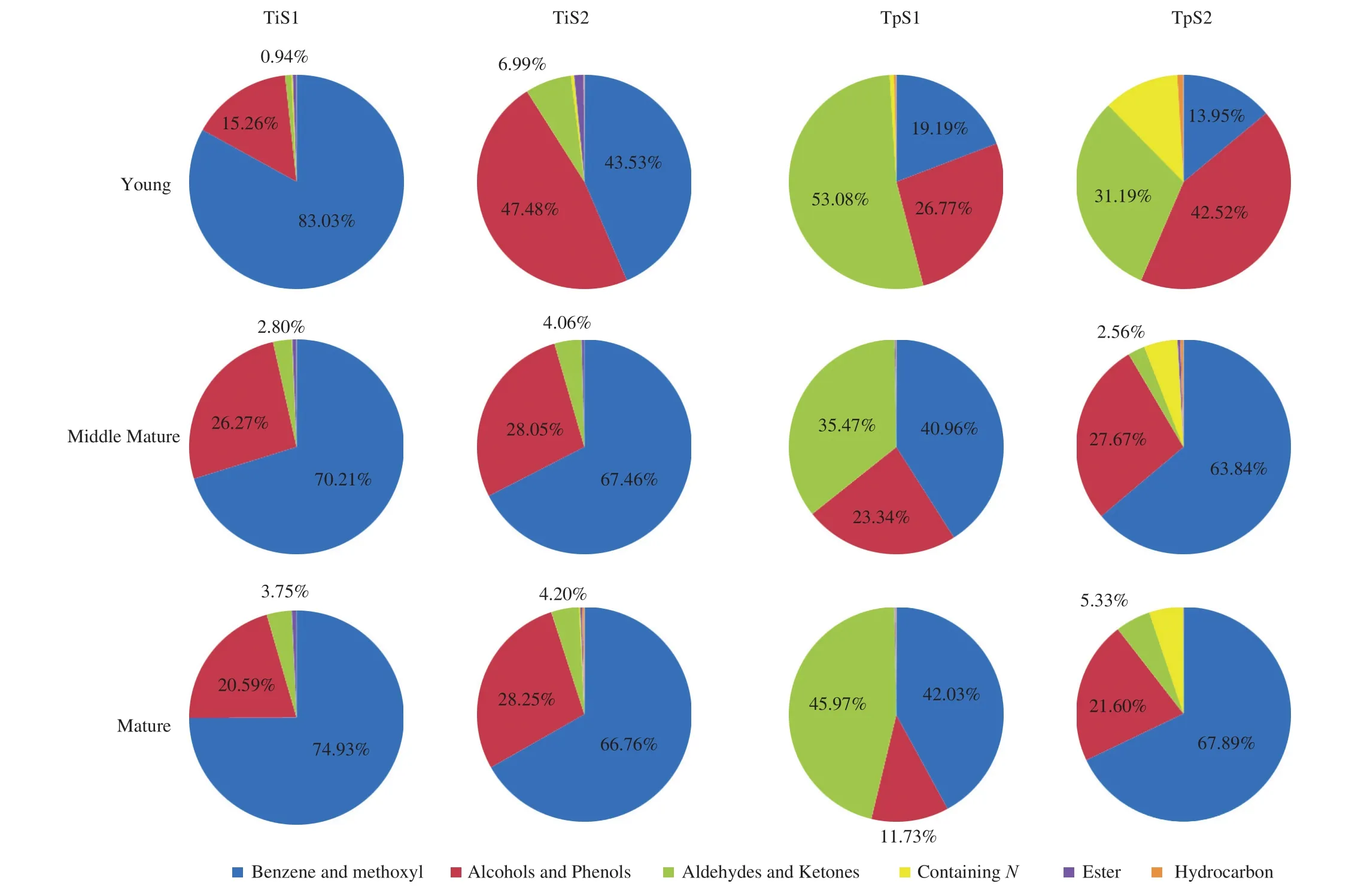
Fig. 6 Chemical classes of VOCs and their relative amount from fruit bodies of T. indicum and T. pseudohimalayense collected in two distinct locations at three stages of maturation (see Fig. 5 for further details). Abbreviations of the truffle species and provenances are described in the legend of Fig. 3.

Table 3Relative contribution of volatiles to aroma in T. indicum at different maturation stages.

Table 4Relative contribution of volatiles to aroma in T. pseudohimalayense at different maturation stages.
4. Discussion
T. indicumandT. pseudohimalayenseare truffles of great commercial interest, in China and beyond (Fig. 7). The truffles are regularly offered on sale in the markets and are collected in the wild,and the two are sold together occasionally. At the moment, there are no certified data on wild truffle yields - that are strongly influenced by the growing environment, natural climate, and other conditions- while cultivation with professional standards has started relatively recently and the output is still limited [15]. Based on the information we gathered, we can safely estimate that the annual output ofT. indicumin China is about 1 000 t, while no plausible data can be currently provided forT. pseudohimalayense.

Fig. 7 Both T. indicum and T. pseudohimalayense are regularly offered on sale in large and small markets in Yunnan and Sichuan, and eagerly bought by locals. (A)T. pseudohimalayense, at the Mushuihua market in Kunming, Yunnan; (B-C) Whole and sliced T. indicum, at the Yongfu farmers market in Panzhihua, Sichuan.
The Asian black truffle,T. indicum, is widely distributed in Himalayan India and China and it looks quite similar toT.melanosporum, although it is not endowed with the same aroma and taste. Starting from the 1990s,T. indicumhas progressively invaded European markets as a cheap alternative to the Périgord black truffle, when not misbranded for it. Some data on the production ofT. indicumin China show a steep decline in the last few years:exportation, for example, dropped from 200-300 t annually from 1995 to 2005 to just 30 t in 2014 [29]. It is generally believed that this species is being over-harvested due to its commercial value, with the concurrent use of destructive harvesting methods and the collection of immature specimens, which in turn leads to habitat damage and production decline, to the point that the conservation status of T. indicum is currently under assessment as ‘near threatened’ (see The Global Fungal Red List Initiative, http://iucn.ekoo.se/en/iucn/welcome). T. pseudohimalayense is an excavated (i.e., produces a distinct cavity at the center of their ascoma) truffle that was originally described on the basis of specimens found in Spanish markets [30]and was later detected in Chinese truffle shipments to Italian markets[31]. Extensive morphological and molecular work has confirmed that T. pseudohimalayense is a good species, distinct from T. indicum and conspecific with T. pseudoexcavatum [32-35], with a wide distribution in Yunnan and Sichuan provinces of southwestern China [36].
Previous work on the volatiles of both T. indicum and T.pseudohimalayense exists, allowing direct comparison. Some of the volatiles identified in T. indicum in early works, like 3-methylbutanal and 2-methylbutanal, were shown to be common to several other Tuber species [11]. Nevertheless, the definition of the aroma profile of a given species that could be used as a fingerprint for recognition has been a much sought-after goal in this field of research. Given the morphological similarity between T. indicum and the more valuable T. melanosporum, considerable efforts have been devoted to the development of identification methods that could avoid commercial frauds. DNA barcoding has been a much-favored approach to this end [37,38], but the differences between the aromatic compounds of both species have also been investigated. As for T. indicum,eight main odorants were identified: 1-octen-3-one, 1-octen-3-ol, ethyl isobutyrate, ethyl 2-methylbutyrate, 3-methyl-1-butanol, isopropyl acetate, and the two sulfides, dimethyl sulfide and dimethyl disulfide [39].This study indicated the important role played by the family of C8compounds in the aroma of T. indicum species, demonstrating the importance in the aromatic profile of this truffle of 1-octen-3-one and 1-octen-3-ol, in particular, both reported as having a characteristic mushroom aroma; intriguingly, these compounds are either absent or of scarce aromatic significance in T. melanosporum [39].Recently, Feng and colleagues [40] analyzed the volatile constituents of three truffle species from Yunnan by flavoromics approach through SPME extraction combined with several GC techniques and aroma recombination. One of the species considered, T. sinense should be treated as a synonym of T. indicum [35]. The study detected a total of 44 substances and 38 aroma-active compounds in this species, with dimethyl sulfide, dimethyl disulfide, octanal and 1-octen-3-one having relatively higher aroma intensities [40].The only work on T. pseudohimalayense aroma components that we could spot in the literature is that performed by Li et al. [19],naming the species as T. pseudoexcavatum. In this study, ten volatiles were identified in the species’ aroma (1-octen-3-ol, phenylethyl alcohol, 3-octanol, 3-octanone, benzene acetaldehyde, (E)-2-octenal,nonanal, tricyclo[5.4.0.0(2,8)]undec-9- ene,2,6,6,9-tetramethyl-,(1R,2S,7R,8R)-, cis-thujopsene, 1-butanamine, 3-methyl-N-(3-methylbutylidene-)), with 1-octen-3-ol and phenyl acetaldehyde(benzene acetaldehyde) as key aroma components [19].
The overall number of volatiles identified in the present study,many of which present at very low level, is very high compared to previous studies, for both T. indicum and T. pseudohimalayense.However, per se this is not unprecedented nor surprising. As back as the early 1990s, 125 volatile constituents were identified by headspace analysis of fresh T. melanosporum [28]. More recently, a Proton Transfer Reaction-Mass Spectrometry (PTR-MS) system was applied to the analysis of VOCs from T. magnatum fruiting bodies, leading to the identification of 111 compounds, some of which were only present in traces [41]. Our data and these examples clearly show how complex VOCs profiles of Tuber spp. are.
During our study, we did not detect any sulfur-containing VOCs.This might be interpreted as a somewhat surprising finding, given the fact that such compounds, like dimethyl sulfide, dimethyl disulfide and bis(methylthio)methane, are characteristic constituents of the aroma of many truffle species, and in some instances have been reported also for T. indicum (see above). However, other meta-analyses revealed that sulfur-containing compounds are not abundant, when present, in T. indicum [11], suggesting that their absence among our results might be due to methodological reasons. In particularly, it has been reported before that the DVB/CAR/PDMS extraction fiber used in this study has a lower sensitivity and reproducibility for the extraction of volatile organic sulfur compounds [42]. Also, given the preeminent role played by the truffle-associated microbiome in the synthesis (either direct or mixed with the fungus) of aroma components [13], it might well be that microbiome composition and therefore its contribution to aroma formation in T. indicum (and possibly T. pseudohimalayense)could largely depend on the provenance and maturation stage of the fruit bodies.
In this study, for the first time, we provide clear evidence that the VOCs production of T. indicum and T. pseudohimalayense, two of the most commercially important Chinese truffles, is dependent on both their maturation stage and provenance. Since the aroma of truffles, besides its biological function, is closely related to the organoleptic properties of the mushroom and hence its economic value, understanding the influence of these factors on the truffle quality under natural conditions (and looking forward, eventually in truffle orchards), is key to the development of a modern and environmentally sustainable truffle industry in China. Our results show that aroma details can distinguish Chinese truffles of different origin, even within the same region, probably because of a mix of genetic polymorphism, identity and metabolic activity of associated microorganisms, and different pedoclimatic conditions, although determining the contribution of each factor is not straightforward[43]. Not surprisingly, the relationship between chemical composition of truffle fruiting bodies and their geographic distributions is not limited to VOCs. Investigating the composition of T. indicum from five geographical regions of China, Li and colleagues [44] reported that truffles collected in Yunnan possessed the highest amount of free sugars, total flavonoids, total phenolics and the highest antioxidant activity. An elevated antioxidant activity, coupled to a high concentration of phenolic compounds, was also reported for T. pseudohimalayense [45].
Other studies have addressed the not trivial matter of using VOCs to distinguish truffles of different origin and maturation stage. Zeppa and colleagues [27] identified and characterized 49 VOCs released during the different stages of T. borchii fruit body maturation.Solid-phase microextraction coupled to gas chromatography/mass spectrometry (SPME-GC/MS) was the technique of choice by Gioacchini and colleagues [46] to characterize the volatile profile of T. magnatum produced in seven geographical areas of Italy, detecting“significant differences in the proportion of volatile constituents from truffles of different geographical areas”. More recently, Vita and colleagues [41] applied PTR-TOF-MS technology to VOCs analysis of T. magnatum, being able to distinguish aroma profiles between fruiting bodies originating from two Italian regions (Piedmont and Tuscany) and also summer and fall/winter growth period.
It will certainly take more time and considerable effort to disclose the secrets of the aromas produced by Chinese truffles. It would be particularly important to address the key issue of the truffle-associated microbiome in the formation of volatiles, and how this correlates with the maturation- and geographic-related changes in the aromatic properties of T. indicum and T. pseudohimalayense described in the present work. Our team is currently conducting research along these lines.
Declaration of Competing Interest
The authors declare no conflicts of interests.
Acknowledgement
This work was supported by the National Key Research and Development Program of China under grant No. 2018YFD0400200 and Guizhou Science and Technology Program under grant No. 4002 (2018). The authors are very grateful to Jianwei Liu,Ran Wang for providing specimens and figures for this study.The authors thank Dr. Mariana Herrera for helping making a geographical map. We are also grateful to Dr. Fei Li (Service Center for Experimental Biotechnology) for his assistance in GCMS analysis.
杂志排行
食品科学与人类健康(英文)的其它文章
- Bioactive compounds and probiotics-a ray of hope in COVID-19 management
- Approaches to evaluate nutrition of minerals in food
- The role of glutamine in supporting gut health and neuropsychiatric factors
- Anti-hyperglycemic effects of dihydromyricetin in streptozotocin-induced diabetic rats
- Stability of phenolic compounds and drying characteristics of apple peel as affected by three drying treatments
- Development of water-soluble zein colloid particles and in situ antibacterial evaluation by multiple headspace extraction gas chromatography
