UPLC-MS/MS法检测人血浆中地塞米松浓度及其临床应用
2021-06-01张佳成詹达强毕重文袁恒杰
张佳成,詹达强,毕重文,袁恒杰
UPLC-MS/MS法检测人血浆中地塞米松浓度及其临床应用
张佳成,詹达强,毕重文,袁恒杰
(天津医科大学总医院,天津 300052)
采用超高效液相-串联质谱联用建立人血浆中地塞米松含量的检测方法.色谱柱为BEH C18,流动相为0.01%甲酸乙腈溶液-0.01%甲酸水溶液,梯度洗脱,流速为0.4mL/min,电喷雾正离子模式,多反应监测.调谐后,地塞米松定量分析检测的离子对为/393.03 → 237.02.地塞米松在1.28~312.12ng/mL(2=0.9988)质量浓度范围内与色谱峰面积线性关系良好;检测限和定量下限分别为0.50ng/mL和1.00ng/mL;日内精密度和日间精密度RSD均小于10.01%,准确度为100.65%~107.82%;提取回收率为80.90%~84.32%;基质效应为102.4%~120.0%;在多种试验条件下,血浆样品稳定性良好,相对误差均在±10%之内.由患有干燥综合征的妊娠母亲抗SSA/Ro或抗SSB/La抗体阳性所引起的免疫性胎儿房室传导阻滞目前临床上采用皮质类固醇治疗,但缺乏与药物疗效及安全性等内容有关的具体数据.本方法首次成功用于患有干燥综合征的妊娠母亲口服地塞米松治疗免疫性胎儿完全性房室传导阻滞病例,在新生儿体内定性和定量检测到DXM原形药物,分娩后同时定量检测母亲及新生儿血浆样品中地塞米松质量浓度分别为25.7ng/mL和5.6ng/mL,比例约为5∶1.本方法灵敏度高、准确性好、方便快捷,可同时测定母亲和新生儿血浆中地塞米松的浓度,为临床治疗提供精准的数据支撑,同时为进行系统全面的数据分析打下基础,确保患者用药安全有效.
地塞米松;房室传导阻滞;超高效液相-串联质谱联用
胎儿房室传导阻滞(atrioventricular block,AVB)属于胎儿缓慢性心律失常.免疫性AVB是其常见类型,此类新生儿死亡率高达34%[1].患有干燥综合征的母亲体内抗干燥综合征抗体(Sjogren syndrome type,SS)A/Ro和SSB/La可跨胎盘转运并损害胎儿心脏传导系统,导致胎儿免疫性AVB[2].研究表明,皮质类固醇可以改善胎儿的心脏血流动力学和传导系统[3],给予抗SSA/Ro或抗SSB/La抗体阳性母体地塞米松(dexamethasone,DXM)可以改善胎儿不完全AVB[4-6],降低新生儿完全AVB发病率[7].DXM脂溶性高,易通过胎盘屏障[8],可用于母体给药,同时治疗母体及胎儿,临床上母体口服DXM可用于治疗胎儿免疫性AVB[9].用高效液相色谱法对支气管肺发育不良新生儿进行DXM血药浓度测定的相关研究鲜有报道,但其服药方法为对早产儿直接给药[10].未见对母亲DXM治疗,同时获得母亲与新生儿血药浓度相关性数据的研究.
目前,DXM的检测方法包括:紫外分光光度 法[11]、高效液相色谱法[12]、气相色谱-串联质谱联用法[13]及高效液相-串联质谱联用法[14-15]等.本研究旨在采用灵敏度更高的超高效液相-串联质谱联用(ultra-high performance liquid chromatography-tandem mass spectrometry,UPLC-MS/MS)法同时定量检测母亲和新生儿血浆样品中DXM的浓度,为母亲口服DXM、预防和治疗胎儿免疫性AVB提供证据,为进一步开展相关治疗、研究及预测新生儿发育奠定基础.
1 材 料
1.1 仪 器
Waters Xevo TQD IVD 液质联用仪(ESI源),杭州奥盛仪器有限公司氮吹仪,Thermo Scientific 涡旋仪,Thermo Scientific Legend Micro 21R冷冻高速离心机(离心半径为8.6cm),Thermo Scientific Myspin 6台式离心机(离心半径为5cm),Benchtop Cleaners 超声仪,梅特勒MS105十万分之一天平,密理博Direct 8超纯水系统,艾本德移液器(10μL、100μL、200μL、1000μL共4种).
1.2 药品与试剂
地塞米松对照品(批号 ZCP-100129),购自中国食品药品检定研究院;甲酸(LC-MS级,批号 A117-50)、乙酸(LC-MS级,批号 A113-50)、甲醇(LC-MS级,批号 A456-4)、乙腈(LC-MS级,批号 A955-4),均购自赛默飞世尔科技(中国)有限公司;乙酸乙酯(HPLC级,批号2019.07.10),购自天津市风船化学试剂科技有限公司;实验室所用纯水均由密理博Direct 8超纯水系统制备;健康人空白血浆由天津医科大学总医院血库提供.
2 方法与结果
2.1 储备液和工作液的制备
精密称定DXM对照品10mg,置于100mL的容量瓶中,甲醇定容,制备成质量浓度为0.1mg/mL的对照品储备液.精密移取上述对照品储备液200μL,甲醇定容至10mL,制得DXM对照品工作液,DXM的浓度为20μg/mL.储备液和工作液均存放于-80℃冰箱备用.
2.2 质控品的制备
用空白血浆逐级稀释DXM对照品工作液,制得3种浓度的血浆质控样品,分别为100ng/mL、50ng/mL和20ng/mL.
2.3 血浆样本处理
将500μL血浆样品移至10mL EP管中,加入250μL醋酸缓冲液(pH=3),涡旋数秒混匀;加入1.5mL乙酸乙酯,涡旋1min,3500r/min离心10min;吸取全部上层有机相,置于1.5mL EP管中,于氮吹仪下挥干;残留物加入200μL乙腈和水体积比为1∶1流动相溶液,涡流振荡10min,14000r/min离心10min,取上清液,移置于进样小瓶中备用.
2.4 色谱条件
本实验采用Waters BEH C18柱(1.7μm,2.1mm×50mm);柱温40℃;流动相为0.01%甲酸乙腈溶液-0.01%甲酸水溶液(0~1min,20∶80→40∶60;1~4min,40∶60→90∶10;4~4.5min,90∶10→10∶90;4.5~4.51min,10∶90→20∶80;4.51~5min,20∶80);流速为0.4mL/min;进样量10μL;进样室温度15℃.
2.5 质谱条件
离子源为电喷雾电离(electrospray ionization,ESI)源,毛细管电压为3.5kV,离子源温度为150℃,脱溶剂气温度为400℃,脱溶剂气流速为800L/h,碰撞能量和锥孔电压分别为21V和23V.检测方法为多反应监测模式,正离子模式分通道扫描.调谐后,DXM定量分析检测的离子对为/393.03→237.02.
2.6 方法学考察
2.6.1 专属性
取空白血浆500μL,按第2.3节方法操作.另配制DXM质量浓度为300ng/mL的血浆样品,按第2.3节方法操作,考察专属性.DXM的保留时间为1.43min,血浆中内源性物质对DXM的测定不构成干扰,结果如图1所示.图1(a)、(b)、(c)中最高色谱峰的响应值分别为326、4.89×105、3.41×104,DXM定量分析检测的离子对均为/393.03→237.02.
2.6.2 线性关系、检测限及定量下限
精密称取对照品工作液13μL,加入至820μL的空白血浆中,体外制得833μL血浆样品.随后采用逐级稀释法连续5次制备,每次依次取上一级833μL血浆样品中的333μL,加入至500μL空白血浆中稀释,共制得6个系列梯度质量浓度的标准曲线血浆样品.按第2.3节方法操作,以稀释后系列梯度质量浓度为横坐标(),进样后药物特征峰的峰面积为纵坐标(),加权(1/2)最小二乘法进行线性回归后,所得直线回归方程即为标准曲线.测量连续5d,每天建立随行标准曲线.标准曲线各点对应质量浓度为:1.28ng/mL、3.2ng/mL、8ng/mL、20ng/mL、50ng/mL、125ng/mL、312.12ng/mL).DXM的回归方程为=105.73+44.223,2=0.9988.DXM的线性范围是1.28~312.12ng/mL.
将DXM标准溶液分别加入空白血浆,充分混匀并逐步稀释.DXM的检测限和定量下限分别为0.50ng/mL(/=3∶1时的浓度)和1.00ng/mL (/=10∶1时的浓度).
2.6.3 准确度和精密度
取空白血浆,配制DXM的高、中、低质量浓度分别为100ng/mL、50ng/mL和20ng/mL的质控样品,按第2.3节方法操作.每天测定这一梯度的质量浓度5份,连续5d.准确度与精密度以每日随行标准曲线计算样品的质量浓度与配制的质量浓度对比得出.准确度与精密度结果见表1.

表1 人血浆中DXM的准确度和精密度
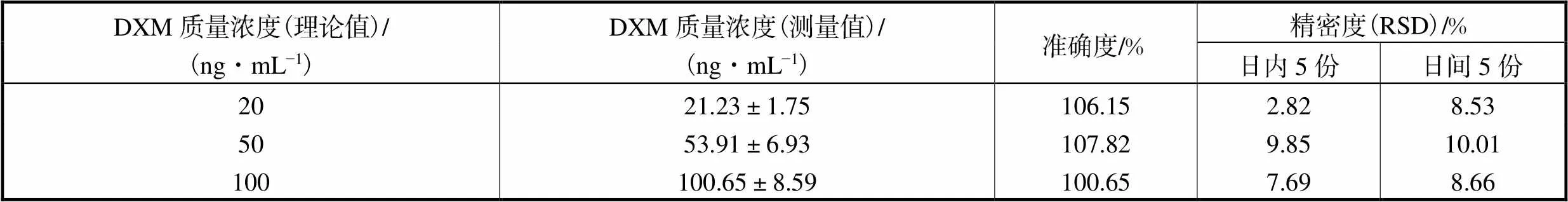
Tab.1 Accuracy and precision of DXM in human plasma
2.6.4 提取回收率
质控样品的配置方法:取空白血浆,配制DXM的高、中、低3种质量浓度的质控样品,按第2.3节方法操作,每个质量浓度制备3批同日测定.对照品溶液的配制方法:取空白血浆500μL,经液液萃取的空白血浆提取液,制备成质量浓度为100ng/mL、50ng/mL和20ng/mL高、中、低3种质量浓度的质控对照品溶液,每个质量浓度制备3批进行同日测定.比较质控样品与质控对照品溶液的色谱峰面积,用于计算提取回收率.DXM的高、中、低3种浓度的提取回收率分别为84.32%、82.18%和80.90%,见表2.
表2 人血浆中DXM的提取回收率和基质效应因子
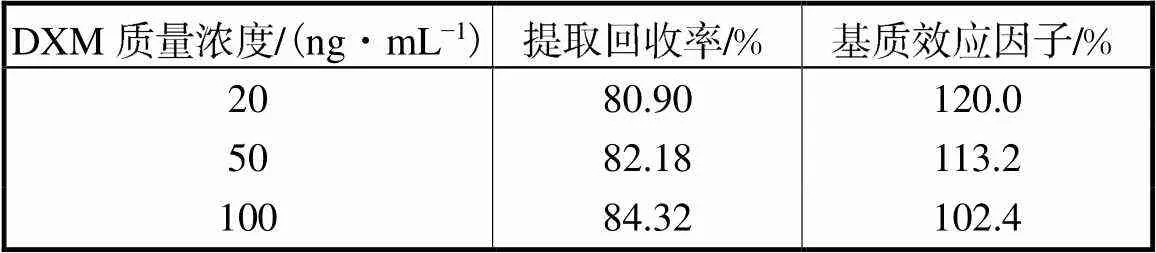
Tab.2 Extraction recovery and matrix effector of DXM in human plasma
2.6.5 基质效应
取空白血浆,以和样品制备相同的方式进行处理.液液萃取后,离心取上清液,将与配制质控血浆样品所需加入的等份对照品工作液,加入到上清液中;并将混合物涡旋1min,然后进样分析,制备5个重复样品并分析质控浓度基质效应.通过比较从加入对照品工作液的空白血浆提取物与相同浓度的对照品溶液的峰面积响应,计算基质效应因子,评估基质效应.DXM的基质效应因子在高、中、低3种浓度分别为102.4%、113.2%和120.0%,见表2.
2.6.6 稳定性
分别考察血浆样品室温放置4h、提取处理后进样器(10℃)放置24h、反复冻融3次的稳定性.每一项稳定性考察时,按第2.2节方法配制高、低两个质量浓度(100ng/mL和20ng/mL)的质控样品各5份,按第2.3节方法制备后,进样分析,并与相应新配制的质控样品比较.结果显示,血浆样品在室温放置4h稳定,提取处理后进样器(10℃)放置24h稳定,4℃冰箱放置24h稳定,-20℃冰箱放置36d稳定,相对误差均在±10%之内,见表3.
表3 在不同处理条件下DXM的稳定性

Tab.3 Stability of DXM under different treatment conditions
3 临床应用实例
患者,女,33岁,主因“孕2产0孕37+2周,血压升高10周,胎儿发育小6周”入院治疗.既往“干燥综合征”2+年,现口服DXM 4.5mg QD控制可.3年前,孕27+周因“胎儿完全性房室传导阻滞”行引产一次,未避孕.“胎儿生长受限,妊娠期高血压”于腰硬联合麻醉下行子宫下段横切口剖宫产术+双侧子宫动脉结扎术,术中剖娩1活婴,体重2400g,身长47cm,Apgar评分9′-10′-10′.分别取母亲和新生儿静脉血3mL,按第2.3节方法操作.测定DXM,母亲和新生儿的血浆样品中地塞米松质量浓度分别为25.7ng/mL和5.6ng/mL,比例约为5∶1.
4 讨 论
本研究采用UPLC-MS/MS定量检测新生儿血浆样品中DXM的浓度.该方法可有效减少样品前处理时间和分析成本,提高了检测的灵敏度、准确度和精密度;与其他方法,如高效液相色谱法[12]相比,该方法检测限更低.本方法可同时检测母亲及AVB新生儿DXM血药浓度,简单高效,便于分析研究.同时,该检测分析时间短,取血量少,对新生儿创伤小,能够更好地满足日常监测的要求.该方法流动相不含缓冲盐,对色谱柱损害较小.在血浆样品处理过程中,本研究采用液液萃取法(liquid-liquid extraction,LLE)来减少检测过程中的残留杂质.
近年来,生物样本中的地塞米松检测为特定疾病的诊断与治疗提供清晰准确的数据支撑,如检测多发性骨髓瘤患者的血清样本中的DXM[16],通过检测眼房水中DXM含量治疗多种眼部疾病[17],以及通过监测人角化细胞中地塞米松浓度变化来确定药物间相互作用[18].本病例中患病母亲抗SSA/Ro抗体阳性,曾经分娩过AVB胎儿,再次妊娠孕娩AVB胎儿的发生率高达12%~20%[19].该患者在妊娠期每日单次口服DXM,新生儿预后良好,生长发育正常.以往文献中仅报道DXM可以通过胎盘[8, 20],本研究首次采用 UPLC-MS/MS 于妊娠母亲给药后定量检测分娩新生儿体内的DXM浓度,获得了母体及新生儿的DXM血药浓度的准确数据.通过同类病例的后续随访,本方法将进一步积累相关数据,探究二者比例关系,建立数学模型,进而通过母亲血药浓度预测AVB胎儿血药浓度,为临床实践中确定妊娠母亲DXM给药剂量、胎儿AVB预防效果以及探讨DXM浓度与新生儿未来发育的联系提供准确可靠的数据支持,确保患者用药安全有效.
5 结 语
本方法首次成功用于患有干燥综合征的妊娠母亲口服地塞米松治疗免疫性胎儿完全性房室传导阻滞病例,在新生儿体内定性和定量检测到DXM原形药物,灵敏度高、准确性好、方便快捷.本方法同时测定母亲和新生儿血浆中地塞米松的浓度,二者的比例关系为5∶1.
[1] 严华林,李一飞. 胎儿免疫性房室传导阻滞研究进展[J]. 临床儿科杂志,2015,33(7):662-667.
Yan Hualin,Li Yifei. Advances in fetal immune mediated atrioventricular block[J]. J Clin Pediatr,2015,33(7):662-667(in Chinese).
[2] Ambrosi A,Wahren-Herlenius M. Congenital heart block:Evidence for a pathogenic role of maternal autoantibodies[J]. Arthritis Res Ther,2012,14(2):208.
[3] Yamada H,Kato E H,Ebina Y,et al. Fetal treatment of congenital heart block ascribed to anti-SSA antibody:Case reports with observation of cardiohemodynamics and review of the literature[J]. Am J Reprod Immunol,1999,42(4):226-232.
[4] Gleicher N,Elkayam U. Preventing congenital neonatal heart block in offspring of mothers with anti-SSA/Ro and SSB/La antibodies:A review of published literature and registered clinical trials[J]. Autoimmun Rev,2013,12(11):1039-1045.
[5] Jaeggi E T,Silverman E D,Laskin C,et alProlongation of the atrioventricular conduction in fetuses exposed to maternal anti-Ro/SSA and anti-La/SSB antibodies did not predict progressive heart block. A prospective observational study on the effects of maternal antibodies on 165 fetuses[J]. J Am Coll Cardiol,2011,57(13):1487-1492.
[6] Friedman M,Kim M Y,Copel J A,et al. Prospective evaluation of fetuses with autoimmune-associated congenital heart block followed in the PR interval and dexamethasone evaluation (PRIDE)study[J]. Am J Cardiol,2009,103(8):1102-1106.
[7] Sunderji S,Jaeggi E,Ryan G,et al. NAFTNET retrospective report on dexamethasone and fetal heart block[J]. Am J Obstet Gynecol,2019,220(1):169-170.
[8] Gilstrap L C,Christensen R,Clewell M E,et al. Effect of corticosteroids for fetal maturation on perinatal outcomes[J]. JAMA,1995,273(5):413-418.
[9] Hutter D,Silverman E D,Jaeggi E T. The benefits of transplacental treatment of isolated congenital complete heart block associated with maternal anti-Ro/SSA antibodies:A review[J]. Scand J Immunol,2010,72(3):235-241.
[10] Schild P N,Charles B G. Determination of dexamethasone in plasma of premature neonates using high-performance liquid chromatography[J]. J Chromatogr B:Biomed Sci Appl,1994,658(1):189-192.
[11] 雷利群,王述蓉,张 昊. 紫外分光光度法测定地塞米松搽剂中主药的含量[J]. 中国药房,2007,18(28):2214-2215.
Lei Liqun,Wang Shurong,Zhang Hao. Determination of dexamethasone acetate in dexamethasone liniment by ultraviolet spectrophotometry[J]. China Pharmacy,2007,18(28):2214-2215(in Chinese).
[12] 韦林洪,陈丽萍,郭 静,等. 高效液相色谱法测定地塞米松及有关物质[J]. 分析实验室,2019,38(12):1454-1458.
Wei Linhong,Chen Liping,Guo Jing,et al. Determination of dexamethasone and its related substances by high performance liquid chromatography [J]. Chinese Journal of Analysis Laboratory,2019,38(12):1454-1458(in Chinese).
[13] 张 瑞,苏小川,雷宁生,等. 气相色谱-质谱联用法测定人体尿液中地塞米松[J]. 应用预防医学,2014,20(6):379-381.
Zhang Rui,Sue Xiaochuan,Lei Ningsheng,et al. Determination of dexamethasone in human urine by gas chromatography-mass spectrometry[J]. Applied Prev Med,2014,20(6):379-381(in Chinese).
[14] Li L J,Ma P C,Wei J,et al. LC-ESI-MS method for the determination of dexamethasone acetate in skin of nude mouse[J]. J Chromatogr B:Anal Technol Biomed Life Sci,2013,933:44-49.
[15] 栾玉静,王瑞花,董 颖,等. 固相萃取-液相色谱-质谱法检验人全血中的地塞米松[J]. 中国法医学杂志,2017,32(3):297-299.
Luan Yujing,Wang Ruihua,Dong Ying,et al. Determination of dexamethasone in human plasma by solid phase extraction with ultra performance liquid chromatography-tandem mass spectrometer [J]. Chin J Forensic Med,2017,32(3):297-299(in Chinese).
[16] Shu C,Zeng T M,Gao S H,et al. LC-MS/MS method for simultaneous determination of thalidomide,lenalido-mide,cyclophosphamide,bortezomib,dexamethan-sone and adriamycin in serum of multiple myeloma patients [J]. J Chromatogr B:Anal Technol Biomed Life Sci,2016,1028:111-119.
[17] Ferreira M S,Marquez C R,Dos Santos D A,et al. Validation of direct method to quantify dexamethasone in human aqueous humor by LC-MS/MS[J]. Bioanalysis,2018,10(17):1361-1370.
[18] Li L J,Li H Y,Wang C,et al. Simultaneous determination the concentration change of ketoconazole and dexamethasone acetate:Application of drug-drug interaction in human keratinocyte[J]. J Pharm Biomed Anal,2020,188:1-6.
[19] 倪 晴,陈 黎,陈文玮. 自身抗体相关性先天性心脏传导阻滞研究进展[J]. 国际生殖健康/计划生育杂志,2016,35(6):486-489.
Ni Qing,Chen Li,Chen Wenwei. Research progress of autoantibody-related congenital heart block[J]. J Int Reprod Health/Fam Plan,2016,35(6):486-489(in Chinese).
[20] Blanford A T,Murphy B E P. In vitro metabolism of prednisolone,dexamethasone,betamethasone,and cortisol by the human placenta[J]. Am J Obstet Gynecol,1977,127(3):264-267.
Determination of Dexamethasone in Human Plasma by UPLC-MS/MS and Its Clinical Application
Zhang Jiacheng,Zhan Daqiang,Bi Chongwen,Yuan Hengjie
(General Hospital of Tianjin Medical University,Tianjin 300052,China)
We aimed to develop an ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS)method to determine the concentration of dexamethasone (DXM)in human plasma. The column was an ethylene bridged hybrid C18eluted with a gradient of 0.01% formic acid in acetonitrile-0.01% formic acid in water as the mobile phase at a flow rate of 0.4mL/min in electrospray ionization positive ion mode with multiple reaction monitoring. After tuning,the ion pair for quantification was DXM:/393.03→237.02. DXM showed a good linear relationship within the range of 1.28~312.12ng/mL(2=0.9988). The detection limit and lower limit of quantification were 0.50ng/mLand 1.00ng/mL,respectively. Relative standard deviations of intra-day and inter-day precision were <10.01%. Accuracy was within the range of 100.65%—107.82%. Extraction recovery was within the range of 80.90%—84.32%. The matrix effects ranged from 102.4% to 120.0%. Plasma samples remained stable before and after treatment under various conditions,with the relative error being within ±10%. Fetal immune mediated atrioventricular block induced by anti-SSA/Ro-positive or anti-SSB/La-positive mothers with Sjogren syndrome were clinically treated with corticosteroids,whereas the effect and security need to be testified. This is the first applied method in cases in which the mother is administered DXM orally. It was found that DXM exists in neonate plasma and that the DXM concentrations of plasma samples of the mother and neonate were 25.7ng/mLand 5.6ng/mL,respectively,with rough ratio being 5∶1.The developed method is sensitive,efficient,and convenient to determine the concentration of DXM in plasma of mother and neonate so as to provide precise data for clinical application.
dexamethasone;atrioventricular block;ultra-high performance liquid chromatography-tandem mass spectrometry
R917
A
0493-2137(2021)09-0956-06
10.11784/tdxbz202008068
2020-08-26;
2020-10-13.
张佳成(1995— ),男,硕士研究生,zhangjiacheng1995@126.com.
袁恒杰,hengjieyuan@163.com.
国家自然科学基金资助项目(81720108015).
Supported by the National Natural Science Foundation of China (No. 81720108015).
(责任编辑:田 军)
