Synthesis,characterization and properties of Ni(II)-based metal-organic framework with biphenyl tetra-carboxylic acid
2021-05-27QIDanyangCHENZizhaoDENGYijiaLIUZixuanCAOMengyaYANGHuayongYANGLirong
QI Danyang,CHEN Zizhao,DENG Yijia, LIU Zixuan,CAO Mengya,YANG Huayong, YANG Lirong
(College of Chemistry and Chemical Engineering, Henan University, Kaifeng 475004, Henan, China)
Abstract: A novel Ni(II)-based metal-organic framework (MOF), namely, {[Ni(H2bptb)0.5(bibp)(H2O)2]·H2O}n(I)(H4bptb = 2,4,4′,6-biphenyl tetra-carboxylic acid, bibp = 4,4′-bis(imidazolyl)biphenyl) was successfully synthesized under hydrothermal conditions.The Ni-MOF was characterized by IR spectroscopy, X-ray single crystal diffraction,thermogravimetric analysis and powder X-ray diffraction. Single-crystal X-ray diffraction indicates that Ni(II) center is six-coordinated with a distorted octahedral geometry of {NiN2O4}. The as-synthesized Ni-MOF is assembled into three-dimensional interpenetrating architecture through the connections of H2bptb2- and bibp ligands as well as hydrogen bonding interactions and π…π stacking.The properties of electrochemistry and magnetism were performed in the work. The CV experiments display a pair of quasi-reversible redox peak in 300-500 mV, which are connected with Ni (Ⅱ)/Ni (Ⅲ) of oxidation reduction process and show the redox processes are controlled by the surface. In addition, the χmT vs T curve obeys the Curie-Weiss law, demonstrating the weak antiferromagnetic interactionis.
Keywords: metal-organic framework; hydrothermal synthesis; cyclic voltammetry; antiferromagnetism
Metal-organic frameworks, a new type of crystalline porous materials, are formed through the coordination reactions between metal ions or metal clusters and organic ligands,which have aroused significant interest originating from their special structure and conducive to functionalization[1]. Due to their special characteristics of porous structures, MOFs have potential applications not only in energy storage[2-3], gas capture[4-8], chemical sensing[9-10], the adsorption and removal of heavy metal ions[11], but also in catalysis[12], proton conducting[13], electrochemical devices, etc.[14-15]. Compared to traditional inorganic porous materials (such as activated carbon, molecular sieve, zeolite), MOFs materials have a large surface area, open the metal sites[16], high porosity[17-18]and excellent stability so that they present promising physical and chemical properties.
MOFs materials based on Mn(Ⅱ), Ni(Ⅱ), Co(Ⅱ) and other elements can be used as candidate materials for electrode modification, electrochemical devices and magnetic devices due to their rich and unique microstructure, high specific surface area and porosity[19].As an important part of connecting metal ions or metal clusters, organic ligands with different sizes, groups, rigidity and flexibility have great influences on the structures and properties of porous MOFs[20].Polycarboxylates are widely used in the synthesis of MOFs, owing to their diversified coordination modes and high structural stability[21-24].
Based on the above factors, a novel Ni-MOF, namely, {[Ni(H2bptb)0.5(bibp)(H2O)2]·H2O}n(I) was prepared using multidentate ligand 2,4,4′,6-biphenyl tetracarboxylic acid (H4bptb) and 4,4′-bis(imidazolyl)biphenyl (bibp) under hydrothermal conditions.Furthermore, its magnetic and electrochemical properties have also been determined and discussed in detail.
1 Experimental section
1.1 Materials, reagents and instruments
All reagents and raw materials (analytical grade, AR) were purchased commercially and used without further purification.IR spectra were obtained from an AVATAR 360 spectrometer in the scan range of 4 000-400 cm-1(KBr pellets). The powder X-ray diffraction (PXRD) data of the coordination polymer was collected using a Bruker-D8 diffraction apparatus with Cu-Kα(λ=0.154 056 nm) in the range of 2θ= 5°-50°. Thermogravimetric analysis (TGA) was performed on a Perkin-Elmer-TGA7 thermal analyzer from environment temperature to 1 000 ℃ at a heating rate 10 ℃/min in N2protected atmosphere.Magnetic properties were investigated with MPMS 3 magnetometer.The electrochemical measurements were carried out on RST-3100 electrochemical work station.
1.2 Synthesis of the Coordination polymer
{[Ni(H2bptb)0.5(bibp)(H2O)2]·H2O}n(I):a mixture of H4bptb(0.1 mmol), bibp (0.1 mmol), Ni(CH3COO)2·6H2O) (0.1 mmol), DMF (2 mL) and H2O (8 mL) was fully stirred for 0.5h at environment temperature and added NaOH(1 mol/L) solution by drop to adjust pH =4.2. And then, the mixture was sealed in 25 mL Teflon reactor and heated to 130 ℃ for 72 h. After cooling, green blocky transparent crystals were obtained after filtration(yield: 60.12%, based on Ni2+). IR (KBr, cm-1): 3 400 (br), 1 606 (s), 1 580 (m), 1 534 (m), 1 447 (m), 1 143 (s), 1 352 (s), 1 218 (m), 1 182 (w), 1 103 (m), 1 069 (s), 991 (w), 863 (m), 813 (s), 796 (w), 718 (s), 705 (m), 635 (m), 572 (w).
1.3 Crystallographic data Collection and refinement
Single crystal diffraction data of Ni-MOF (Ι) was investigated on a Bruker Apex-Ⅱ CCD X-ray single-crystal diffractometer with graphite monochromatic Mo Kαfor excitation light at 296(2)K. Multi-scan empirical absorption corrections were employed to obtain crystallographic data by the SADABS. The crystal structure was recorded with a direct method by the crystallographic software package of SHELXTL.The positional and anisotropic parameters of non-hydrogen atoms were refined by the full-matrix least-squares method based onF2.The crystallographic data of Ni-MOF are summarized in Table 1 and selected bond lengths and angles are listed in Table 2. CCDC:1887229.

Table 1 Summary of crystallographic data for MOFΙ
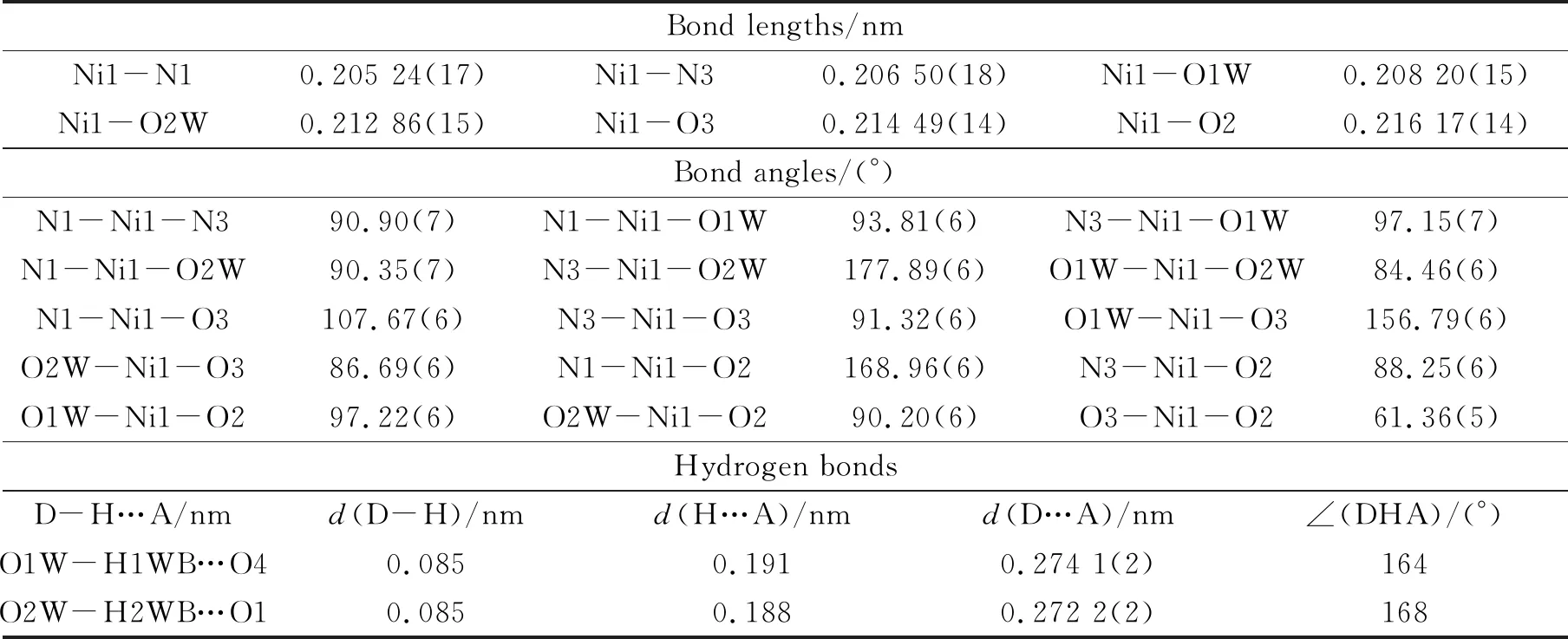
Table 2 Selected bond lengths and bond angles for Ι
2 Results and discussion
2.1 Structural description of Ni-MOF
{[Ni(H2bptb)0.5(bibp)(H2O)2]·H2O}n(Ι):Ni-MOF(Ι) crystallizes in monoclinic crystal system withC2/cspace group. Asymmetric unit ofΙcontains one crystallographically independent Ni(Ⅱ) ion. The pH value of the reaction system is an important factor in the synthetic process of MOFs.Strong acidic environment is not conducive to deprotonation of carboxylic acid ligands, and too strong alkaline environment is easy to lead to metal precipitation.In a weak acidic environment with pH=4.2, the H4bptb ligands are partially deprotonated, which cause the exposed metal site to bind with Ni(Ⅱ) ions to form a distorted octahedral structural unit.The Ni(II) center is six-coordinated with a distorted octahedral geometry of {NiN2O4} involving center Ni(Ⅱ) atom, two O atoms from one H2bptb2-ligand, two N atoms from two different bibp ligands and two O atoms from water molecules (Fig.1a).The H2bptb2-ligands adopt the coordination mode ofμ2-η1,η1,η1,η1.The lengths of the Ni-Ocarboxyland Ni-OW bonds are in the ranges of 0.215(14)-0.216(14) nm and 0.208(15)-0.213(15) nm, while the lengths of the Ni-N bond are in the range of 0.205(17)-0.207(18) nm, which are consistent with the reported bond lengths of the nickel coordination polymers[25-26].Moreover, H2bptb2-and bibp ligands coordinate with Ni(Ⅱ) centers to form a one dimensional (1D) ribbon-like structure, based on which to fabricate a two-dimensional (2D) layer through π…π stacking between the adjacent and parallel ones (e.g.Ring 1: N1-C27-N2-C28-C29 and ring 2: C21-C22-C23-C24-C25-C26, the center to center distance is 0.383 06(16)nm, see
Fig.1b,1c). Further, the 3D architecture is cross-linked by hydrogen bonds among the neighboring 2D layers along thec-axis direction (O3W-H3WA…O4, dO3W-O4=0.303 5(2)nm,Fig.1d). The 3D skeleton ofΙis stabilized by the synergistic effect of hydrogenbondingand π…π interactions.

Fig.1 (a)Coordination environment in Ι; (b) The π…π stacking of between adjacent 1D chains; (c) The hydrogen bonds between adjacent 1D chains; (d) The 3D structure of Ι
2.2 PXRD and TGA analysis
PXRD pattern was measured at room temperature, as shown in
Fig.2b. The experimental result was basically consistent with the simulated pattern, indicating that the sample phase was of high purity. To further explore the stability of the polymer, thermal stability was analyzed in detail. The TGA curve ofIexhibits two weight loss stages(Fig.2a). The first step of weight loss at 30-167 ℃, which could be ascribed to the departure of three molecules of water (calcd: 9.61%, obsd: 9.29%). In the temperature range of 167-340 ℃, the framework ofIremains integrated.Thereafter,the title Ni-MOF begins to collapse with temperature increasing and the final remnant is nickel metal oxide.
2.3 Cyclic Voltammetry
To explore the properties ofI, the glassy carbon electrode modified with coordination polymerI(I-GCE) was used as the working electrode, platinum electrode was selected as the auxiliary electrode, and saturated calomel electrode was used as the reference electrode. The CV experiments ofΙbulk-modified glassy carbon electrode(GCE) were performed in 0.1 mol/L KOH aqueous solution.The CV responses ofIshowed a pair of reversible redox peak in 300-500 mV, which are attributed that Ni2+are subjected to oxidation-reduction reactionsby electron transfer in 0.1 mol/L KOH aqueous solutionby electron transfer(see
Fig.3a). Dueing to the potential difference of the redox peak is greater than 0.06 V(0.373 V, 0.438 V), the electron transfer process is reversible[27]. NiOOH+H2O+e-↔Ni(OH)2+OH-.Due to the difference between the coordination model and the ligand structure, diverse Ni-MOFs have different electrochemical performance[28].In view of the effect of scanning rates on electrochemical properties, the CV ofIwere tested in the range of 20-200 mV/s. When the sweep rates are increasing, the peak potentials of anode are increasing in the positive direction and while the peak potentials of cathode are decreasing in the negative direction. The corresponding linear relationship were obtained by fitting the relationship between the peak current and the square root of the sweep rate, indicating that the redox process ofIis controlled by the surface[29](See
Fig.3b).
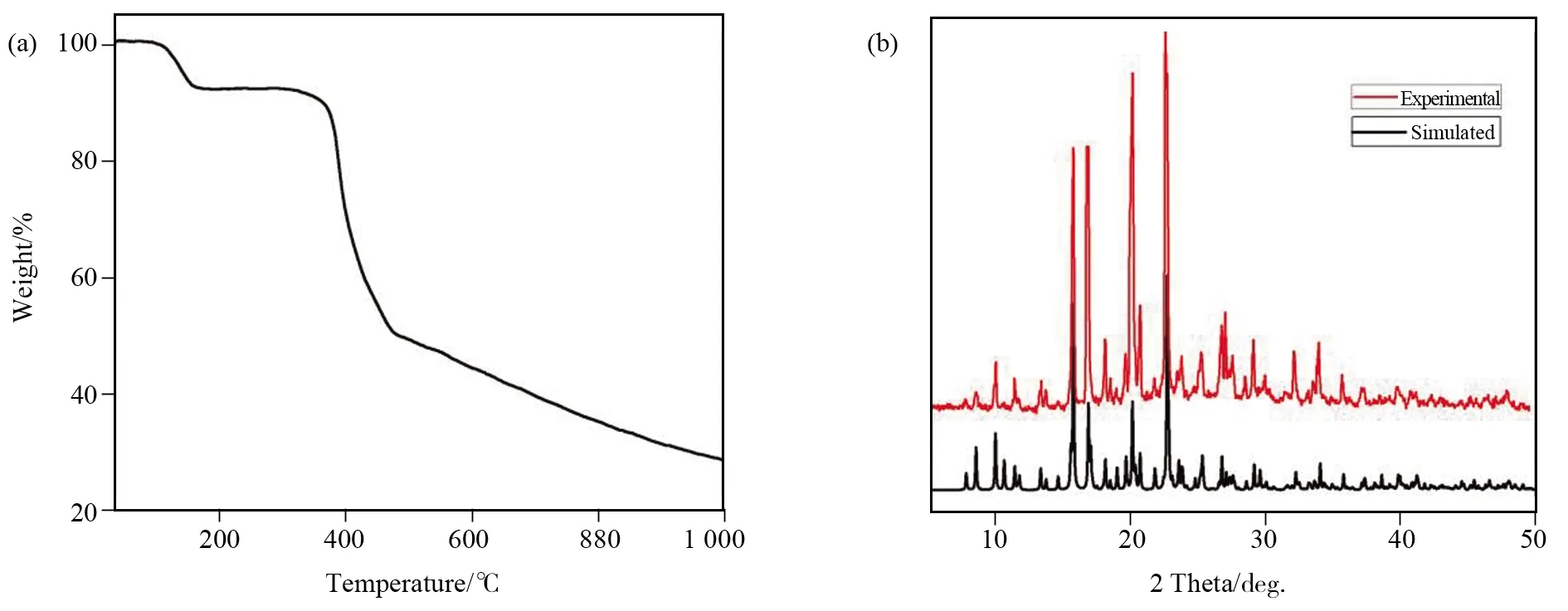
Fig.2 (a) Thermogravimetric curve of Ι; (b) The XRD patterns of Ι

Fig.3 (a) The CV curves of I in a 0.1 mol/L KOH electrolyte solution at different scanning rates; (b) the linear relationship between peak currents and square root of sweep rates
2.4 Magnetic analysis
The magnetic susceptibility data of Ni-MOF was measured in the range of 2-300 K with applied magnetic fields of 1 kOe.The plots ofχmTandχmversusTare shown in
Fig.4a. At 300 K,χmTvalue is 1.734 cm3mol-1K, lower than the isolated high-spin Ni(Ⅱ) ion of the theoretical value (2 cm3mol-1K,s= 1,g= 2.0)[30].TheχmTvalue gradually reduces with the decrease of temperature, and ultimately it reaches the minimum value of 0.408 cm3mol-1K at 2 K. The curve ofχmTvsTobeys the Curie-Weiss law with the temperature ranges from 2 K to 300 K(C= 1.754 cm3mol-1K,θ= -8.033 K), which indicates dominant intracluster antiferromagnetic coupling between the Ni(II) ions and H4bptb.Simultaneously,χmTvalue and the relationship between temperature and negativeθfurther illustrate the weak antiferromagnetic interaction[31].

Fig.4 (a) Thermal variation of χm and χmT for I; (b) Plot of thermal variation of χm-1 for Ι
3 Conclusions
In summary, a novel Ni-MOF(I) was successfully synthesized under hydrothermal condition, which displays an interpenetrating 3D framework.The 3D skeleton of Ni-MOF(I) is stabilized by the synergistic effect of hydrogen-bond and π…π interactions.MOFImaintains high thermodynamic stability up to 340 ℃.The cyclic voltammetry experiments ofIperforming in 0.1 mol/L KOH aqueous solution reveal that the redox process arecontrolled by the surface.In addition,magnetic measurement indicates that compoundIshows weak antiferromagnetic interaction.
