Burden of venous thromboembolism in patients with pancreatic cancer
2021-05-25CorinneFrere
Corinne Frere
Abstract
Key Words: Pancreatic cancer; Venous thromboembolism; Low-molecular weight heparin;Direct oral anticoagulant; Multi-language mobile application; Risk-assessment models;Thromboprophylaxis
INTRODUCTION
Pancreatic cancer (PC) is a devastating disease with fewer than 10 % of patients being alive at 5 years[1 ]. Its prevalence continues to increase worldwide[2 ]. In most cases,there is no effective treatment. Given its dismal prognosis[3], there is an urgent need to improve patient quality of life by integrating best supportive care[4 ,5 ].
Venous thromboembolism (VTE) is a frequent but still underrecognized complication in PC patients[6 ]. According to a recent large population-based cohort study[7 ],the incidence of PC-associated VTE has doubled from 1997 to 2017 , due to increase in PC prevalence, improved survival, advanced age of PC patients, and better detection of incidental VTE with the routine use of computed tomography scans. Primary thromboprophylaxis is a supportive care with a well-documented clinical benefit,which remains unfortunately underused nowadays. Since 2013 , the International Initiative on Thrombosis and Cancer (ITAC), a multidisciplinary group of experts from across the globe committed to improve the management of patients with cancerassociated thrombosis through dissemination of educational initiatives to health professionals, strives to raise awareness on this important issue[8].
Anticoagulation therapy is the mainstay of the VTE prevention and treatment, but its management is particularly challenging for the treating physicians in these patients who already suffer from multiple co-morbidities such as renal failure, hepatic failure,thrombocytopenia, and who undergo complex cancer treatment protocols[9 ,10 ].
Herein, we discuss the most recent data on the incidence and risk factors of VTE in PC patients, as well as evidence from recent clinical trials of low-molecular-weight heparin (LMWH) and direct oral anticoagulants (DOAC) for the primary prophylaxis and treatment of cancer-associated VTE that support current clinical practices guidelines (CPGs)[8 ,11 ,12 ].
EPIDEMIOLOGY OF VTE IN PC AND IMPACT ON SURVIVAL
Cancer has been demonstrated to be an independent major risk factor for VTE[13 ]. The extent of this risk mainly depends on cancer type and disease stage. Among all cancer types, PC carries the highest risk for VTE[7 ,14 ]. In retrospective cohorts of PC patients,the reported incidence of VTE varies broadly from 5 % to 57 %[15 -33 ], depending on the study population, the duration of follow-up, the definition of VTE and the methods used for diagnosing VTE.
Due to their large sample sizes, multicenter prospective design, and systematic follow-up, phase 3 randomized control trials (RCTs) conducted in PC patients are expected to provide reliable data on the true incidence of VTE. However, a recent systematic review and meta-analysis of chemotherapeutic and thromboprophylaxis RCTs conducted in PC patients highlighted that VTE events were underreported in chemotherapeutic RCTs[6 ]. The pooled rate of VTE in chemotherapy studies (n = 13 ,5694 patients) was 5 .9 % [95 % confidence interval (CI): 3 .9 -9 .0 ; I² = 94 %] and significantly lower than the corresponding 16 .5 % (95 %CI: 11 .7 -23 .3 ; P < 0 .001 ) reported in thromboprophylaxis studies (n= 9 , 631 patients, I² = 69 %). Importantly, 30 eligible chemotherapy RCTs (n= 9000 patients) were excluded from this meta-analysis because they did not report VTE as adverse events[6], which reveals quite clearly a lack of awareness on the burden of VTE among oncologists.
The incidence and risk factors for VTE was recently assessed in a large prospective multicenter cohort of patients with newly diagnosed PC[34 ], providing real-life contemporary estimates. In this study, 152 out of 731 (20 .79 %) patients developed a VTE event, with a median time from PC diagnosis to VTE of 4 mo. In competing-risk analysis, the cumulative rates of VTE were approximatively 13 % and 20 % at 6 mo and 1 year, respectively[34 ].
The most common VTE events occurring in PC patients are deep vein thrombosis(DVT) and pulmonary embolism (PE)[35 ], but incidental PE and incidental visceral vein thrombosis (VVT) are increasingly diagnosed, accounting now for approximatively 50 % of all reported VTE events[23 ,30 ,34 ,36 ]. In the BACAP-VTE study[34 ],DVT, PE, VVT, and combined events were observed in 26 %, 17 %, 30 % and 21 % of patients, respectively. Overall, 46 % of VTE events were symptomatic and 54 % of them were asymptomatic[34 ].
Early retrospective studies reported no association between VTE and overall survival (OS) in PC patients[21 ,27 ]. However, all patients included in these studies had metastatic disease with a short life expectancy. By contrast, later studies reported that the onset of VTE was associated with a poorer prognosis. In a retrospective cohort of 227 patients with unresectable PC, VTE during the course of chemotherapy was associated with a 2 .5 -fold decrease in progression-free survival (PFS) and a 1 .6 -fold risk decrease in OS[17 ]. Similarly, in a small cohort of 135 PC patients, the onset of VTE was significantly associated with increased mortality[23 ]. Importantly, survival was significantly improved in patients with VTE receiving anticoagulant therapy compared to those who did not receive anticoagulant therapy [hazard ratio (HR) 0 .30 ,95 %CI: 0 .12 -0 .74 , P = 0 .009 ][23 ]. Retrospective studies focusing on incidental VTE in PC patients also reported an association between VVT and mortality[36 ,37 ]. Similarly,in a prospective cohort of 731 newly diagnosed PC, patients who developed asymptomatic or symptomatic VTE during follow-up had significantly shorter PFS(HR 1 .74 ; 95 %CI: 1 .19 -2 .54 ; P = 0 .004 ) and OS (HR 2 .02 ; 95 %CI: 1 .57 -2 .60 ; P < 0 .001 )compared to those who did not developed VTE[34 ].
RISK FACTORS FOR VTE AND RISK STRATIFICATION IN PC PATIENTS
Several studies have demonstrated that the most important risk factor for VTE in PC patients is the presence of a metastatic disease[16 ,18 ,27 ,31 ,34 ,38 ,39 ]. In a recent retrospective cohort of 165 PC patients, metastatic disease was associated with a 4 .8 -fold increase in the risk for VTE; 41 out of 51 patients who developed VTE had metastasis at diagnosis[39 ]. Similarly, in the BACAP-VTE study[34 ], metastatic tumors were associated with a 2 .5 -fold increased risk for VTE compared to non-metastatic tumors.
Major abdominal surgery is also an important risk factor for VTE in PC patients. In an early observational study of 1915 patients with exocrine PC, 127 out of 383 (33 .1 %)patients requiring pancreatic surgery developed postsurgical VTE[22 ]. Similarly, 31 out of 209 (14 .8 %) patients requiring pancreatic surgery developed postsurgical VTE in a large retrospective study of 1 ,115 conducted in East Asian population[27 ].
Chemotherapy increases the risk of VTE in cancer patients[40 ]. Nevertheless, as recently highlighted by Chiasakulet al[6], the rates of VTE were underreported in PC chemotherapy RCTs and data on the respective risk of various chemotherapy regimens remain scarce. In recent retrospective or prospective cohorts of PC patients,the rate of VTE did not differ between those receiving gemcitabine-based chemotherapy and those receiving FOLFIRINOX[30 ,34 ]. In the subgroup of 273 PC patients included in the CASSINI trial[41 ], the rates of VTE did not differ between patients treated with 5-fluorouracil-based regimenvsgemcitabine-based regimen.
Systematic screening of VTE is not recommended in daily clinical practice.However, all PC patients should receive verbal and written information on the risk factors for VTE, as well as on the signs and symptoms of VTE to promote selfdiagnosis and reporting of VTE symptoms.
Over the past ten years, many efforts have been made to develop risk assessment models (RAM) aiming to select cancer patients at highest risk for VTE, and therefore expected to have the best benefit from thromboprophylaxis. However, none of these RAM was designed to specifically assess this risk in PC patients.
The Caprini score is the most widely RAM to assess the risk of VTE in patients undergoing surgery. It has been validated in several types of cancers[42 ]. However,this model was unable to identify patients at highest risk for VTE in a retrospective cohort of 426 PC patients undergoing preoperative treatment followed by surgical resection[43 ].
Furthermore, the Khorana score[44 ] is the most widely used RAM to assess the risk of VTE in ambulatory cancer patients. It was developed ten years ago[44 ]. It assigns 1 to 2 points to 5 simple clinical and laboratory variables (primary tumor site, platelet count ≥ 350 × 109 /L, hemoglobin concentration ≤ 10 g/dL or use of erythropoiesisstimulating agents, leukocyte count ≥ 11 × 109 /L, body mass index (BMI) ≥ 35 kg/m2 .Patients are classified as being at “low-risk” (Khorana score = 0 ), “intermediate-risk”(Khorana score = 1 -2 ), or “high-risk” (Khorana score ≥ 3 ). All PC patients are classified as being at intermediate- or high-risk. Unfortunately, this model did not discriminate between these two risk categories, neither in retrospective studies of PC patients undergoing chemotherapy[25 ,28 -30 ,32 ,39 ,45 ], nor in the large prospective BACAPVTE study[34 ], nor in the subgroup of 273 PC patients included in the recent CASSINI trial[41 ] (Table 1 ), questioning its relevance in this specific population.
Several modifications to this RAM by the addition of other variables to the model have been proposed. The PROTECHT score[46 ], which includes treatment with cisplatin or carboplatin-based chemotherapy or gemcitabine was found to perform better than the Khorana score in a retrospective analysis of the PROTECHT study,decreasing the number needed to treat (NTT) from 50 to 17 . However, this score has not been externally validated in PC patients. More recently, the ONKOTEV score[47 ]was developed in a prospective cohort of 843 various cancers patients in Italy and Germany, including 253 patient with gastroenteric cancer. The ONKOTEV score assigns one point to four variables, namely: a Khorana score > 2 , a history of previous VTE, a metastatic disease, and a compression of vascular structures by the tumor. The ONKOTEV score demonstrated a significantly higher predictive power compared to the Khorana score in the original development cohort and was recently externally validated in a retrospective single-center cohort of 165 PC patients treated in Portugal with promising results[39 ]. Ninety-two (55 .8 %) patients had a metastatic disease at diagnosis and 109 (66 .1 %) received systemic chemotherapy. At inclusion, 18 .2 % of patients had an ONKOTEV score of 0 , 38 .2 % of patients had an ONKOTEV score of 1 ,33 .3 % of patients had an ONKOTEV score of 2 , and 10 .3 % of patients had an ONKOTEV score > 2 . During a median observation period of 6 .3 mo, 51 out 165(30 .9 %) PC patients developed VTE. The cumulative incidence of VTE was 82 .4 % in patients with an ONKOTEV > 2 compared to 3 .3 % in those with an ONKOTEV score of 0 [39 ]. These results suggest that the ONKOTEV score could be of help to better stratify PC patients having the highest risk for VTE but deserve further confirmation in prospective cohorts of ambulatory PC patients.
Integration of relevant biomarkers into current RAMs might improve their ability to predict VTE. Failleet al[38 ] recently assessed the diagnosis performances of several biomarkers to predict VTE in a prospective cohort of 50 PC patients, including Factor VIII, D-dimers, von Willebrand factor, free tissue factor pathway inhibitor, microvesicle-tissue factor (MV-TF) activity and CA 19 .9 . In multivariate analysis, baseline Ddimers ≥ 2 .16 μg/mL (HR 4 .9 ; 95 %CI: 1 .0 -23 .1 ), baseline MV-TF activity 2 .37 pg/mL(HR 10 .5 ; 95 %CI: 1 .5 -72 .4 ), and baseline CA 19 .9 ≥ 2153 U/mL (HR 9 .5 ; 95 %CI: 1 .5 -60 .2 ) were significantly associated with VTE after adjustment for age and sex, with the best sensitivity and specificity in predicting VTE obtained for CA 19 -9 [38 ]. However,these associations were no more significant after adjustment for the presence of metastasis, suggesting once again that the presence of a metastatic disease is the most important risk factor for VTE in PC patients.
The clinical-genetic Thrombo inCode-Oncology (TiC-Onco) score was developed in a prospective cohort of 391 ambulatory patients with various cancers initiating systemic chemotherapy, including 72 (18 .5 %) patients with PC[48 ]. Seventy-one out of 391 (18 %) patients developed VTE within 6 mo. The prespecified variable selection process selected both clinical variables (tumor site, family history of VTE, BMI ≥ 25 kg/m2) and genetic variables (germline polymorphisms in the F5 , F13 and SERPINA10 genes) for inclusion in the score. In the derivation cohort, the TiC-Onco score performed better than the Khorana score in predicting VTE at 6 mo (sensitivity 49 %vs22 %, specificity 81 % vs 82 %, positive predictive value 37 % vs 22 %, and negative predictive value 88 % vs 82 %)[48 ]. Importantly, patients suffering from PC had higher rates of VTE (40 %) than patients with other type of cancers (18 %), suggesting that PC has a major impact on the accuracy of the TiC-Onco score. However, this model has not yet been externally validated in a cohort of PC patients.
The CATS/MICA score[49 ] includes two variables, namely: tumour-site risk category (very highvshigh and highvslow or intermediate) and continuous D-dimer levels. It was developed in the prospective Vienna Cancer and Thrombosis Study(CATS) cohort of 1423 ambulatory cancer patients undergoing chemotherapy,including 118 (8 %) patients with PC[49 ]. During a median follow-up of 6 mo, 80 out of1423 patients (6 %) developed VTE. In the CATS cohort, the C-index of the model was 0 .66 (95 %CI: 0 .63 -0 .67 ) compared to 0 .61 (95 %CI: 0 .51 -0 .70 ) for the Khorana score[49 ].The score was then validated in the prospective Multinational Cohort Study to Identify Cancer Patients at High Risk of Venous Thromboembolism (MICA) cohort (n= 832 ),including 116 (14 %) patients with PC[49 ]. Using this RAM, all PC patients are classified at intermediate or high risk of VTE. Of note, the CATS/MICA score has not yet been externally validated in a cohort of PC patients.

Table 1 Studies assessing the predictive values of risk assessment models in pancreatic cancer patients
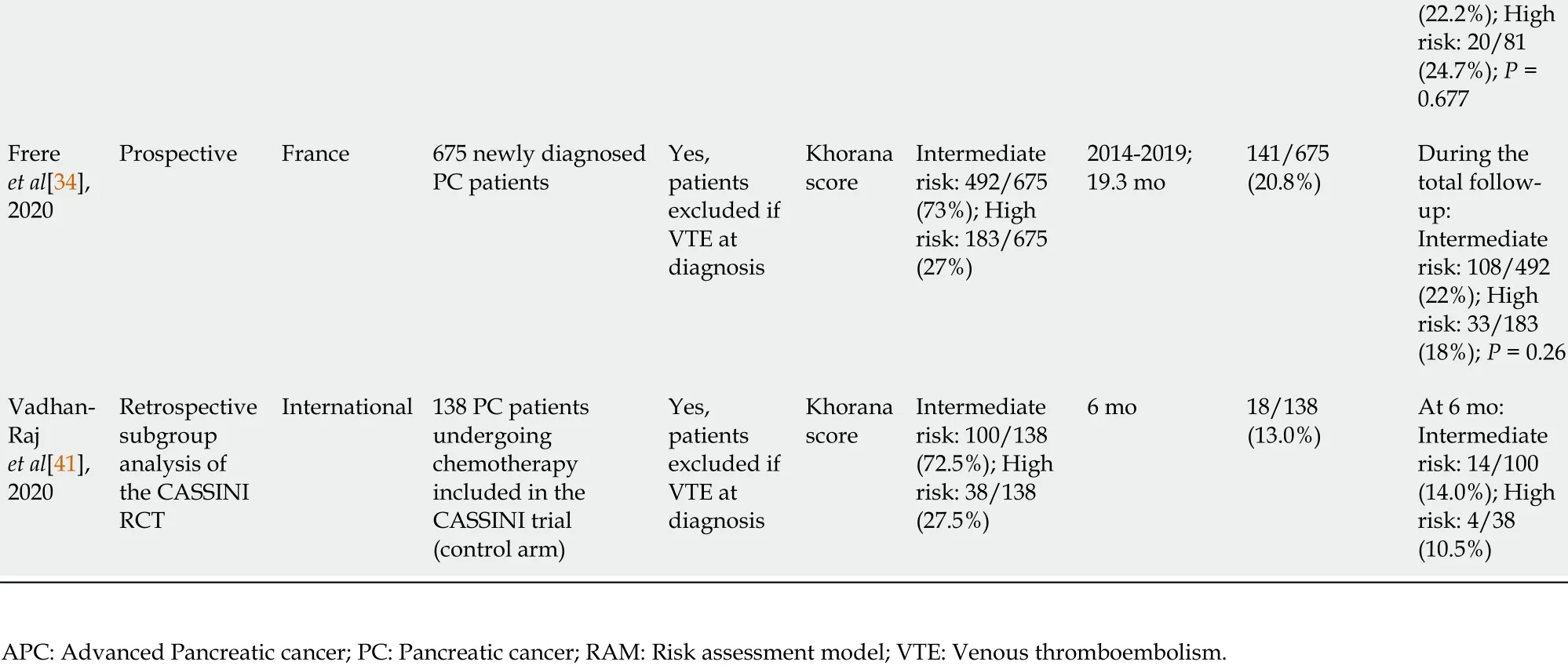
(22 .2 %); High risk: 20 /81(24 .7 %); P =0 .677 Frere et al[34 ],2020 Prospective France 675 newly diagnosed PC patients Yes,patients excluded if VTE at diagnosis Khorana score Intermediate risk: 492 /675(73 %); High risk: 183 /675(27 %)2014 -2019 ;19 .3 mo 141 /675(20 .8 %)During the total followup:Intermediate risk: 108 /492(22 %); High risk: 33 /183(18 %); P = 0 .26 Vadhan-Raj et al[41 ],2020 Retrospective subgroup analysis of the CASSINI RCT International 138 PC patients undergoing chemotherapy included in the CASSINI trial(control arm)Yes,patients excluded if VTE at diagnosis Khorana score Intermediate risk: 100 /138(72 .5 %); High risk: 38 /138(27 .5 %)6 mo 18 /138(13 .0 %)At 6 mo:Intermediate risk: 14 /100(14 .0 %); High risk: 4 /38(10 .5 %)APC: Advanced Pancreatic cancer; PC: Pancreatic cancer; RAM: Risk assessment model; VTE: Venous thromboembolism.
Finally, machine learning methods are increasingly used for the development of prediction models. Two recent studies conducted in various cancer patients[50 ] or in ovarian cancer patients[51 ] have demonstrated that such models could improve the prediction of VTE compared to conventional methods.
WHEN SHOULD WE CONSIDER PRIMARY PROPHYLAXIS IN PC PATIENTS?
Surgical PC patients
Prolonged thromboprophylaxis following major abdominal surgery has been shown to decrease the rate of VTE by approximately 50 %[52 ]. Accordingly, all current CPGs recommend using thromboprophylaxis in surgical PC patients[8 ,11 ]. In those undergoing laparotomy or laparoscopic surgery without contraindications to LMWH,the highest LMWH prophylactic dose should be used for an extended duration of 4 wk(Grade 1 A)[8 ]. External compression devices alone should be used only in patients with contraindications to anticoagulants (Grade 2 B)[8 ]. Inferior vena cava filters should not be used systematically in this setting (Grade 1 A)[8 ]. The risks of VTE should be balanced by the competing risk of bleeding. Numerous factors such as advanced or metastatic disease, older age, anemia, thrombocytopenia, renal impairment, liver dysfunction, and concomitant anticancer therapies may potentiate the overall bleeding risk and should be taken into account. The careful evaluation of each individual profile is warranted for overcoming management challenges.
Hospitalized PC patients
Acute medical illness and bed rest constitute transient factors increasing the risk of VTE in hospitalized cancer patients. Although there is no large RCT specifically demonstrating the benefit of thromboprophylaxis in cancer inpatients, RCTs conducted in non-cancer inpatients have demonstrated that LMWH improves survival and reduces VTE in general medical patients hospitalized with acute medical conditions, and recommendations for cancer patients have been extrapolated from these RCTs. The ITAC CPGs[8] recommend using LMWH at prophylactic doses or unfractionated heparin (UFH) or Fondaparinux in PC inpatients without contraindications to anticoagulants (Grade 1 B)[8 ]. Due to the lack of data on the efficacy and safety of DOAC in this setting, they should not be used (Best clinical practice)[8].
Ambulatory PC patients
Most cancer patients develop VTE in the outpatient setting[53 ]. The net clinical benefit of primary thromboprophylaxis in advanced PC patients has been firmly established in two pivotal RCTs[54 ,55 ] which specifically addressed the efficacy and safety of LMWH in this setting (Table 2). Based on the results of these two trials, the ITAC CPGs recommend using primary thromboprophylaxis with LMWH in ambulatory advanced PC patients receiving chemotherapy with a Grade 1 B evidence level since 2013 [8 ,56 ,57 ].
The FRAGEM trial randomized 123 advanced PC patients to receive gemcitabine plus weight-adjusted therapeutic doses of dalteparin for 12 wk or gemcitabine alone[54 ]. The coprimary endpoints were the rate of symptomatic or incidentally diagnosed VTE events during the 12 -wk anticoagulation period and the rate of symptomatic or incidentally diagnosed VTE events during the overall follow-up period. The rate of VTE was significantly lower in the dalteparin arm (3 .4 % vs 23 % in the control arm, risk ratio 0 .145 , 95 %CI: 0 .035 -0 .612 , P = 0 .002 ), resulting in a NNT of 6 patients to prevent 1 VTE event. No VTE-related deaths occurred in the dalteparin arm compared to 5 (8 .3 %) VTE-related deaths in the control arm. The rates of major bleeding did not differ between the 2 arms and were lower than 3 %, with only 2 patients experiencing a major bleeding requiring anticoagulation discontinuation. Of note, patients in the dalteparin arm experienced more minor bleeding such as skin bruising or epistaxis (9 % vs 3 % in the gemcitabine alone arm)[54 ]. There was no difference in PFS or OS between the two arms.
The PROSPECT-CONKO 004 trial randomized 312 advanced PC patients to receive supra-prophylactic doses of enoxaparin during the first 3 mo of chemotherapy or chemotherapy alone[55 ]. Unlike in FRAGEM, incidental VTE events were excluded from the analysis. The cumulative incidence rate of symptomatic VTE within the first 3 mo was 1 .3 % in the enoxaparin arm compared to 10 .2 % in the control arm (HR 0 .12 ,95 %CI: 0 .03 -0 .52 ), resulting in a NNT of 11 patients to prevent 1 VTE event. The rates of major bleeding events were similar in both arms. PFS and OS did not differ between the 2 arms[55 ].
Two additional phase III double-blinded placebo-controlled trials (the PROTECHT[58 ] and the SAVE-ONCO studies[59 ]) evaluated the efficacy and safety of primary thromboprophylaxis with prophylactic doses of other LMWH in ambulatory cancer patients receiving chemotherapy. In the PROTECHT study (n= 1150 )[58 ], while nadroparin reduced the rate of VTE from 3 .9 % to 2 .0 % (P = 0 .02 ) without difference in major bleeding in the overall population, the rates of VTE did not differ between the two arms in the subgroup of 53 PC patients (P = 0 .755 ). In the SAVE-ONCO study (n=3221 )[59 ] the rate of VTE was 1 .2 % in the semuloparin arm compared to 3 .4 % in the placebo arm (HR 0 .36 , 95 %CI: 0 .21 -0 .60 ; P < 0 .001 ) in the overall population, without difference in major bleeding (HR 1 .05 , 95 %CI: 0 .55 -1 .99 ). The absolute VTE risk reduction with semuloparin appeared to be much higher in the subgroup of 254 PC patients. The magnitude of the VTE risk reduction was similar to that obtained with therapeutic doses of dalteparin in the FRAGEM study[54 ] or with supra-prophylactic doses of enoxaparin in the PROSPECT-CONKO 004 study[55 ].
More recently, two randomized placebo-controlled trials assessed the efficacy and safety of primary thromboprophylaxis with prophylactic doses of DOACs (apixaban 2 .5 mg twice daily for up to 6 mo in the AVERT trial[60 ]; rivaroxaban 10 mg once daily for up to 6 mo in the CASSINI trial[61 ]) in cancer patients with a Khorana score ≥ 2 undergoing chemotherapy. Results from a subgroup of PC patients were reported only for the CASSINI trial[41 ]. Among the 273 PC patients included in this prespecified subgroup analysis, 214 (78 %) had a locally advanced or metastatic PC and 271 (99 .3 %)were receiving cytotoxic chemotherapy (fluorouracil-based in 47 .6 % of cases and gemcitabine-based in 44 .7 % of cases). Rivaroxaban did not significantly reduce the rates of the primary efficacy endpoint of symptomatic DVT, asymptomatic proximal DVT, any PE and VTE-related death within the 6 mo observation period (absolute difference of 3 .4 %,P= not significant). However, most of VTE events occurred after discontinuation of rivaroxaban (61 .5 %) compared to placebo (22 .2 %). During the intervention period, rivaroxaban significantly reduced the rates of the primary efficacy endpoint from 10 .1 % to 3 .7 % (absolute difference of 6 .4 %, HR 0 .35 , 95 %CI: 0 .13 -0 .97 ,P= 0 .034 ), resulting in a NTT of 16 patients to prevent 1 event. Importantly, 2 out of 5 events in the rivaroxaban arm and 5 out of 14 events in the placebo arm were asymptomatic lower-extremity proximal DVT diagnosed by ultrasound screening during the follow-up, leading to overestimate the rates of VTE in both arms. The rates of major bleeding and all-cause mortality did not differ between the two arms[41 ].

Table 2 Studies assessing the clinical benefit of anticoagulants for the prevention of venous thromboembolism in ambulatory pancreatic cancer patients

(4 ∙3 %); Arm B: 16 /381(4 .2 %); P = not significant. PC subgroup: not significant HR 1 .01 (95 %CI:0 .87 -1 .38 ); P =0 .44 Arm B: 23 .8 %; HR 0 .83(95 %CI 0 .62 -1 .11 ); P = 0 .213 .PC subgroup: Arm A: 34 /135(25 .2 %); Arm B: 33 /138(23 .9 %)CI: Confidence interval; CRNMB: Clinically relevant non-major bleeding; HR: Hazard ratio; ISTH: International society of thrombosis and haemostasis;KPS: Karnofsky performance status; o.d.: Once daily; NTT: Number needed to treat; OR: Odds ratio; PC: Pancreatic cancer; RR: Risk ratio.
Recently, a systematic review and meta-analysis aggregated the data from the 1003 PC patients enrolled in the 5 above-mentioned RCTs[62 ]. Primary thromboprophylaxis was estimated to significantly reduce the risk of symptomatic VTE by approximately 69 %, resulting in a NTT of 11 .9 to prevent one VTE event, without increase in the risk of major bleeding. Sensitivity analyzes showed that primary prophylaxis with LMWH or DOAC, and prophylactic doses or supra-prophylactic doses of anticoagulants reduced the risk of VTE with the same magnitude.
In light of the results from the AVERT and CASSINI trials, the ITAC[8] and ASCO[11 ] CPGs now recommend thromboprophylaxis with apixaban or rivaroxaban in cancer outpatients undergoing chemotherapy having a Khorana score ≥ 2 , no bleeding risk and no drug-drug interactions (Grade 1 B)[8 ]. Since the Khorana score assigns + 2 points for PC, thromboprophylaxis with DOAC or LMWH may be now offered in all ambulatory PC patients. Decisions to initiate thromboprophylaxis should be made based on a multidisciplinary patient-centered approach, after close discussion with the patient.
Nevertheless, primary thromboprophylaxis has not been yet widely adopted in PC outpatients, mainly due to fear of bleeding in otherwise frail subjects and inherent costs for such therapy.
HOW TO TREAT VTE IN PC PATIENTS?
A step-based adapted approach
For many years, monotherapy with LMWH has been the standard of care to treat cancer-associated VTE, based on the results of 5 landmark RCTs comparing LMWH to vitamin K antagonists[63 -67 ]. However, positive results from 4 recent RCTs comparing DOAC to LMWH monotherapy for the treatment of cancer-associated thrombosis[68 -71 ] (Table 3 ) prompted current updated CPGs to include DOACs as a new first-line option in selected patients, but not all[8 ,11 ].
The ability to now use oral-only anticoagulation strategies, precluding the need for long-term daily injection and dose adjustment, may seem appealing but adds to the complexity of decision making. Appropriate selection of anticoagulants appears more than ever as a critical element of high-quality care for cancer patients with VTE, and numerous factors must be taken into consideration when choosing one anticoagulant rather than the other[72 ]. A personalized approach is warranted.
The ITAC CPGs recommend using LMWH for the initial and long-term treatment of established VTE when creatinine clearance is ≥ 30 mL per min (Grade 1 B)[8 ]. For patients without risk of gastrointestinal or genitourinary bleeding, rivaroxaban (in the first 10 d) or edoxaban (started after at least 5 d of parenteral anticoagulation) can also be used (Grade 1 B)[8 ]. UFH provides an alternative option when LMWH or DOACs are contraindicated, or not available (Grade 2 C)[8 ]. Anticoagulation should be continued for at least 6 mo (Grade 1 A) or indefinitely while cancer is active or treated[8].
LMWH are the preferred option in patients with VVT due their short half-life and possible dose reduction in case of esophageal varices.
Briefly, DOAC are a reasonable option in ambulatory PC patients with DVT or PE with an intact upper gastrointestinal tract, without nausea or vomiting, with a low risk of bleeding, with a platelet count > 50000 /mm3 , with a creatinine clearance > 30 mL/min, without severe hepatic impairment and for whom no surgical intervention is planned. They should not be used in patients with creatinine clearance < 30 mL/min,luminal gastrointestinal lesion, platelet count < 50000 /mm3 , high bleeding risk, recent or planned surgery, or potential drug-drug interactions[73 ,74 ].
A step-based adapted approach (Figure 1), incorporating tumor type, careful examination of bleeding risk, potential drug–drug interactions, and patient preferences, has been proposed by several authors[73 ,74 ]. The multi-language web-based mobile application developed by the ITAC (downloadable for free at www.itaccme.com) based on such decision-tree algorithms is paramount to help clinicians in decision making[8].
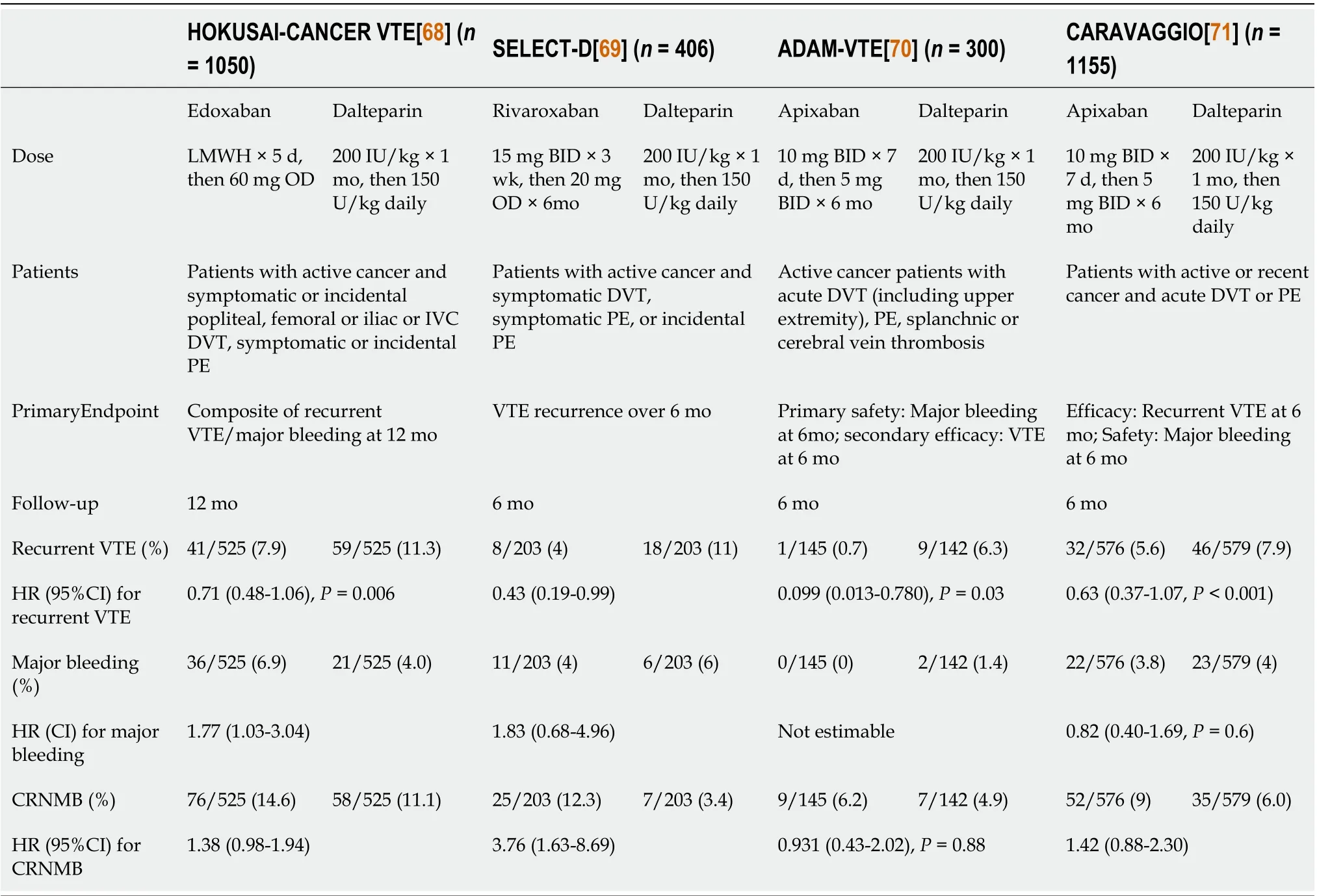
Table 3 Randomized trials assessing the efficacy and safety of direct oral anticoagulants in cancer patients with venous thromboembolism
Patients should be actively involved in treatment decisions and those treated with anticoagulants should be educated on the rationale for their treatment, the potential treatment safety concerns, and the risk of drug-drug interactions to ensure optimal adherence and treatment outcomes.
Incidental VTE is associated with high risks of recurrent VTE and VTE-related mortality[75 -77 ] and should be treated as symptomatic VTE[8 ].
CONCLUSION
VTE is a common and potentially life-threatening complication in PC patients. Strict adherence to current evidence-based guidelines and dedicated patient education programs are warranted to optimize both the primary thromboprophylaxis and the treatment of VTE in PC patients. Clinical innovative tools, such as the multi-language web-based mobile application developed by the ITAC (downloadable for free at www.itaccme.com) will be paramount to assist clinicians in rigorously implementing updated CPGs and further decrease the burden of VTE in PC patients.
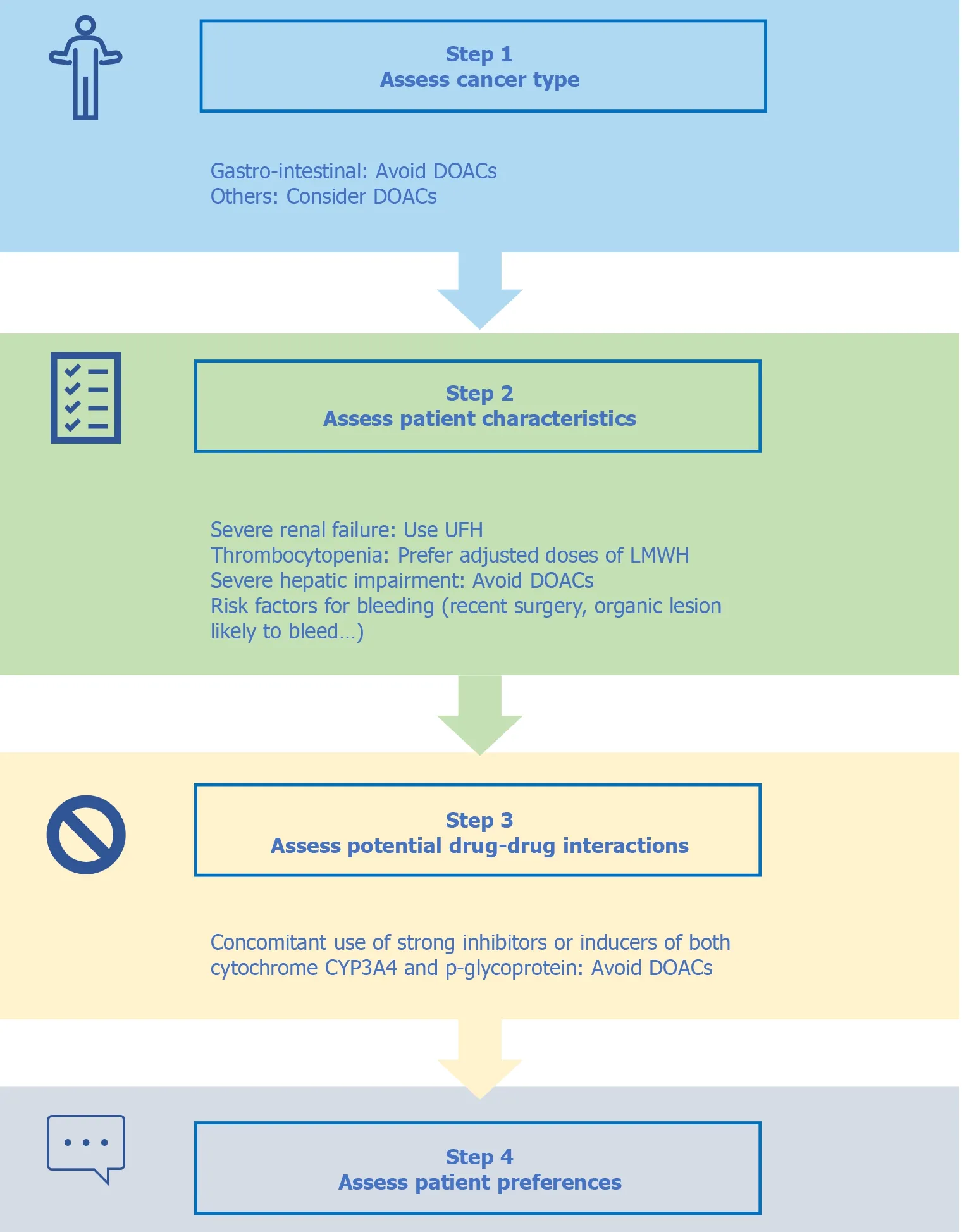
Figure 1 Four step adapted approach for the treatment of cancer-associated venous thromboembolism. DOACs: Direct oral anticoagulants;UFH: Unfractionated heparin; LMWH: Low-molecular-weight heparin; CYP3 A4 : Cytochrome P4503 A4 .
杂志排行
World Journal of Gastroenterology的其它文章
- Celiac Disease in Asia beyond the Middle East and Indian subcontinent: Epidemiological burden and diagnostic barriers
- Biomarkers in autoimmune pancreatitis and immunoglobulin G4-related disease
- Risk of hepatitis B virus reactivation in patients with autoimmune diseases undergoing non-tumor necrosis factor-targeted biologics
- Risk factors and prognostic value of acute severe lower gastrointestinal bleeding in Crohn’s disease
- Changes in the nutritional status of nine vitamins in patients with esophageal cancer during chemotherapy
- Effects of sepsis and its treatment measures on intestinal flora structure in critical care patients
