Advancements in plant regeneration and genetic transformation of grapevine (Vitis spp.)
2021-05-23ZHANGXiumingWUYifeiLIZhiSONGChangbingWANGXiping
ZHANG Xiu-ming,WU Yi-fei,LI Zhi,SONG Chang-bing,WANG Xi-ping
1 Key Laboratory of Horticultural Plant Biology and Germplasm Innovation in Northwest China,Ministry of Agriculture/State Key Laboratory of Crop Stress Biology in Arid Areas/College of Horticulture,Northwest A&F University,Yangling 712100,P.R.China
2 College of Biological Science and Engineering,North Minzu University,Yinchuan 750021,P.R.China
Abstract Grapevine (Vitis spp.) is one of the most economically important fruit crops worldwide,and there is considerable interest in improving its major agronomic and enological traits in response to ever-changing agricultural environments and consumer demands. Molecular genetic techniques in particular,associated with rapid technological advancements,provide an attractive alternative to conventional breeding approaches for developing new grapevine varieties with enhanced yield performance,quality,stress tolerance and disease resistance. To date,several grapevine varieties have been transformed with genes associated with diverse functions through biolistic bombardment and/or Agrobacterium-mediated transformation,and transgenic grape lines have been obtained using established regeneration systems. Nevertheless,a wide range of factors,including genotype,explant source and culture medium,have been shown to affect the efficiency of plant regeneration.Moreover,the selection and use of acceptor materials,bacterial strain and cell density,selectable markers and selection methods also influence transformation efficiency. This paper provides an overview of recent advances in grapevine regeneration and genetic transformation and in-depth discussion of the major limiting factors,and discusses promising future strategies to develop robust plant regeneration and genetic transformation in grapevine.
Keywords:grapevine,organogenesis,somatic embryogenesis,plant regeneration,genetic transformation
1.Introduction
Grapevine (Vitisspp.) is an economically important perennial fruit crop that is grown in many regions of the world as a source of fresh fruit,raisins and a variety of processed products,such as jam,jellies,juice,vinegar,wine and grape seed oil. Efforts to improve grape production and quality have continued throughout the history of grape cultivation;however,conventional breeding approaches,such as interspecific hybridization,are exceptionally timeconsuming due to grape’s long life cycle,as well as the heterozygous nature of genome (Gray and Meredith 1992).In recent years,transgenic technologies have successfully improved many other crops through the introduction of insect or disease resistance. Similarly,the addition of specific desirable characteristics into existing varieties,viagene insertion technologies,may provide an alternative for grapevine improvement.
The establishment of an efficient plant regeneration system is key to successful genetic transformation and the consequent generation of transgenic plants. Grapevine regeneration methods that have already been developed involve shoot organogenesis and somatic embryogenesis.An early study by Colbyet al.(1991b) showed the developmental anatomy of direct shoot organogenesis from leaf petioles,using light microscopy,and such anatomical information has shed light on the potential suitability of related regeneration systems. Colbyet al.(1991a) stated that genetically modified cells,visualized by β-glucuronidase(GUS) expression,were most frequently observed either at the cut surface in vascular bundles,or in inner cortical cells of the petiole,but none of these regions produced adventitious shoots. However,shoot organogenesis from meristematic bulk tissue has been used in the transformation of the grapevine cultivars Thompson Seedless,Silcora and Chardonnay,as well as other cultivars and rootstocks(Mezzettiet al.2002;Xieet al.2016;Sabbadiniet al.2019a,b).
Somatic embryogenesis has been widely utilized in micro-propagation and genetic transformation of many woody perennial plant species. In general,the efficiency of somatic embryos (SE) induction has been extremely low and is dependent on the developmental stage of the explants (Maillotet al.2016). Furthermore,the long-term maintenance of regenerated embryogenic callus (EC),proembryonic masses (PEM) and SE is also crucial,and in this regard a circulatory system for embryogenic culture maintenance and transformation of Thompson Seedless was recently reported (Zhouet al.2014). Thus far,the most successful method for the genetic transformation of grapevine has beenAgrobacterium-mediated transformation coupled with PEM and SE. Over the past decade,many investigators have endeavored to optimizeAgrobacteriummediated transformation protocols in order to improve transformation efficiency. Such incremental technical improvements will help realize the potential breeding efforts that largely target the enhancement of abiotic stress tolerance,fruit quality and resistance to various devastating pathogens (Saportaet al.2016;Wanget al.2018a).
Some grapevine cultivars and rootstocks have been transformed,either through biolistic bombardment and/orAgrobacterium-mediated transformation,and transgenic lines can be generated through protocols that are now established;however,a number of factors have been identified that can substantially affect the efficiency of plant regeneration and transformation. Here we provide an overview of recent developments and important variables involved in grapevine plant regeneration and transformation.
2.Grapevine plant regeneration and the underlying factors
Of the two core approaches to grapevine regeneration,shoot organogenesis is technically easier than somatic embryogenesis. To our knowledge,Favreet al.(1977)reported the firstin vitroadventitious bud formation from leaf explants of grapevine.Since then organogenesis has been achieved with several grapevine cultivars and rootstock species using different explants,including petioles (Zhanget al.2011),young leaves (Nicholsonet al.2012),internode segments (de Carvalhoet al.2013) and fragmented shoot apices (Xieet al.2016;Sabbadiniet al.2019a,b) (Table 1).However,one limitation of this method is that it has the propensity to give rise to chimeras since the adventitious buds formed during organogenesis are derived from multiple cells (Colbyet al.1991a). To avoid such chimeras,a commonly adopted regeneration method involves somatic embryogenesis from a single cell. The first report of somatic embryogenesis using unfertilized ovulesofV.viniferaL.cv.Cabernet-Sauvignon was published by Mullins and Srinivasan (1976). To date,SE have been successfully obtained from other cultivars,as well as from various explant types,including zygotic embryos (Emershad and Ramming 1994),tendrils (Salunkheet al.1997),filaments(Nakajima and Matsuta 2003;Nakajimaet al.2020),stigmastyles (Morganaet al.2004),stem nodal sections (Maillotet al.2006),whole flowers (Gambinoet al.2007;Alavijehet al.2016),buds (Arayaet al.2008),stamens (Liet al.2014),gynoecia (Xuet al.2014),mature seeds (Peiróet al.2015),ovaries (Carraet al.2016),leaves (Soliman 2018),carpels (Cardosoet al.2019),protoplasts (Bertiniet al.2019) and anthers,the last of which is the most extensively used explant type (Jayasankaret al.1999;Gribaudoet al.2004;Pradoet al.2010;Xuet al.2014;Alavijehet al.2016) (Table 2). In all these reports,embryogenesis is achievedviainduction of embryogenic callus from cultured explants,followed by the embryo differentiation,embryo germination and plant regeneration in a different medium(Fig.1). Several factors have been reported to influence the efficiency of grapevine initial inducing embryogenic callus and adventitious buds in plant regeneration. These include the grapevine genotype,explant source,culture medium and others. Examples of these variables are described below.
2.1.Grapevine genotype
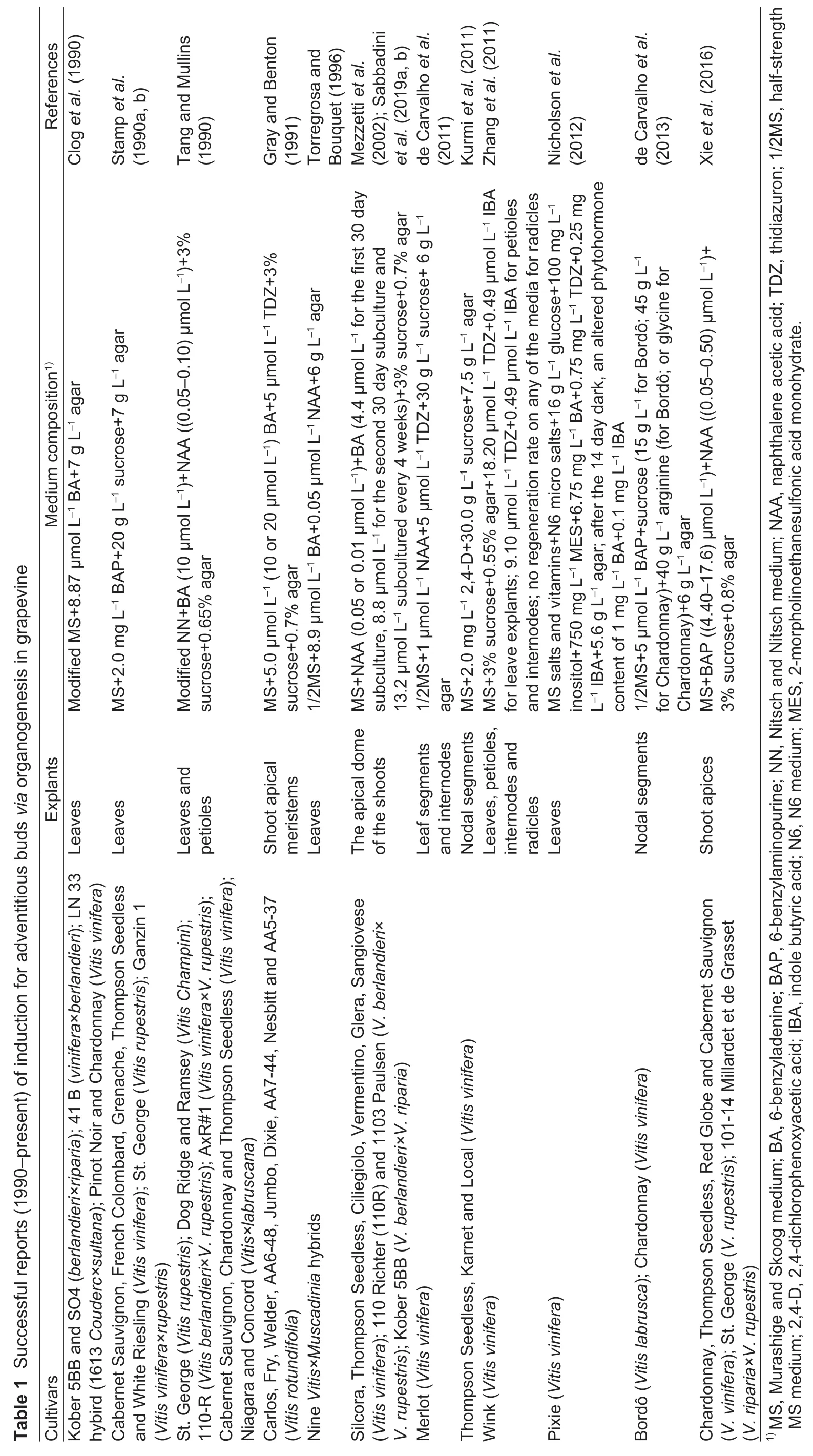
The effects of genotype on the efficiency of plant regeneration have been extensively studied.It is well known that the regeneration capacity of rootstock varieties is higher than that ofV.viniferahybrids and cultivars in both organogenesis and somatic embryogenesis.Among those cultivars,Thompson Seedless has the highest induction rate of embryogenic calli. For shoot organogenesis,three grapevine rootstock varieties(Kober 5 BB,41 B and SO 4),one LN 33 hybrid and twoV.viniferacultivars (Pinot Noir and Chardonnay)were compared,and all tested genotypes showed different regeneration capacities. Kober 5 BB gave the best results,with 39% of explants producing organogenic calli and all rootstocks giving rise to rooted plants. In contrast,the twoV.viniferacultivars only yielded greenish calli and produced no buds (Cloget al.1990). It was also found that adventitious bud formation from leaves of 20V.viniferacultivars averaged 37%of cultured explants,and large differences in the formation were oberseved among cultivars(Péroset al.1998). With regard to somatic embryogenesis,the effect of genotype on embryogenic culture was tested using eightVitis. Of the investigated genotypes,the interspecific rootstock,G 28,appeared to be the best with a moderate regeneration efficiency and a tendency for recurrent somatic embryogenesis (Mozsár and Viczián 1996). In another study,Carraet al.(2016) showed that grapevine genotype was the key factor for induction of SE,and a 50-fold difference in the percentage of somatic embryogenesis was observed among eight Italian grapevine cultivars.
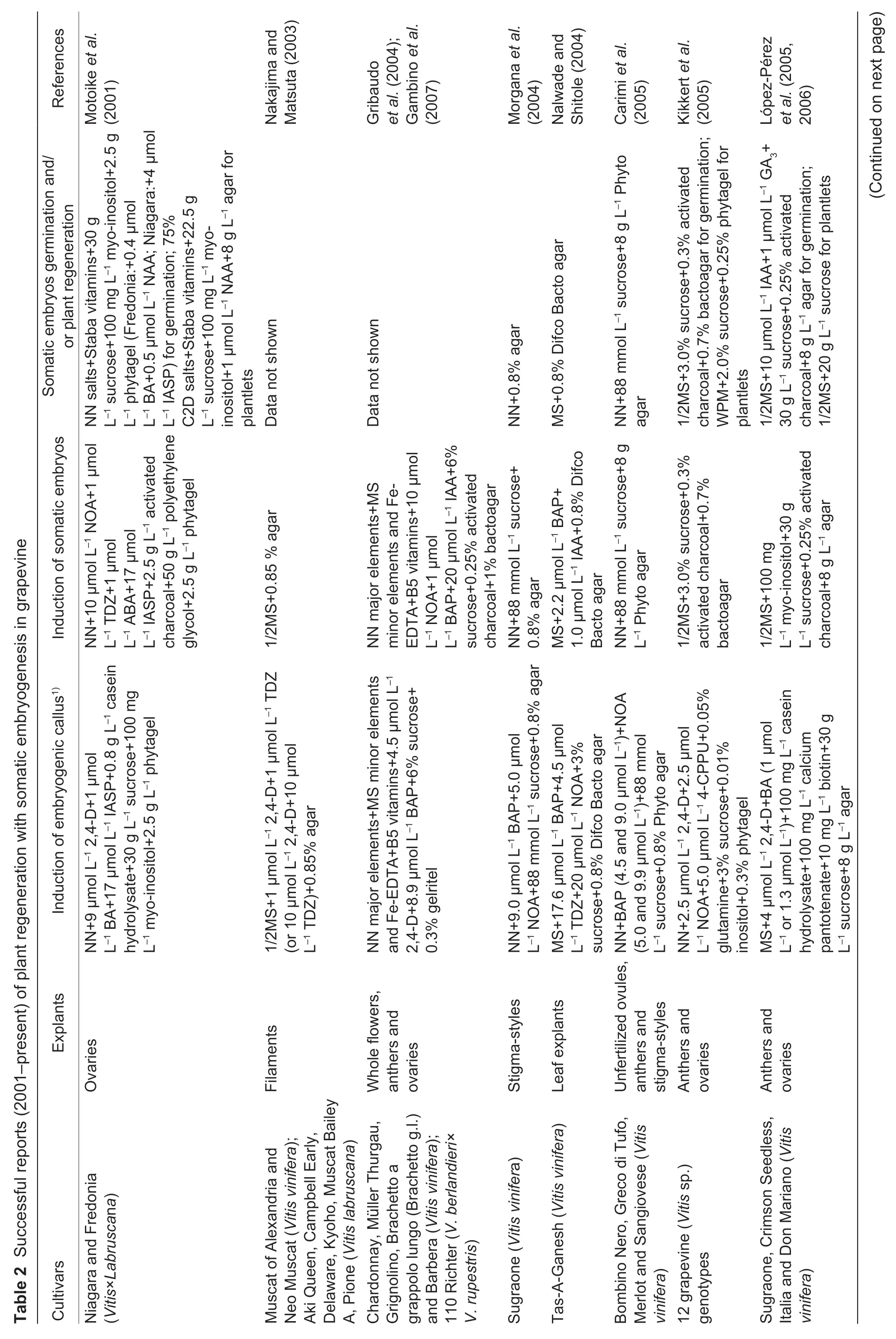
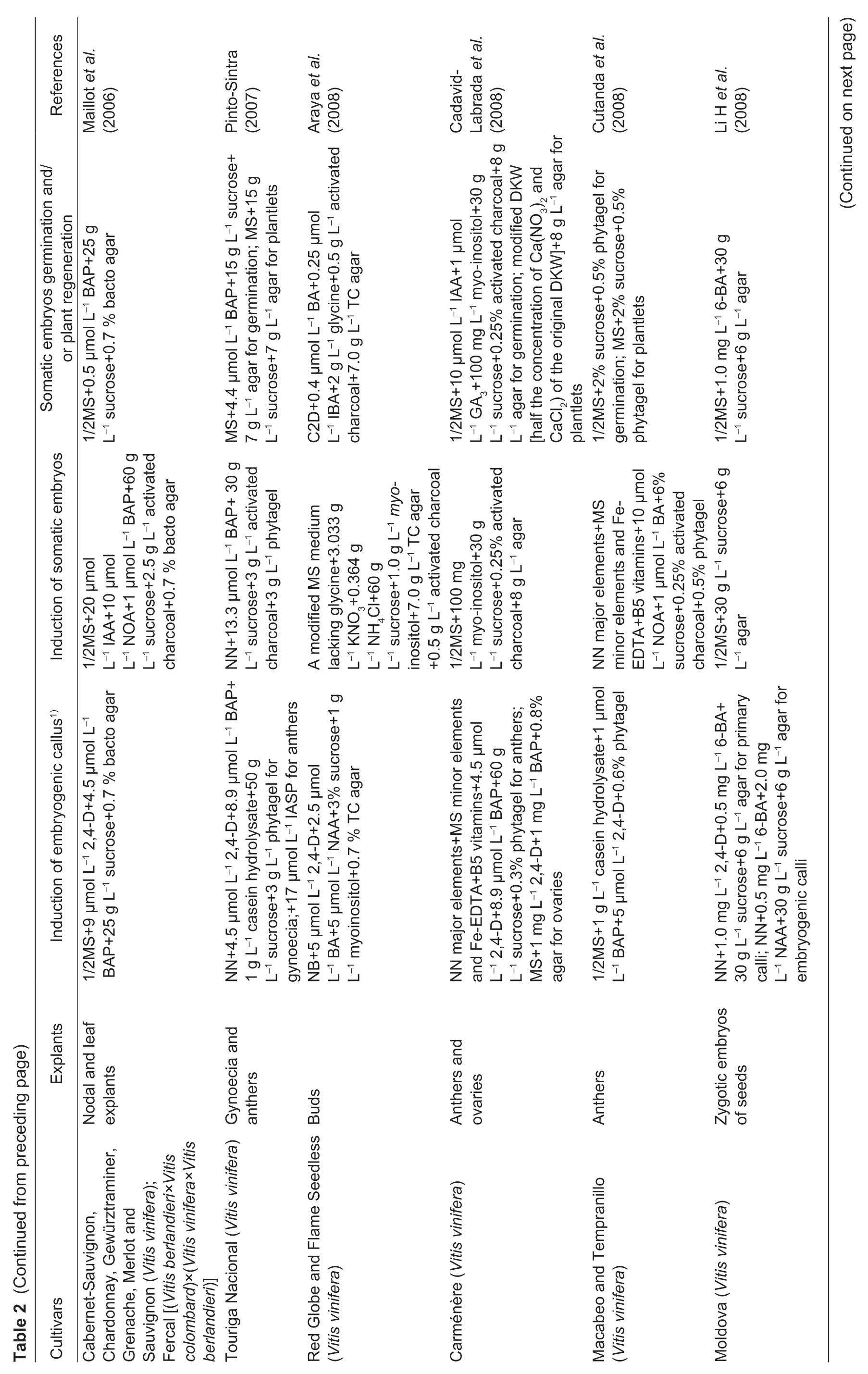
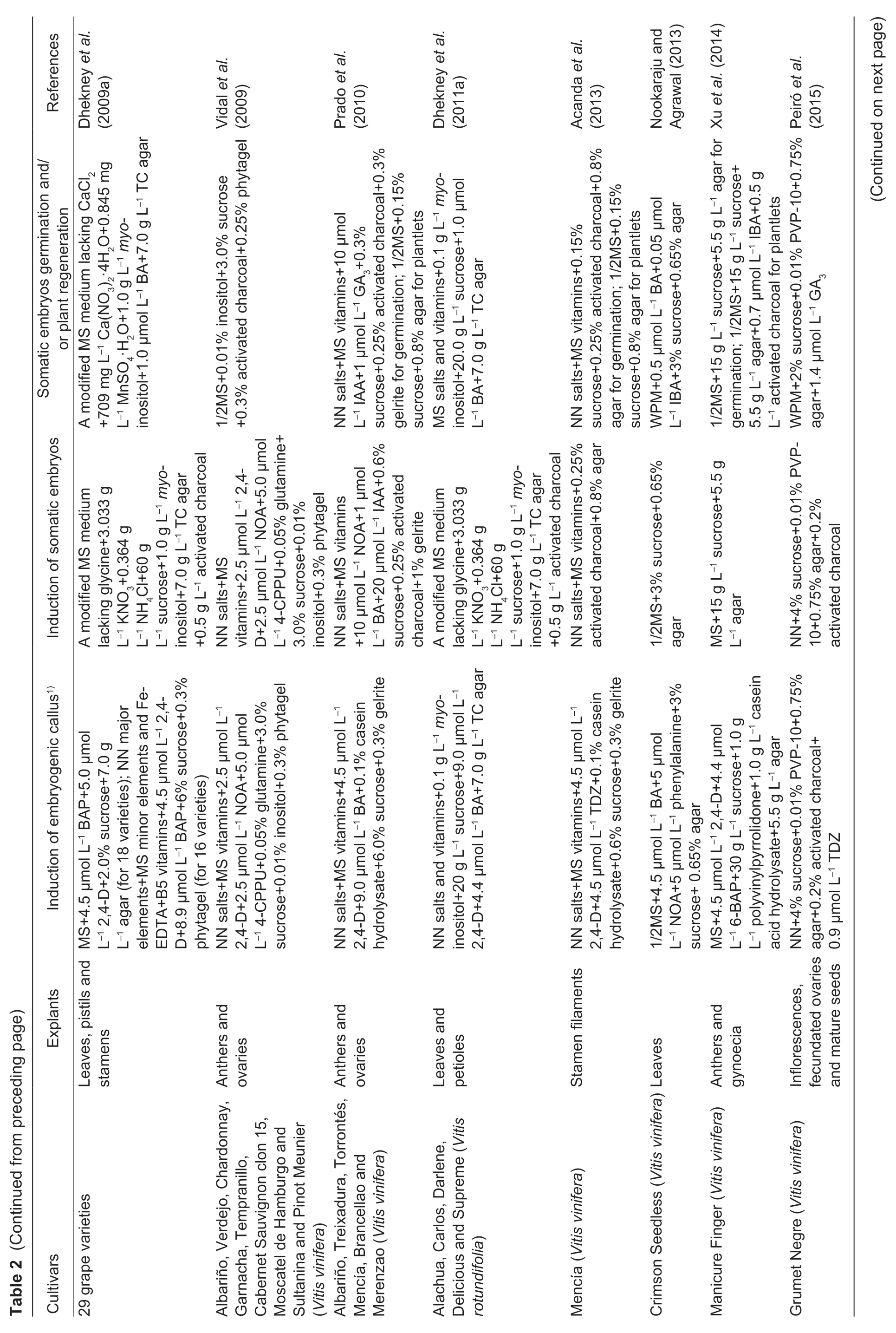
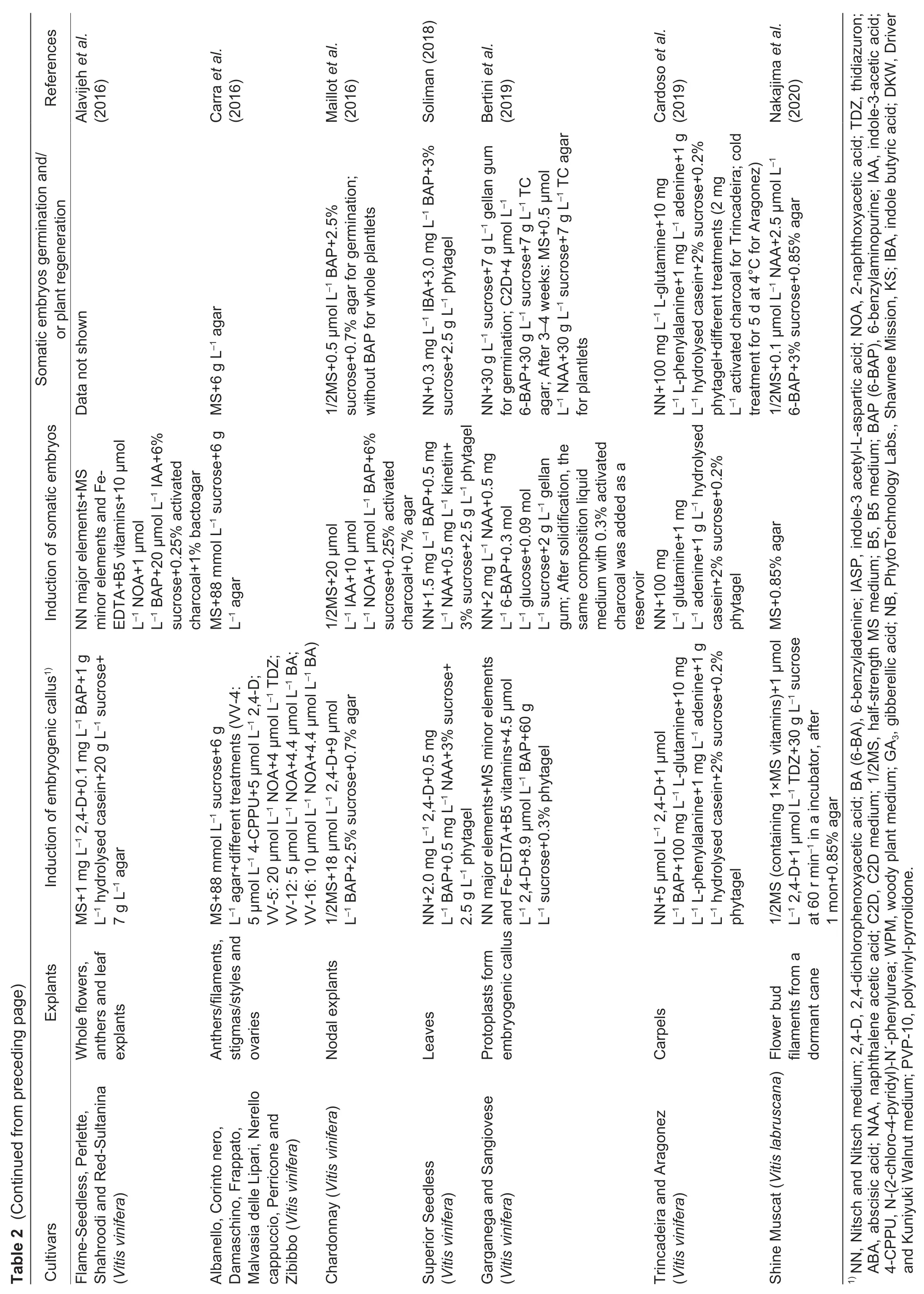
Fig.1 The plant regeneration system of somatic embryogenesis in Vitis vinifera L.cv.Thompson Seedless. A,inflorescence. B,flower bud. C,anther. D,ovary. E,embryogenic callus induction from anthers. F,embryogenic callus induction from ovaries.G,no-embryogenic callus induction from anthers. H,browning and death flowers. I,proembryonic masses. J,somatic embryos.K–L,the plant regeneration from germinated somatic embryos.
2.2.Explant source
Generally,the induction rates of different explants of the same varieties often varied greatly in shoot organogenesis.In one study,internodes were reported to have a larger organogenic capacity than leaf segments (de Carvalhoet al.2011),while in another,leaf explants showed a higher regeneration rate than did petiole and internode explants,although the latter produced stronger adventitious shoots;and radicles did not regenerate any plants on any tested medium when the cultivar Wink was testedinvitro,using leaves,petioles,internodes and radicles (Zhanget al.2011). Additionally,phyllotactic position was found to have a strong influence on the regenerative potential of leaves(Nicholsonet al.2012). It has been suggested that shoot organogenesis increases substantially on regeneration medium with decreased leaf maturity (Stampet al.1990b).There is also evidence that enhanced regeneration capacity can be achieved by pre-conditioning the leaf explant material under dark conditions and/or in liquid medium prior to excision from the plant (Nicholsonet al.2012).
The average percentage of somatic embryogenesis can also vary based on the organ/tissue types. For example,the values obtained from ovaries (10.1%) were reported to be about 2-fold higher than those from anthers (4.9%)(Vidalet al.2009). A recent report also described the use of mature seeds as explants for SE induction (Peiróet al.2015),which found that SE could only be obtained from seeds that were cut prior to being cultured for five months.The efficiency of SE induction is known to be influenced by the developmental stage of the explants,as well as by the genotypes tested. Vidalet al.(2009) showed that earlier flower developmental stages (II–III) more often underwent embryogenic induction from anthers,while later stages(III–V) did so more often from ovaries. Pradoet al.(2010)identified three flower developmental stages -R1,R2 and R3,among which R1 and R2 correspond to stages V and VI as defined by Gribaudoet al.(2004),while R3 is a later binucleate microspore stage. One study by Pradoet al.(2010) concluded that four cultivars (Albarñio,Treixadura,Torrontés and Merenzao) obtained the best results at the R2 stage,while another two cultivars (Mencía and Brancellao)were best used at the R3 stage. Finally,Gambinoet al.(2007) reported that the highest percentages of embryogenic calli were obtained from ovary-derived calli from four of five cultivars tested. They also found that whole flowers gave similar,or better,results for initiating embryogenic cultures than did anthers for the 110 R,Chardonnay and Grignolino varieties (Gambinoet al.2007).
2.3.Culture medium composition
The culture medium provides critical nutrients for the growth of explants at different developmental stages,and its composition is a major factor determining the probability of successful plant regeneration. To date,many different types of basal culture media have been used by investigators,such as MS medium (Murashige and Skoog 1962),1/2MS medium (half-strength MS medium),LS medium (Linsmaier and Skoog 1965),NN medium (Nitsch and Nitsch 1969),WPM medium (Lloyd and McCown 1980),C2D medium(Cheeet al.1984) and DKW medium (Driver and Kuniyuki 1984). Of these,MS and NN media are the most widely used in inducing embryogenic callus and adventitious buds,while the induction of somatic embryos,embryo germination and plant development use MS and 1/2MS media the most(Tables 1 and 2).
The effects of the concentrations of individual mineral elements in media have rarely been reported,mainly because most basal media contain high levels of such elements that are sufficient for callus initiation and plant development. However,a decrease in concentration of ammonium has been shown to promote SE induction in some media (Perrinet al.2001). Sucrose is preferentially used as the sole carbohydrate source in media for grape embryogenic culture initiation and SE induction and development (or adventitious bud formation) with concentrations reportedly ranging from 10 to 180 g L–1and being most widely used at 30–60 g L–1(Tables 1 and 2). Since dehydration of grapevine SE may improve plant formation (Gray 1989),sucrose is frequently employed as an osmoticum during cultivation for SE germination and plant regeneration (Elidemiret al.2007). Comprorr and Gray(1996) found that grape SE germination and subsequent development were enhanced by incubation on medium with a relatively high sucrose concentration (150 g L–1) before transfer to germination medium with benzyladenine. Other grapevine regeneration protocols also involve the addition of amino acids,such as L-glutamine,phenylalanine and glycine,to media (de Carvalhoet al.2013;Nookaraju and Agrawal 2013) to increase the regeneration rate.
Moreover,present studies have tested how grapevine regeneration is influenced by plant hormones and hormone analogs,such as 2,4-dichlorophenoxyacetic acid(2,4-D),N-(2-chloro-4-pyridyl)-N´-phenylurea (4-CPPU),6-benzyladenine (6-BA,BA),6-benzylaminopurine (6-BAP,BAP),gibberellic acid (GA3),indole-3-acetic acid (IAA),indole butyric acid (IBA),naphthalene acetic acid (NAA),2-naphthoxyacetic acid (NOA),and thidiazuron (TDZ)(Tables 1 and 2). It was found that the addition of one or several hormones could dramatically improve the induction efficiency. For instance,the addition of 2,4-D (18 μmol L–1) and BAP (9 μmol L–1) to basal media was effective in inducing SE (Maillotet al.2016). In addition,recallusing was achieved when a single embryo was transferred to MS medium supplemented with indole-3-aspartic acid(IASP) and 2,4-D due to the synergistic hormonal effects(Perlet al.1995). Therefore,combinations of hormones can be advantageous. Although plant hormones/growth regulators are widely used to induce shoot organogenesis and somatic embryogenesis,they can serve different roles at different stages,such as embryogenic culture initiation and maintenance,SE development and germination (or adventitious bud formation). For example,GA3has mainly been used at the SE germination and plant regeneration stage (Pradoet al.2010;Peiróet al.2015),in contrast to 2,4-D,which is used in the embryogenic callus stage (Bertiniet al.2019;Cardosoet al.2019;Nakajimaet al.2020).López-Pérezet al.(2006) reported that the percentages of regenerated plants of the Crimson Seedless grapevine variety were 63.8% for SE cultured on a medium with IAA(10 μmol L–1) and GA3(1 μmol L–1),and 49.9% for those cultured without growth regulators. It has also been suggested that the incorporation of additives other than hormones in medium,such as phosphinothricin (Hébert-Souléet al.1995) or polyvinylpyrrolidone (PVP) (Passoset al.1999),could stimulate embryogenesis,while the addition of methylglyoxal bis-(guanylhydrazone) (MGBG)(1–10 mmol L–1) was reported not to promote growth and development,but rather to inhibit embryo development and to reduce embryo quality (Comprorr and Gray 1996).
2.4.Other factors
An increase in the regeneration frequency and mean number of regenerated shoots has also been shown to result from a two-week dark culturing period (Zhanget al.2011). Other treatments to improve the regeneration efficiency include the application of activated charcoal (López-Pérezet al.2005),chilling pre-treatment (Li Het al.2008),cotyledon excision(Li Z Tet al.2008;Dhekneyet al.2009b),and varied pH values (Parket al.2001).
3.Methods for genetic transformation of grapevine
Biolistic bombardment andAgrobacterium-mediated transformation have emerged as the preferred methods for grapevine transformation (Table 3). Moreover,it has been shown that embryogenic cultures,which provide maximum cell exposure to bombardment that are capable of forming embryos,may be the best target tissue for transformationviamicro-projectile bombardment. It was reported that up to 850 transformed callus colonies were obtained,though no plants were regenerated,when embryogenic suspensions of the interspecific hybrid grapevine cultivar,Chancellor,were bombarded with tungsten particles coated with a plasmid (pBI426) containing the GUS marker together with the neomycin phosphotransferase II (nptII) gene for kanamycin resistance (Hébertet al.1993). However,in a subsequent study,transformed plants were obtained using the same biolistic transformation approach,and Southern blot analysis was used to confirm genomic integration of thenptIIgene in all 3 GUS positive plants tested (Kikkertet al.1996). This suggests that biolistic bombardment can be an effective method for producing transgenic grapevine.These experiments paved the way for biolistic transformation of embryogenic cultures or suspensions of theV.viniferaL.cultivars Merlot and Chardonnay (Kikkertet al.2000;Vidalet al.2003,2006a,b) with exogenous genes associated with disease resistance. Moreover,use of minimal cassette (linear DNA comprising promoter+open reading frame+terminator) technology for genetic transformation of grapevine cellsviabiolistics (Sanjurjoet al.2013) provides a method for producing transgenic cells/tissues without inserting unwanted sequences. In this system,the authors found that 3´-end cassette protection for successful protein expression was necessary due to the nuclease activity of the target plant material.
Agrobacterium-mediated transformation that utilizes eitherA.rhizogenesorA.tumefaciensis generally more widely used than biolistic bombardment for inserting foreign genes into plants. These bacteria are capable of transferring a DNA segment (T-DNA) from their Ti (tumor inducing) plasmids into the nuclear genome of the host plant. Following the DNA transfer,transformants can be obtained through plant regeneration. Genetically transformed grapevine roots have been obtained usingA.rhizogenesafter inoculation ofin vitrogrown whole plants (Guellecet al.1990) or leaf segments (Nakanoet al.1994);however,attempts to regenerate transgenic plants after transformation only resulted in embryogenic calli.Subsequently,Gribaudoet al.(1995) demonstrated that root production took place at all inoculation sites (scraped internodes,decapitated stems,petioles without leaf blades)and for all tested grapevine cultivars (Nebbiolo,Moscato and Barbera) and bacterial strains (A4,15834,NCPPB2659 and 8196). When three interspecific rootstocks and six hybrids were used to generate hairy root cultures from plants co-inoculated withA.rhizogenes,plant regeneration did not occur. Nevertheless,an alternative approach was suggested involving graftingin vitrotransgenic roots onto non-transformed shoot systems. Grafted plants were established under greenhouse conditions,which could permit rapid testing of the resistance induced by nepovirus coat protein in roots of genotypes that are recalcitrant toA.tumefaciens-mediated transformation (Torregrosa and Bouquet 1997).Agrobacterium rhizogenes-transformed roots from different grapevine genotypes can also be used as a source of grapevine viruses for isolation. In one study,all three grapevine phloem-limited viruses tested (grapevine fleck virus,grapevine virus A,grapevine virus B) were able to proliferate and persist in root cultures,and were then used for viral particle purification. Indeed,it has been proposed that grapevine roots may be useful for culturing and purifying other non-mechanically transmissible grapevine viruses(Lupoet al.1994).
To date,most reports of stable genetic transformation depend on the acceptor materials of co-cultivation withA.tumefacienin grapevine (Table 3 and Fig.2).This technique has been applied to other plant species.Furthermore,Agrobacteriumcells can also be infiltrated into grapevine leaves using vacuum infiltration or a needleless syringe. Santos-Rosaet al.(2008) reported that the infiltration of grapevine leaves using a needleless syringe was not efficient because only the leaf area in direct contact with the syringe was infiltrated;while a higher efficiency resulted from vacuum infiltration of detached grapevine leaves fromin vitrogrown plants. Agro-infiltration of leaf tissues is typically the method of choice for transient gene expression,usually implemented onin vitrocultured plants(Zottiniet al.2008). However,Ben-Amaret al.(2013)demonstrated that non-detached leaves of greenhousegrown grapevine plants were the most efficient target materials for vacuum infiltration regardless of genotype.Despite the reported differences,the infiltration system provides a powerful tool for rapid analysis of gene function in grapevine.

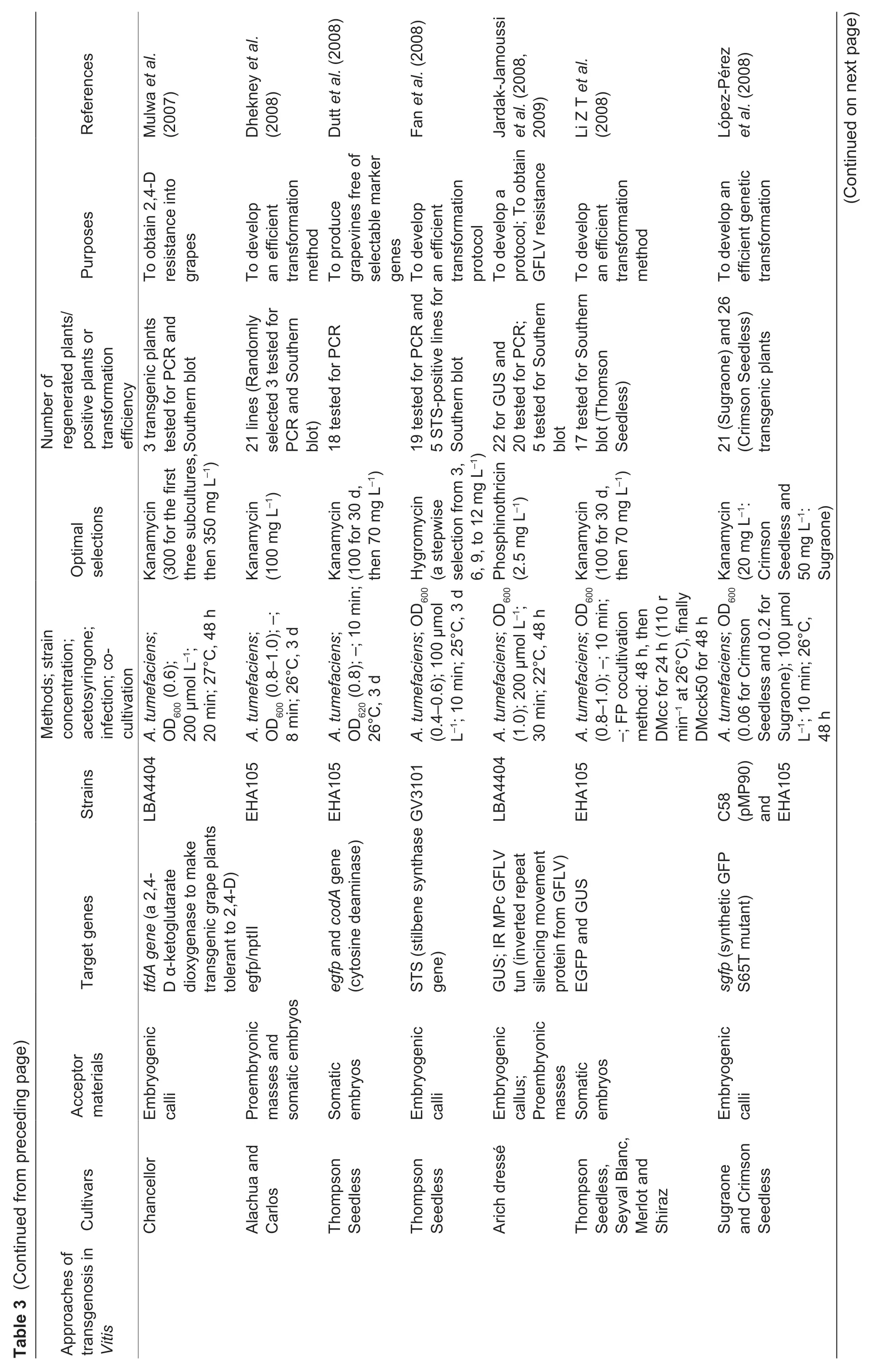
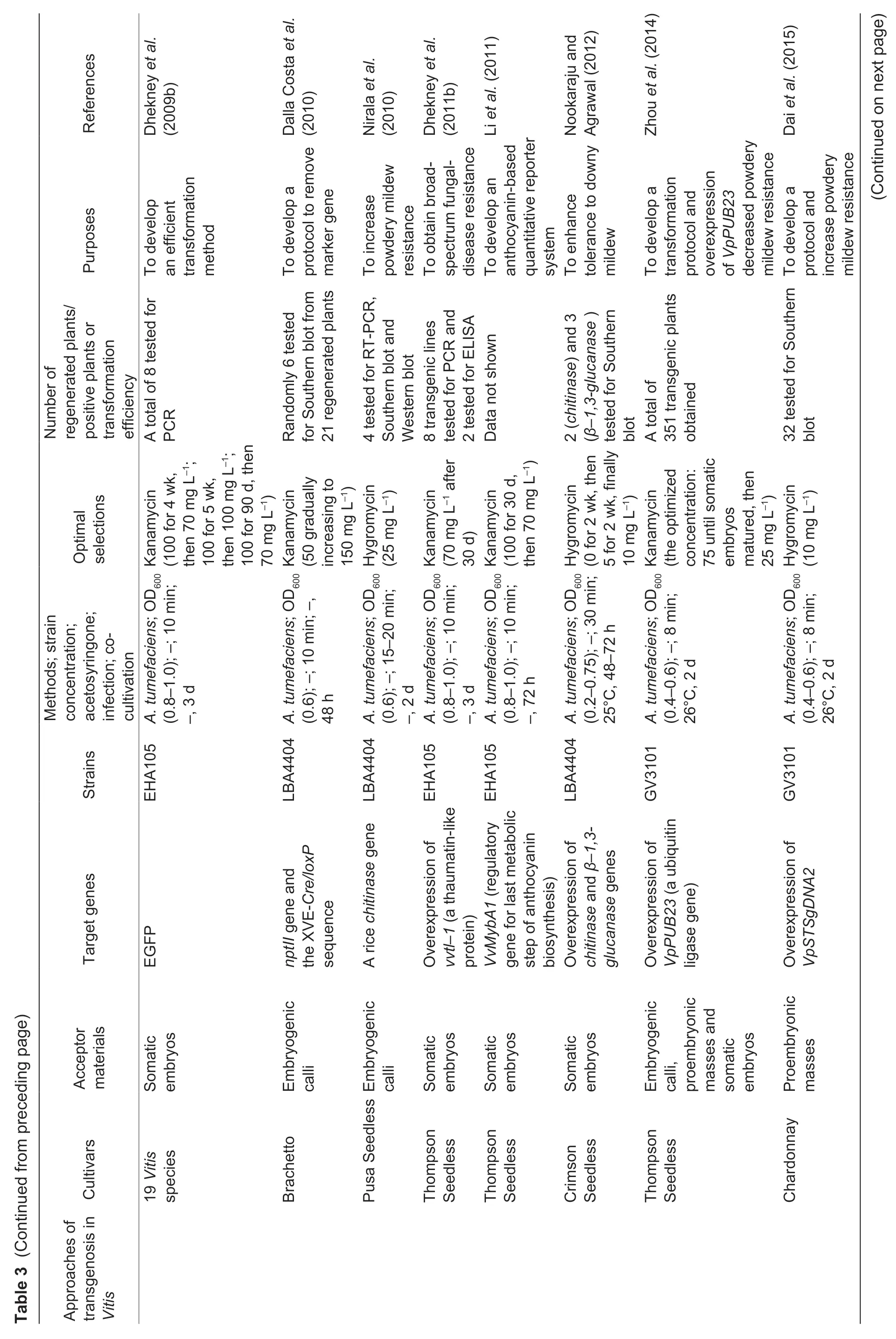
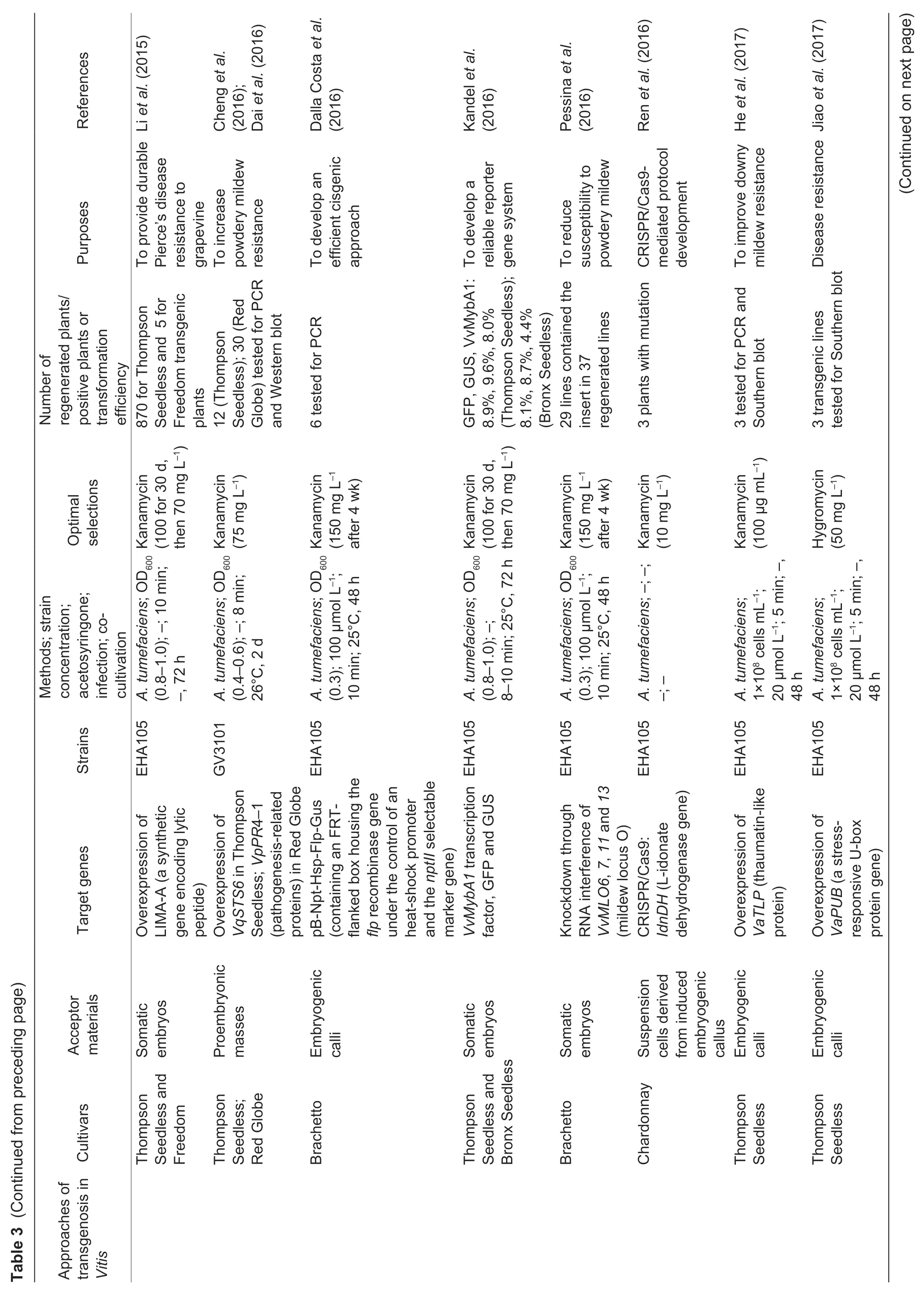
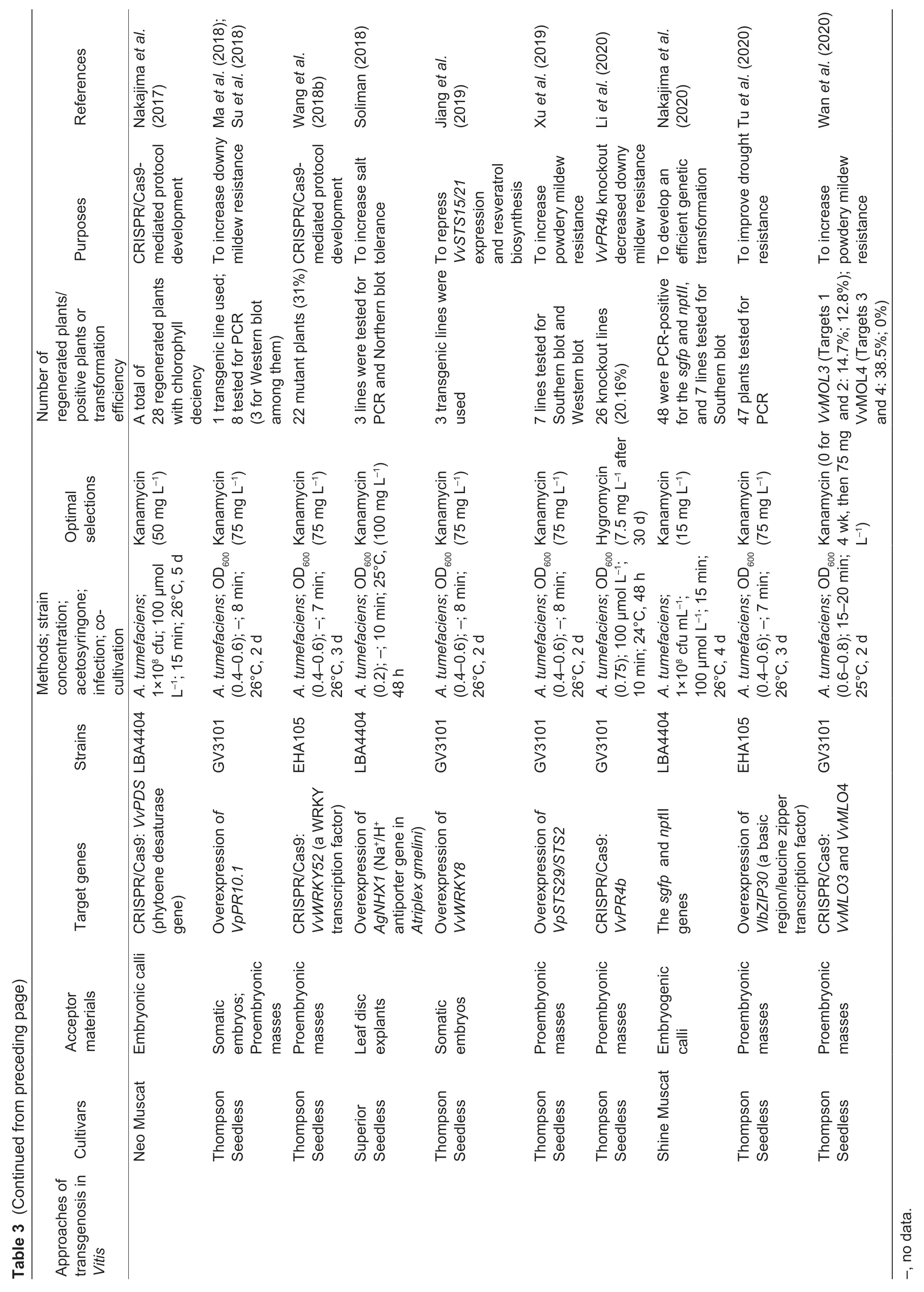

Fig.2 The genetic transformation system via somatic embryogenesis in Vitis vinifera L.cv.Thompson Seedless. A,pre-cultured proembryonic masses (PEM). B,PEM submerged in A.tumefaciens. C,co-cultivation on two layers of sterile filter paper. D,PEM eliminated A.tumefaciens. E,positively selected somatic embryos. F,the resistant plantlet. Bar=1 cm.
A combination of biolistic bombardment andAgrobacterium-mediated transformation has also been tested. Transformants were recovered from SE that had been bombarded twice with gold particles and then exposed toA.tumefaciens,suggesting that biolistic bombardment followed by exposure toA.tumefaciensis a valid approach for transforming grapevine somatic embryos (Scorzaet al.1995,1996). It has also been reported that differentiated SE from different Tunisian autochthonous grapevine cultivars were preferably transformed after being wounded by a scalpel (Bouamamaet al.2000),which resulted in a degree of damage to the surface of the explants that is similar to that caused by gene gun bombardment.This damage might facilitate subsequentAgrobacteriuminfection.However,it has been reported that tissue injuries caused by needle (Martinelli and Mandolino 1994) or particle bombardment (Mozsáret al.1998) did not necessarily increase transformation efficiency.
Moreover,plasmid-mediated genetic transformation with grapevine protoplasts has also been reported. Nevertheless,this method is chiefly used for the transient expression,such as protein subcellular localization assays,protein-protein interaction studies,and evaluation of the gene editing efficiency or standardization of a stepwise protocol for the CRISPR system (Wanget al.2015;Malnoyet al.2016;Zhaoet al.2016;Osakabeet al.2018). Therefore,transient transformation of protoplasts can provide a prompt strategy to study gene function and avoid the time-consuming process of stable transformation plants in grapevine.
A highly efficient and simple transgenosis method used to study gene function is still critical in grapevine,although the genetic modification technologies have been performed more than three decades up till the present moment.Biolistic bombardment can be widely used for various tissues and organs,and there is no need to construct specialized vectors for genetic transformation. However,the genes of interest must be coated with spherical gold or tungsten bullets,resulting in an expensive spend. The bombardment process also tends to cause loss of molecular integrity of the inserted DNA fragment,multiple copies of gene insertions,and emergence of chimeric genetically modified plantlets (Altpeteret al.2005;Hwanget al.2017).Protoplast transformation is not commonly used to obtain stable transgenic plants,but for transient transformation,because it is difficult to regenerate plantlets (Malnoyet al.2016). Fortunately,theA.tumefaciens-mediated genetic transformation method possesses several advantages compared with other transformation approaches,including easy implementation with relatively low cost,minimal experimental condition,generally the low gene transfer copy number,and a relatively stable DNA inserted into plant genome (Gaoet al.2008;Hwanget al.2017). Hence,theA.tumefaciensmethod still representes the most widely used and efficient approach for obtaining transgenic plants in grapevineviaexogenous gene transfers.
4.Factors influencing the efficiency of grapevine genetic transformation
4.1.Acceptor materials
Different explant sources and quality of the callus used in the transformation process have significant effects on the rate of transformation. Shoot apical meristems are the most widely used material forAgrobacterium-mediated transformation of grapevineviashoot organogenesis (Xieet al.2016;Sabbadiniet al.2019a,b). However,other types of embryogenic tissues that have been utilized for somatic embryogenesis include embryogenic callus,proembryonic masses and somatic embryos (Table 3). To date,proembryonic masses and somatic embryos are widely considered the most suitable explants forAgrobacteriummediated transformation since the regenerative cells on the surface of these tissues are susceptible toAgrobacterium,and the single cell origin gives rise to non-chimeric transformants. Dhekneyet al.(2008) reported that both PEM and SE at the mid-cotyledonary stage of development were suitable target tissues for co-cultivation withAgrobacterium,as evidenced by transient GFP expression. However,the transformation efficiency of PEM and SE was not compared in this study. Recently,three types of embryogenic tissues(compact EC,creamy loose PEM and SE) were used in a study of transformation ofV.viniferacv.Chardonnay,which found that the transgenic SE lines from EC and SE did not regenerate into plants (Daiet al.2015). In contrast,earlier studies reported that EC and/or SE were effective explant sources for transformation and plant recovery (Liet al.2006;Li Z Tet al.2008;López-Pérezet al.2008).Furthermore,embryogenic cell suspension cultures are important materials for co-cultivation withA.tumefaciensto develop an efficient transformation protocol and to obtain overexpression or gene editing plants (Saportaet al.2014;Chuet al.2016;Renet al.2016;Osakabeet al.2018).Sometimes,the cultures are also used for microprojectile bombardment (Kikkertet al.1996;Vidalet al.2003,2006a,b). Since suspension cultures can also provide maximum cell exposure to bombardment,in an appropriately grown embryogenic suspension culture,a large number of cells are competent to form SE and plants (Hébertet al.1993). More recently,suspension cultures ofV.Vinifera cv.Chardonnay at the single cell stage derived from a proembryonic mass were used as explants to optimize anAgrobacteriummediated transformation system (Wuet al.2012).
4.2.Bacterial strain and concentration
To date,most grapevine transformation has been based onAgrobacterium spp,the strain and cell density of which can have a significant effect on transformation efficiency.Many different bacterial strains have been used to transform diverse grape varieties,such asA.tumefaciensstrains C58 (pMP90),EHA101,EHA105,GV3101,and LBA4404(Table 3) andA.rhizogenesstrains 8196,15834,A4,A5,A13,D6,and K599 (Nakanoet al.1994;Jittayasothornet al.2011;Wuet al.2012). Of those,EHA105 was reported for a high transformation rate. In a comprehensive study,López-Pérezet al.(2008) reported thatAgrobacteriumstrain EHA105 yielded better transformation efficiency than C58 (pMP90) in both Sugraone and Crimson Seedless(V.viniferaL.),which was consistent with results obtained by Torregrosaet al.(2002).
An excess of bacteria can cause acceptor material death,whereas too low a cell density often results in a reduced infection rate. It has been reported that an optical density value of 0.2–1.0 (at OD420,OD550,OD600or OD630) and 108–109bacterial cells mL–1is effective whenAgrobacteriumis cocultured with the recipient materials (Table 3). More recently,López-Pérezet al.(2008) showed that co-cultivation with lower bacterial densities (0.06 or 0.2 at OD600) avoided tissue necrosis and could lead to transgenic grapevine plants of both the cultivars Sugraone (Superior Seedless)and Crimson Seedless.
4.3.Culture and selection methods
Concurrent with efforts to optimize transformation protocols,culture methods,inoculation and co-cultivation have also been investigated. A previous study indicated that transformation efficiency could be increased through modifications of co-cultivation and subsequent washing procedures (Li Z Tet al.2008). To select transformants,the selectable markernptIIgene,which confers resistance to the antibiotic kanamycin,has been widely used,in combination with concentrations of kanamycin ranging from 10 to 150 mg L–1(Table 3). It is important to employ an appropriate concentration of antibiotics to select transformed tissues because false negatives may be obtained at concentrations that are too high,and false positives at concentrations that are too low (Martinelli and Mandolino 1994;Duttet al.2007). Recently,the hygromycin phosphotransferase II (hptII) gene (Guanet al.2013;Daiet al.2015) and phosphinothricin-Nacetyl transferase(bar) gene (Jardak-Jamoussiet al.2008,2009),conferring resistance to hygromycin and phosphinothricin,respectively,have also been used as selectable markers in grapevine.In addition,the use of genes that yield visible markers,such as GUS and enhanced GFP (EGFP),permits visual screening of transformed cultures (Table 3). In Dhekneyet al.(2008),SE proliferation was carried out in the presence of 50 or 75 mg L–1kanamycin and a relatively small number of transformants were obtained.However,the growth of non-transformed tissue was reduced by using 100 mg L–1kanamycin,and a greater percentage of transformed SE was also obtained,resulting in the recovery of transgenic plants.Although a few non-transformed SE developed when using 100 mg L–1kanamycin,they were easily identified by the absence of GFP-fluorescence. Therefore,a combination ofnptIIalong withegfporgusis an effective method to screen the stable transformants. More recently,a grapevine-derived anthocyanin marker system was developed (Liet al.2011;Kandelet al.2016). In this system,the ectopic expression of the grapevine gene,VvMybA1,allowes the production of anthocyanin in transformed and otherwise non-pigmented cells,without relying onnptIIexpression or kanamycin selection. As a visible reporter marker,VvMybA1,provides a relatively efficient and reliable system for screening for transgenic plants.
Recently,different strategies have also been developed to obtain cisgenic plants without selectable marker genes.Duttet al.(2008) reported a co-transformation system to produce transgenic grapevines that are free of selectable marker genes,where a mixture of twoAgrobacteriumstrains were used to inoculate SE ofVitis viniferaL.‘Thompson Seedless’. One strain harbored a binary plasmid with the targetegfpgene between the T-DNA borders,while the other strain contained a positive selectionnptIIgene and a negative selection cytosine deaminase (codA) gene. The two genes were linked by a bi-directional dual promoter complex. Dalla Costaet al.(2010) tested a XVE-Cre/LoxPsystem in which thenptIIgene was removed as a result of the expression of the 17-β-estradiol inducingCrerecombinase gene. More recently,a system was reported to involve a binary vector containing a heat-shock-inducible promoter activating a recombinase gene to facilitate the excision of a FRT-flanked box harboring both thenptIImarker gene and the recombinase itself. Consequently,the downstreamgusreporter gene could be expressed after the removal of this cassette (Dalla Costaet al.2016). Using this system,when grapevine embryogenic callus tissues from Brachetto were co-cultured withA.tumefaciens,transgenic plants resulted after heat-shock induction. The removal of thenptIIgene was subsequently verified by quantitative PCR analysis of genomic DNA;and GUS activity was measured by a fluorimetry assay. It shows this system is a promising method for obtaining transformants without the selectable marker gene.
4.4.Culture medium type
As a general rule,there are four primary stages in plant regeneration following grapevine transformation:1) embryogenic culture (or meristematic bulk) initiation and maintenance;2) genetic transformation and selection of putative transformed lines;3) transgenic SE development and germination (or adventitious bud formation);and 4) plant development and analysis of transformants (Fig.2).In the first three phases,different culture media,either liquid,solid,or a combination of the two,are often used to tailor the growth of explant cultures at different developmental stages. A number of commonly used basal media for callus induction and/or embryos proliferation have been modified to give rise to NB medium (Le Gallet al.1994),GS1CA and PIV media (Frankset al.1998),DM and X6 media (Liet al.2001),and 1/2MSAC medium (López-Pérezet al.2005). The X6 medium is exclusively used at the third development stage in the process of somatic embryogenesis to obtain transformants,while the media most often used in the first two stages are those modified from MS and NN basal media. Several studies have examined the effects of specific callus induction media on different phases.For example,a significantly greater percentage of stable transgenic calli from co-cultivated embryogenic cultures of Ramsey,Harmony,and Richter 110 was observed when cultures were maintained on DM and GS1CA media,than on NB medium (Dhekneyet al.2009b).
While both solid and liquid media have been used,solid media is generally preferred. Liquid media are often used for the proliferation of proembryonic masses (Jayasankaret al.1999;Dhekneyet al.2008),co-cultivation of explants withAgrobacterium tumefaciens(Li Z Tet al.2008) and inhibition ofAgrobacterium tumefaciengrowth (Duttet al.2008). For instance,Li Z Tet al.(2008) reported a method by which explants were co-cultivated withA.tumefaciensand then transferred to liquid DMcck medium (DM medium containing 200 mg L–1each of carbenicillin and cefotaxime and 50 mg L–1kanamycin) for 2 days,followed by transfer to solid DMcck medium. This method was also successfully utilized by other investigators (Dhekneyet al.2009b,2011b;Liet al.2015) in spite of the different kanamycin concentration and co-cultivation time. Finally,it has been observed that incubation of explants in liquid medium,followed by cultivation on solid medium,resulted in a higher degree of SE induction thanviaincubating exclusively on solid medium (Nakajima and Matsuta 2003).
4.5.Other factors
The compounds Pluronic F-68 (Mulwaet al.2007) and acetosyringone (AS) are often added to bacterial cultures to activateAgrobacteriumbefore infection of grapevine explant materials (Table 3). Another factor that can influence transformation efficiency is tissue browning and necrosis followingAgrobacteriuminfection;however,this can be avoided to some degree by pre-culturing acceptor material for 7 d (Liet al.2006) and adding antioxidants,such as dithiothreitol (DTT) and polyvinylpolypyrrolidone (PVPP)(Perlet al.1996;Daset al.2002;Liet al.2006;Dhekneyet al.2007;Mulwaet al.2007). Additionally,Nookaraju and Agrawal (2012) reported that transformation efficiency and secondary embryo survival were enhanced 2.5–3 folds by incorporating antioxidants/anti-necrotic agents in cocultivation medium. Finally,sonication of SE for 2–3 s at 60 kHz in a bacterial suspension ofA.tumefacienswas found to significantly increase transformation efficiency.
5.Inserting functional genes for trait improvement in grapevine
Genes that confer desirable characteristics can be introduced into the genome of existing grapevine cultivarsviagenetic transformation,while preserving the genetic identity of the plants. Accordingly,the identification and characterization of more genes that function to enhance resistance to various biotic and abiotic stresses are highpriority targets for grapevine research. In recent years,there have been a number of reports describingA.tumefaciensmediated transformation of various grapevine rootstocks and varieties with various target genes,including reporter genes and genes conferring traits related to enhancing resistance/tolerance to disease,pests,abiotic stresses and improving fruit quality (Table 3). Mullinset al.(1990)reported the first successful regeneration of transgenic grapevine plants through co-cultivation of hypocotyl explants derived from SE of rootstock,Rupestris St.George (Vitis rupestris),with a disarmedA.tumefaciensstrain encoding the GUS reporter gene.The same result was achieved later using a gene conferring kanamycin resistance (Martinelli and Mandolino 1994). Subsequently,genes with different functions were similarly transferred into grapevine.They included the coat protein of grapevine chrome mosaic nepovirus (GCMV-CP) (Le Gallet al.1994) and grape fanleaf virus (GFLV-CP) that confer virus resistance,a chitinase gene that enhances resistance to fungal pathogens,antifreeze genes,the green fluorescent protein andGUSreporter gene,a 2,4-D α-ketoglutarate dioxygenase (tfdA)gene that confers tolerance to 2,4-D,a stilbene synthase gene (STS) for synthesizing the phytoalexin,resveratrol,theV.viniferathaumatin-like protein (vvtl-1) gene conferring fungal disease resistance,theV.pseudoreticulataglyoxal oxidase (VpGLOX) gene,the stress-responsive U-box protein genePUB,theV.pseudoreticulata PRgene(VpPR4-1),andchitinaseandβ-1,3-glucanasegenes suppressing powdery mildew in a susceptible grapevine genotype (Table 3). More recently,a pathogenesis-related gene (VpPR10.1) from the wild Chinese grapevine,V.pseudoreticulata,was transferred into grapevinecv.Thompson Seedless for functional evaluation. In that study,transgenic plants overexpressingVpPR10.1exhibited increased resistance to downy mildew,compared to nontransgenic plants (Maet al.2018;Suet al.2018). When the stilbene synthase gene,VpSTS29/STS2,was transferred into Thompson SeedlessviaA.tumefaciens-mediated transformation,the transformants showed elevated levels of stilbene and H2O2,leading to reduced growth of powdery mildew (Xuet al.2019). Finally,RNA interference system(Pessinaet al.2016) and CRISPR/Cas9-based genome editing system have also been introduced into grapevine cells usingAgrobacterium-mediated transformation to improve the quality of grape (Nakajimaet al.2017;Osakabeet al.2018;Wanet al.2020). In conclusion,A.tumefaciensmediated transformation has been established as an effective and the predominant method for generating transgenic grapevine plants.
6.Conclusion and future developments
Genetic engineering is an attractive approach for enhancing the commercial value and yield of grapevine.To date,the transformed acceptor materials obtainedviabiolistic bombardment and/orAgrobacterium-mediated transformation have been developed into stable transgenic plants using the established regeneration systems. The genotypes of grapevine species,explant source and culture medium are important factors influencing the efficiency of plant regeneration. In addition,acceptor materials,the bacterial strain and cell density of bacterial suspension,and selection methods also influence transformation efficiency.Grapevine is relatively recalcitrant to plant regeneration,as are many other woody crops.A highly efficient protocol for plant regeneration,though being a critical prerequisite for genetic transformation,has yet to be achieved. As in Table 3,Agrobacterium-mediated transformation is still the best effective and recorded method for stable transformation of grapevine. In the procedure ofAgrobacterium-mediated transformation,target materials precultured in the suitable medium were lightly placed on two layers of sterile filter paper after inoculation withAgrobacteriumat different concentrations for 5–30 minutes,and co-cultured for 2–5 days at a suitable temperature in the dark. After this co-cultivation treatment,the explants were washed several times with cefotaxime and/or carbenicillin at matching concentrations to inhibit the growth ofAgrobacterium,and finally transferred to the plantlet regeneration medium with a selection agent to screen the positive transgenic plants.The specific steps of theAgrobacterium-transformation process are shown in Figs.2 and 3.
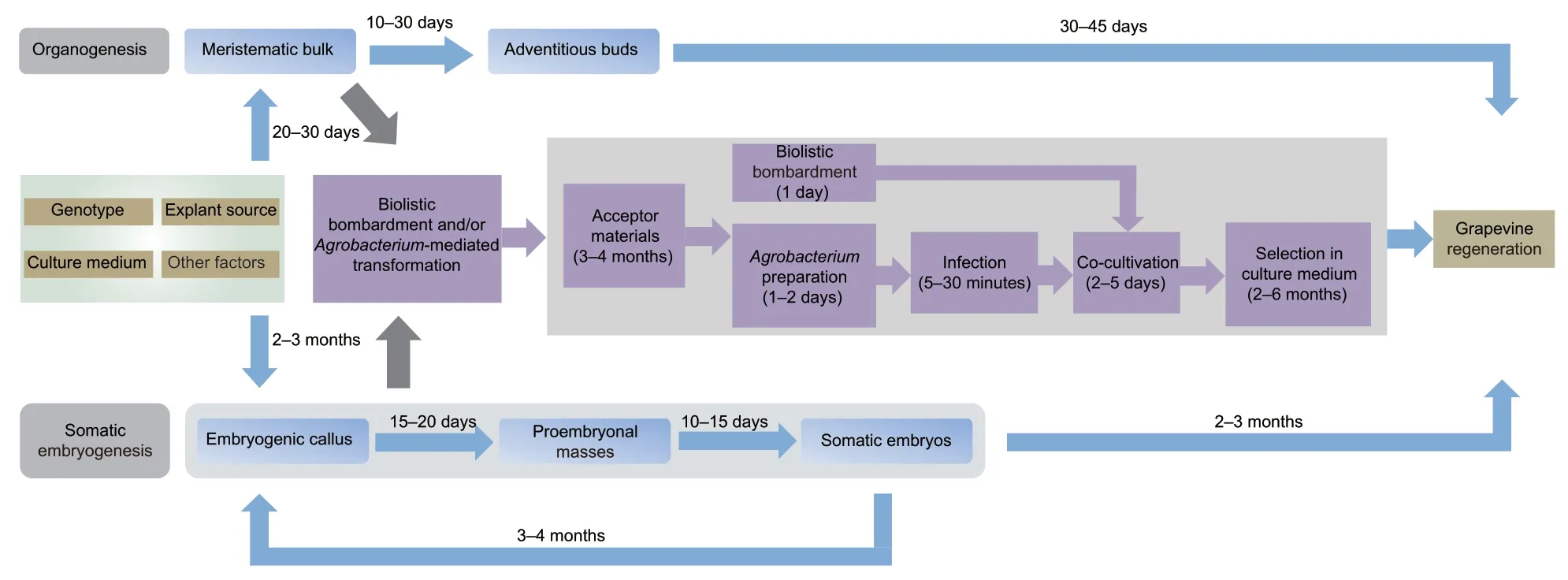
Fig.3 General diagram for grapevine plant regeneration and genetic transformation.
In spite of efforts to genetically engineer grapevine,few varieties,mostly Thompson Seedless and Chardonnay up to now (Table 3),have been successfully transformed and further studies are needed to improve transformation efficiency. Consequently,establishment of high efficiency plantlet regeneration and genetic transformation systems are still the main limitation for grapevine researchers.Exhilaratingly,a few recent breakthrough reports have emerged as a promising solution where developmental regulators are used to enhance the regeneration and transformation frequency in several plants. Simultaneously overexpressing the morphogenic genesBaby boom(Bbm)andWuschel2(Wus2) from maize was able to dramatically stimulate the plantlet regeneration and transformation efficiency in sorghum,sugarcane and rice,and even in the recalcitrant maize inbred lines (Loweet al.2016,2018). It is worth noting that increased developmental regulators,such asWOX9-1(Tvorogovaet al.2019),GRF5(Konget al.2020) and SHOOT MERISTEMLESS (STM) (Maheret al.2020),have also been reported to boost the regeneration and transformation capacity of recalcitrant plants. More recently,Debernardiet al.(2020) reported that GRF-GIF chimera protein,GROWTH-REGULATING FACTOR 4and its cofactorGRF-INTERACTING FACTOR 1improved regeneration and transformation in monocots and dicots.We conclude that the benefits of the developmental regulators technology can be rapidly extended to other species,including grapevine.
Additionally,there are several disadvantages associated with using theAgrobacterium-mediated,biolistic-mediated and protoplasts transformation methods to obtain transgenic plantlets. Thus,developing new genetic transformation methods to overcome the current bottlenecks,such as limitation of genotype and low transformation efficiency,is also needed. In recent years,new nanoparticle-mediated delivery systems that may be helpful in the future to obviate plant tissue culture have been extensively utilized in Arabidopsis,cotton,maize,tobacco and other species(Doyleet al.2019;Lvet al.2020). We hold the opinion that these novel delivery methods will promptly accelerate the quality trait improvement in grapevine. Nonetheless,these promising delivery methods are mostly used for transient expression.Thus,future research needs to be done in conjunction with other new technologies to produce stably transformed transgenic plants. Maybe some new genetic transformation systems based onBbm-andWus2-orGRF-GIF-mediated plantlet regeneration will solve this problem.
All in all,both developmental genes and new delivery systems are the key to improving plant regeneration and genetic transformation in grapevine. We anticipate that genetic improvement of grapevine will undergo dramatic changes by combining the CRISPR/Cas-mediated genome editing with highly efficiencient plant regeneration and genetic transformation in the near future.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (U1603234),the 948 Project from the Ministry of Agriculture of China (2012-S12),the Project for the Key Science and Technology Innovation Team of Shaanxi Province,China (2013KCT-25) and the Key Research and Development Plan of Ningxia Hui Autonomous Region,China (2019BEF02005).
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Low glycemic index:The next target for rice production in China?
- Effects of different types of slow-and controlled-release fertilizers on rice yield
- Indica rice restorer lines with large sink potential exhibit improved nutrient transportation to the panicle,which enhances both yield and nitrogen-use efficiency
- Effects of nitrogen management on the ratoon crop yield and head rice yield in South USA
- Response of grain-filling rate and grain quality of mid-season indica rice to nitrogen application
- Determining nitrogen status and quantifying nitrogen fertilizer requirement using a critical nitrogen dilution curve for hybrid indica rice under mechanical pot-seedling transplanting pattern
