中药植物紫草天然产物的生物合成及其功能研究进展
2021-05-19林红燕王煊何聪周紫玲杨旻恺文钟灵韩洪苇陆桂华戚金亮杨永华
林红燕,王煊,何聪,周紫玲,杨旻恺,文钟灵,韩洪苇,陆桂华,戚金亮,杨永华
中药植物紫草天然产物的生物合成及其功能研究进展
林红燕,王煊,何聪,周紫玲,杨旻恺,文钟灵,韩洪苇,陆桂华,戚金亮,杨永华
南京大学医药生物技术国家重点实验室,植物分子生物学研究所,生命科学学院,南京 210023
紫草为我国传统的重要药用植物资源,其根部代谢产生的紫红色萘醌类天然产物—紫草素及其衍生物,临床上常被用于治疗疮疡和皮肤炎症。数十年来,紫草因具高效的多重生物活性、药理作用、良好的临床疗效、较高的利用价值,引起了国内外研究者的重视与关注,正由于此种原因,其野生植物种质资源常遭到大量采挖,生长环境受到严重威胁。随着植物天然产物的生物合成、分子代谢及其生物技术的发展,药用植物天然产物生物活性功能与药理作用研究手段的不断创新,紫草的生物合成途径和相关调控基因的研究取得了显著的进展,紫草素药理作用及其机制得到深入阐明或解析,极大地推进了紫草素的基础性研究及其临床应用开发的进程。本文从紫草分类、紫草素的结构与组成及其生物合成途径、调控紫草素生物合成代谢的功能相关基因以及紫草素生物活性与药理功能等方面综述了相关研究进展,并对未来可能的发展趋势进行了展望,以期为促进我国重要中药材源的药用天然产物的深度挖掘与开发提供有益参考,推动我国传统中药学的现代化发展。
紫草;紫草素;生物合成;基因调控;药理活性
紫草(Zi Cao)是我国传统中药材,始载于《神农本草经》,列为中品,曰:“紫草,味苦、寒,主心腹邪气五疸,补中益气,利九窍,通水道,一名紫丹,一名紫芙,生山谷”。《中华人民共和国药典》(2020年版)收载的以干燥根为中药材的紫草来源于紫草科软紫草属植物—新疆紫草((Royle)Johnst)或内蒙紫草(Bunge);2000年及其之前的药典版本中则收载了紫草科紫草属植物–紫草(Sieb. et Zucc.)等物种。这些紫草物种的根部均含有紫红色的萘醌类药用天然产物—紫草素及其衍生物(以下简称紫草素),具有抗菌、消炎、活血等功效,且可应用于食品、高级化妆品、日化产品与塑料制品的着色等。近年来,国内外的研究发现紫草素不仅具有抗菌消炎、抑制人类免疫缺陷病毒(human immunodeficiency virus, HIV)等多种药理活性,还可通过活化Caspase-3和抑制拓扑异构酶活性来诱导癌细胞凋亡,是继喜树碱、紫杉醇之后又一类极具潜力的天然抗肿瘤药物。本文就紫草分类、紫草素结构、生物合成及其基因调控以及紫草素生物活性与药理功能等研究进展,进行了较为全面的概述,期望加强我国紫草素的基础性研究及其应用开发。
1 紫草的分类
按照《中国植物志》第64(2)卷(1989)及其英文修订版“”·Vol.16(1995)的形态分类系统,紫草科()约包含100多个属,2000多个物种,广泛分布于世界各地,以欧洲地中海区为分布中心[1,2]。目前,紫草科主要被划分为紫草亚科(Subfam.)、天芥菜亚科(Subfam.)、破布木亚科(Subfam.)、厚壳树亚科(Subfam.)这四个亚科,其中紫草亚科为其主要类群。紫草亚科中的紫草族(Trib.)主要包含了紫草属()、软紫草属()、滇紫草属()、蓝蓟属()以及紫筒草属()等5属。其中,紫草属与软紫草属、滇紫草属,可能具有较近的亲缘关系;按花器官分类,滇紫草属可能处于较高的进化地位,其次是软紫草属,而紫草属则处于较原始状态。上述5属植物共有约50个物种,根据形态分类系统,广义上可合并为紫草族,狭义上又可拆分为紫草属与拟紫草属()[3]。
2 紫草素的结构、组成及其生物合成途径
2.1 紫草素结构与组成
“紫草”中药材主要特指能入药或做药的“紫草科”植物富含紫草宁及其衍生物的紫红色根,包括软紫草属的新疆紫草、内蒙紫草,以及紫草属的紫草和滇紫草属的滇紫草等,根据其根部主要药用成分的含量差异,新疆紫草和内蒙紫草的根主要为药用,紫草和滇紫草等可替代入药,也被广泛用做染料[4~6]。紫草素是一类萘醌化合物,其母核为5, 8-二羟基-1, 4-萘醌(5, 8-dihydroxy-1, 4-naphthoquinone),并具异己烯基侧链。根据其旋光性不同,紫草素类化合物被分为两种旋光异构体,即左旋紫草素(阿卡宁,-型,alkannin)与右旋紫草素(紫草宁,-型,shikonin) (图1)[7~10]。
2.2 紫草素的生物合成途径
紫草素的生物合成以产生香叶基–对羟基苯甲酸(m-geranyl-p-hydroxybenzoic acid, GBA或GHB)为节点,可分为上游部分与核心部分;上游部分包含了两个途径,第一个是由萜类骨架途径(terpenoid backbone biosynthesis)合成香叶酯焦磷酸(geranyl pyrophosphate, GPP),第二个是由苯丙素途径(phenylpropanoid biosynthesis, PP)产生对羟基苯甲酸(4-hydroxybenzoate acid, PHB) (图2)[7~10]。
在生物体中,萜类前体骨架途径包括了两个途径,即细胞质中的甲羟戊酸代谢途径(mevalonate pathway, MVA)和质体中的去氧木酮糖途径(2-C- methyl-D-erythritol-4-phosphate pathway, MEP)[11~14]。在紫草素生物合成的研究中,Gaisser 等[15]发现抑制紫草MVA途径中的限速酶甲戊二羟酸单酰辅酶A还原酶(hydroxymethylglutaryl-coenzyme A reductase, HMGR)活性,几乎能够完全抑制紫草素的生物合成。由此,研究者推断紫草的紫草素合成途径中GPP主要来源于MVA途径。但是,近年Singh等[16]发现,当软紫草MEP途径中的限速酶5-磷酸–去氧木酮糖还原酶(5-phosphoric acid-deoxyxylulose reductase, DXR)活性被抑制后,软紫草中的紫草素合成同样受到抑制。由此可见,紫草素生物合成过程中,MVA和MEP这两个途径之间的关系还有待进一步研究和确定。

图1 紫草中的紫草素类天然产物
根据参考文献[9]修改绘制。A:紫草宁类;B:阿卡宁类。R为取代基团;1:紫草素;2:去氧紫草素;3:乙酰紫草素;4:ɑ-甲基-正丁酰紫草素;5:异丁酰紫草素;6:异戊酰紫草素;7:β-羟基异戊酰紫草素;8:β,βʹ-二甲基丙烯酰紫草素;此外,4与6又属于同分异构体。
PHB是紫草素生物合成的另一重要前体,在生物体中,其可由两个途径而来,分别是PP与莽草酸途径(Shikimate pathway)[17,18]。研究发现,莽草酸途径不仅可直接合成分支酸,经由分支酸-丙酮酸合成酶(chorismate-pyruvate lyase, CPL或UbiC)直接合成PHB,还是PP的上游途径,能够为PP途径提供苯丙氨酸(Phenylalanine)。然而,PP中4-香豆酸(4-Coumarate)经4-香豆酸辅酶A连接酶(4-coumarate: CoA ligase, 4CL)催化合成4-Coumaroyl-CoA之后,最终合成PHB的过程中仍有几步反应十分模糊,相应的酶基因也是未知的。在紫草素生物合成中,一般认为PHB只能来自于PP,因为目前在高等植物中似乎并没有找到与UbiC酶同源的基因与酶。此外,Sommer等[19]和Kohle等[20]将大肠杆菌的UbiC酶基因转入了紫草毛状根中进行高表达,虽然紫草毛状根中生产了较多的UbiC酶,但相应的紫草素类化合物并没显著升高。
最新研究发现,紫草宁是由PP代谢途径的产物PHB和MVA代谢途径形成的GPP这两个重要的前体,经对羟基苯甲酸香叶基转移酶(p-hydroxybenzoate geranyltransferase,PGT)催化形成GBA,再经转换为香叶基氢醌(geranyl hyrdoxyquinone, GHQ),继而羟基化为3ʺ-羟基香叶基氢醌(3ʺ-hydroxy-geranyl hyrdoxyquinone, GHQ-3ʺ-OH)等一系列酶促反应,最后在内质网中形成,并通过胞外分泌作用将形成的紫草宁微粒运输到原生质体外的细胞壁中[15,21,22]。此过程中所涉及的部分酶促反应目前尚不清楚,亟需探究。Wang等[23,24]通过紫草细胞的转录组分析与生化检测,发现CYP76B74催化紫草素生物合成中的香叶基氢醌3ʺ的羟基化,为进一步探索开环反应、萘醌骨架的生成鉴定了一个关键位置羟化酶。同时,Song等[25]通过转录组分析与GHQ为底物的酶催化反应,证实CYP76B100在C-3ʺ位置催化GHQ的香叶基侧链羟基化,形成GHQ-3ʺ-OH,CYP76B101在C-3ʺ位置进行GHQ的氧化反应产生GHQ的3ʺ-羧酸衍生物(GHQ-3ʺ-COOH)以及GHQ-3ʺ-OH。此外,在以紫草宁为前体的紫草宁衍生物合成通路研究中,Oshikiri等[26]通过蛋白质组学分析鉴定到两个关键的对映体特异性酰基转移酶LeSAT1和LeAAT1,其能分别将乙酰基-CoA、异丁酰基-CoA和异戊酰基- CoA识别为酰基供体,产生其相应的紫草宁/链烷烃衍生物即乙酰紫草宁/阿卡宁、异丁酰紫草宁/阿卡宁和异戊酰紫草宁/阿卡宁。这些研究都为进一步探究紫草素的生物合成提供了有效信息。

图2 紫草素的生物合成途径
根据参考文献[21]修改绘制。 ACTH: acetoacetyl-CoA thiolase,乙酰乙酰辅酶A硫解酶;C4H: cinnamic acid 4-hydroxylase,肉桂酸-4-羟化酶;4-CL: 4-coumarate:CoA ligase,4-香豆酸辅酶A连接酶;CDPMEK: 4-(cytidine 59-diphospho)-2-C-methyl-D-erythritol 2-phosphokinase,4-二磷酸胞苷-2-C-甲基-D-赤藓醇激酶;DXPS: 1-deoxy-D-xylulose-5-phosphate synthase,1-脱氧-D-木酮糖-5-磷酸合酶;DXR: 5-phosphoric acid-deoxyxylulose reductase,5-磷酸–去氧木酮糖还原酶;GDPS: geranyldiphosphate synthase,焦磷酸香叶酯合酶;GHQH: geranylhydroquinone 3’-hydroxylase,香叶基氢醌3ʹ-羟化酶;HDR: 1-hydroxy-2-methyl-2-(E)-butenyl-4-diphosphate reductase,1-羟基-2-甲基-2-(E)-丁烯基-4-焦磷酸还原酶;HDS: 1-hydroxy-2-methyl-2-(E)-butenyl-diphosphate synthase,1-羟基-2-甲基-2-(E)-丁烯基-4-二磷酸合酶;HMGR: hydroxymethylglutaryl-coenzyme A reductase,甲戊二羟酸单酰辅酶A还原酶;HMGS: 3-hydroxy-3-methylglutaryl-CoA synthase,3-羟基-3-甲基–戊二酰辅酶A合成酶;MCT: 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase,2-C-甲基-D-赤藓糖醇4-磷酸胞苷转移酶;MECDPS: 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase, 2-C-甲基-D-赤藓糖醇2,4-环焦磷酸合成酶;MVAK: mevalonate 5-phospho kinase,甲羟戊酸5-磷酸激酶;MVDD: mevalonate diphosphate decarboxylase,甲羟戊酸5-焦磷酸脱羧酶;PAL: phenylalanine ammonia-lyase,苯丙氨酸解氨酶;PGT: p-hydroxybenzoate geranyltransferase,对羟基苯甲酸香叶基转移酶;PMVK: 5-phosphomevalonate phosphokinase,磷酸甲羟戊二酸激酶;SAT: shikonin acetyltransferase,紫草宁乙酰转移酶。
3 调控紫草素生物合成的功能相关基因
先前的研究者通过抑制差减杂交(suppression subtractive hybridization, SSH)及cDNA末端快速扩增(rapid-amplification of cDNA ends, RACE)等技术,已经成功筛选和克隆了一系列紫草、软紫草以及滇紫草中与紫草素生物合成直接或间接相关的酶基因和调控基因[16]。
3.1 紫草素生物合成直接相关的酶基因
由于紫草素生物合成核心部分不明确,目前已经鉴定和克隆的与紫草素合成直接相关酶基因仅在上游部分,主要包括MVA途径和PP途径的一些酶基因以及催化GBA合成的基因。
在MVA途径中,鉴定与克隆的软紫草基因较为完整。其中软紫草的乙酰乙酰辅酶A硫解酶基因(acetoacetyl-coenzyme A thiolase gene,)、甲戊二羟酸单酰辅酶A合成酶基因(hydroxy-methylglutaryl CoA synthase gene,)、甲戊二羟酸单酰辅酶A还原酶基因(hydroxymethylglutaryl-coenzyme A reductase gene,)、甲羟戊酸焦磷酸激酶基因(phosphomevalonate kinase gene,)、甲羟戊酸激酶基因(mevalonate kinase gene,)、甲羟戊酸5-焦磷酸脱羧酶基因(mevalonate disphosphate decarboxylase gene,)、异戊烯焦磷酸异构酶基因(isopentenyl pyrophosphate:dimethyllallyl pyrophosphate isomerase gene,)以及焦磷酸香叶酯合酶基因(geranyl diphosphate synthase gene,)均已成功获得[16]。但是,到目前为止,在紫草中仅鉴定和克隆了紫草甲戊二羟酸单酰辅酶A还原酶基因(hydroxymethylglutaryl-coenzyme A reductase gene,)[27]。而MVA途径中,HMGR酶可能是唯一的限速酶,直接影响到紫草素的合成[15,16]。
在PP途径中,目前已知的酶基因在紫草和软紫草中均已经被鉴定和克隆,即苯丙氨酸解氨酶基因(phenylalanine ammonia-lyase gene,)、肉桂酸-4-羟化酶基因(cinnamic acid 4-hydroxylase gene,)和基因[28~30]。其中,酶基因被认是控制PP途径的关键起始酶基因[30];而酶基因与酶基因可能不直接影响紫草素的合成[28,30]。
作为紫草素合成核心部分的关键起始酶基因,具有非常重要的作用,其在紫草与软紫草中均已经被鉴定与克隆。有研究报道,许多调控因素,如光信号、植物激素(如二氯苯氧乙酸(dichlorphenoxyacetic acid, 2, 4-D)与茉莉酸甲酯(methyl jasmonate, MJ))以及无机离子(如NH4+),均直接作用于,从而调控紫草素的生物合成[31]。
3.2 紫草素生物合成间接相关的调控基因
除了与紫草素合成直接相关的酶基因以外,研究者还在新疆紫草和紫草中克隆了许多间接相关的调控基因,其中主要包括一些调控因素的潜在受体基因或转录因子基因,以及这些调控因素合成相关的酶基因。
Yazaki等[32]通过SSH技术鉴定与克隆了一系列紫草黑暗诱导基因(dark-inducible gene,)。其中,只有基因受到光信号的严格调控,在黑暗中特异表达。基因产物与拟南芥的脂质转移蛋白(lipid-transfer protein, EARLI 1)同源,可能具有稳定紫草素合成场所(即细胞内小囊泡)的功能,但具体功能目前尚不明确。
花卉分生组织/乙烯响应转录因子(APETALA2/ ethylene-responsive factor,AP2/ERF)基因家族与乙烯不敏感蛋白3/类乙烯不敏感蛋白(/protein, EIN3/EIL)基因家族是乙烯信号传导途径中关键的转录因子[33,34]。研究发现,紫草基因可能参与光信号和乙烯对紫草素合成的调控[35]。Fang等[36]将基因在紫草毛状根中高表达,构建出了一个高产紫草素的毛状根体系。
Fe+结合的顺式还原酮加双氧酶基因(acireductone dioxygenase gene,)被证明参与到乙烯与多胺的合成途径中[37]。Qi等[38]发现紫草基因可能参与调控紫草素的生物合成。此外,有研究报道,多胺合成途径中的精氨酸脱羧酶基因(arginine decarboxylase gene,)也可能参与紫草素合成的调控[35]。
Yazaki等[39]鉴定了一个与病程相关蛋白(pathogenesis related protein, PR)同源的基因,其可能和紫草素合成有关。Yamamura等[40]在紫草细胞系中鉴定得到一个质外体(细胞壁)表达的色素愈伤组织特异性基因(pigment callus-specificgene,),并推测其可能与紫草素的分泌相关。另外,研究者还鉴定、克隆了部分紫草中MYB和MYC转录因子基因,认为它们的功能可能也与紫草素合成有关[18,41,42]。
4 紫草素的生物活性及其药理功能
根据国内外研究报道,紫草素及其衍生物具有显著的抗肿瘤、抗炎、抗菌、抗病毒、抗氧化等多重药理作用,因而具有广阔的开发应用前景[43~45]。
4.1 抗肿瘤活性作用
大量研究表明,紫草素及其衍生物(图3)对不同的肿瘤细胞表现出显著的细胞毒性,其抗癌作用牵涉到多个靶点,抗癌机制包括促细胞凋亡、诱导细胞坏死、抑制DNA拓扑异构酶活性、抑制酪氨酸激酶磷酸化、抑制血管再生及调控多条与肿瘤相关的信号通路等。
SH-7是在紫草宁的结构基础上修饰的来的一种新的萘醌类化合物,它通过抑制Topo II的活性达到抗肿瘤效果,且抑制效果远比其母体化合物紫草宁好[46]。SH-7有效稳定Topo II-DNA复合物并提高了磷酸化的组蛋白H2AX的表达量,同时,它对Topo I也有抑制作用,但效果不如Topo II。早在1995年,Ahn等[47]就合成了一系列乙酰紫草宁类似物,并发现这些类似物是DNA Topo I的良好抑制剂。Qiu等[48]合成的紫草素衍生物PMMB172对三阴性乳腺癌细胞MDA-MB-231的增殖有良好的抑制作用,它通过靶向信号转导与转录激活因子(signal transduction and transcriptional activators, STAT3)的SH2结构域来抑制STAT3的入核以及在细胞核中的定位,进而抑制其下游基因的表达。
对紫草宁及其衍生物的抗肿瘤机制研究最多的要数微管蛋白。2011年,Acharya等[49]报道了萘醌能够使微管蛋白解聚,造成纺锤体微管组织混乱,导致大部分细胞都被阻滞在G2/M期。考虑到紫草宁也属于萘醌类化合物,关于它是否为微管蛋白抑制剂的研究便成为热点。2014年,Wang等[50]合成了一系列杂环羧酸紫草宁酯,并从中筛选获得3-吲哚丙酸紫草宁酯(compound 3)和3-噻吩乙酸紫草宁酯(compound 8)两种对HeLa细胞增殖抑制效果明显的化合物,并推测其为良好的微管蛋白抑制剂。Guo等[51]在紫草宁支链羟基上引入了苯氧苯乙酸,并证明它(compound 16)是通过抑制微管蛋白的聚合来有效抑制HepG2细胞增殖的。Baloch等[52]也对紫草宁进行结构修饰,并筛选出化合物3j,认为它能够干扰微管蛋白的聚集使得HepG2细胞周期抑制在G2/M期,同时激活caspase导致细胞凋亡。Lin等[53]通过硫原子作为桥,在紫草宁支链和萘醌环上连接巯基糖,并通过实验证明连接两个木糖的紫草宁巯基糖衍生物IIb的抗癌活性最佳,而且它也是通过靶向微管蛋白而发挥作用的。Sun等[54]合成获得的苯甲酰丙烯酸紫草宁酯PMMB317可通过靶向表皮生长因子受体(epidermal growth factor receptor, EGFR)和微管蛋白,发挥双重抗肿瘤作用。

图3 具有抗癌活性的代表性紫草宁衍生物
SH7, Compound 3, Compound 8, Compound 16, 3j, PMMB172, PMMB317, Naphthazarin, IIb均为化合物在文献中的名称及编号。
相比之下,关于阿卡宁及其衍生物的抗肿瘤活性研究相对较少(图4)。2004年,Huang等[55]利用多种亲核物质对β, β-二甲基丙烯酰阿卡宁进行还原烷基化和氧化共轭加成反应,所得产物8、11b、12b、14b相比于母体化合物β, β-二甲基丙烯酰阿卡宁(1)和乙酰阿卡宁(2),对人肺腺癌细胞株GLC-82、人鼻咽癌细胞株CNE2、人肝癌细胞株Bel-7402和人白血病细胞株K562具有更高的体外细胞毒性。另外,作者推测,细胞组分(亲核试剂)与醌(亲电试剂)的共轭加成和还原烷基化可能正是醌类结构具有细胞毒性的原因。2010年,Deng等[56]报道一个新型的阿卡宁衍生物SYUNZ-16,称其可通过抑制蛋白激酶B(Protein kinase B, PKB/AKT)激酶活性、阻断蛋白激酶B/叉头状转录因子O (Protein kinase B/forkhead box O, AKT/FOXO)信号通路来诱导细胞凋亡和抑制肿瘤的生长。Zhang等[57~59]报道了二甲基化阿卡宁衍生物S-2a、阿卡宁肟衍生物S-11、DMAKO-05等小分子具有很好的体外抗肿瘤活性。此外,Chang等[60]发现阿卡宁可诱导活性氧(reactive oxygen species, ROS)水平升高从而对DNA造成氧化损伤,同时联合聚腺苷二磷酸–核糖聚合酶(poly- ADP-ribose polymerase, PARP)抑制剂奥拉帕尼在无毒剂量下,显著抑制体内外结肠癌的生长。
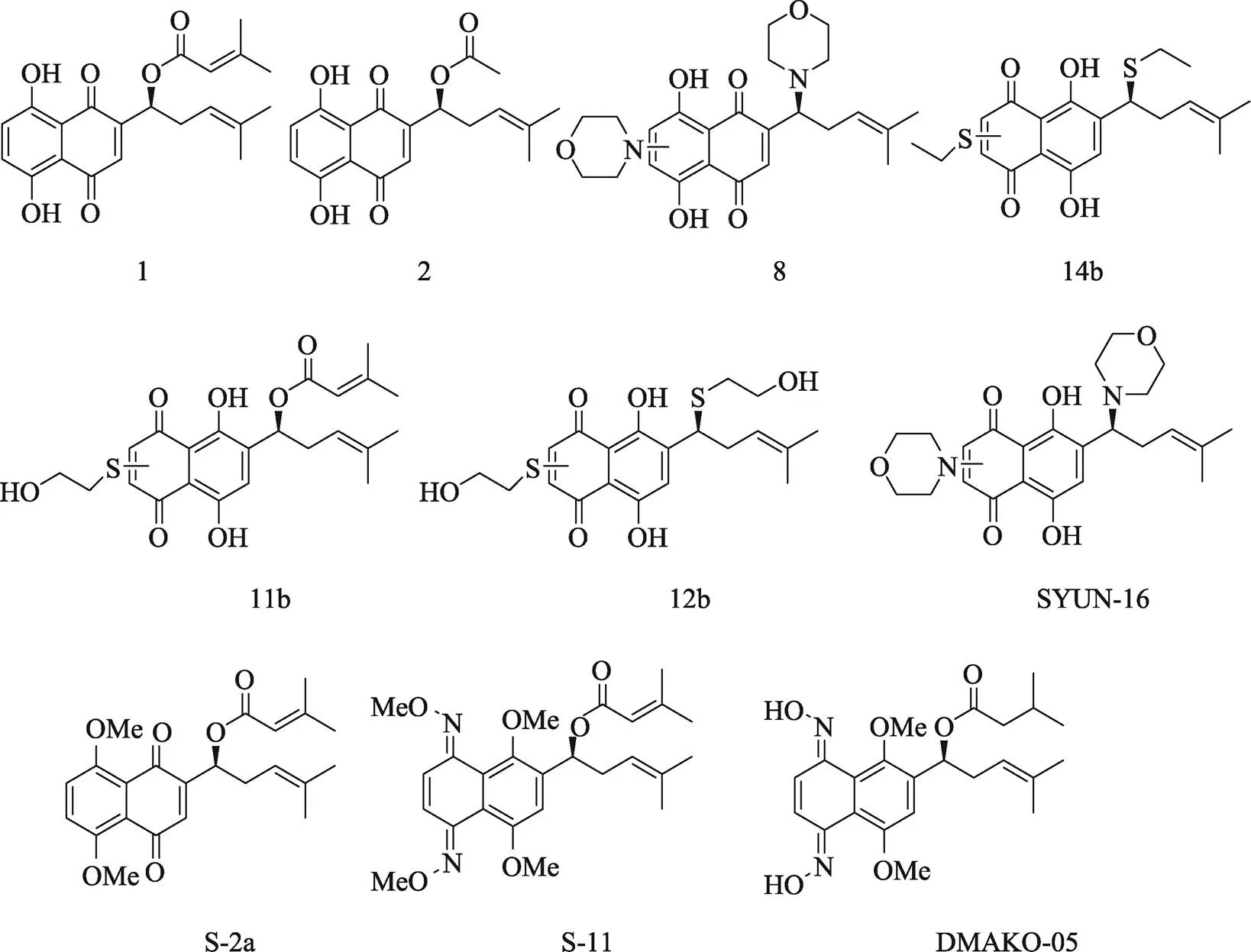
图4 具有抗癌活性的代表性阿卡宁衍生物
1, 2, 8, 14b, 11b, 12b, SYUN-16, S-2a, S-11, DMAKO-05均为化合物在文献中的名称及编号。
4.2 抗炎活性功能
Yang等[61]通过研究紫草宁的抗炎机制,发现紫草宁通过干扰素和核因子κB (nuclear factor kappa-B, NF-κB)信号通路,抑制RAW264.7细胞中脂多糖(lipopolysaccharid, LPS)诱导的高迁移率族蛋白B1 (high mobility group box 1, HMGB1)的释放。此外,紫草宁和β-羟基异戊酰基紫草宁(β-valeryl,β-HIVS) (图5),通过抑制血管内皮生长因子受体(vascular endothelial growth factor receptor, VEGFR)与三磷酸腺苷(triphosadenine, ATP)非竞争性的方式抑制血管生成[62]。萘醌化合物CMEP-NQ可以在不影响细胞活力的前提下,下调LPS诱导的诱导型一氧化氮合酶(inducible nitric oxide synthase, iNOS)和环氧合酶-2(cyclooxygenase-2, COX-2)表达,从而抑制一氧化氮(nitric oxide, NO)和前列腺素E2 (prostaglandin E2, PGE2)的产生[63]达到抗炎作用。乙酰紫草宁具有抗过敏和抗炎作用,能有效降低过敏性鼻炎小鼠模型的过敏性炎症,主要是通过抑制辅助型T细胞2 (T helper 2 cell, Th2)相关卵清蛋白(ovalbumin, OVA)特异性免疫球蛋白E (immune globulin, IgE)、IgG1的产生,同时抑制Th2细胞产生白细胞介素4 (interleukin-4, IL-4)、IL-5、IL-13和肥大细胞产生组胺;此外,乙酰紫草宁可减轻炎症细胞浸润和杯状细胞增生程度[64]。自噬在乙酰紫草宁(图5)通过腺苷酸活化蛋白激酶/哺乳动物雷帕霉素靶蛋白(adenosine monophosphate activated protein kinase/mammalian target of rapamycin, AMPK/Mtor)通路对非酒精性脂肪性肝炎的治疗起着关键作用,Zeng等[65]发现乙酰紫草宁不仅可以改善脂肪变性,还可能通过诱导饮食缺乏蛋氨酸胆碱的小鼠肝脏自噬来减轻肝脏炎症、肝损伤和肝纤维化。Cui等[66]研究证明乙酰紫草宁可明显减弱动脉粥样硬化模型小鼠斑块内炎性细胞(T淋巴细胞、中性粒细胞、巨噬细胞)浸润,IL-1、IL-6、肿瘤坏死因子-α (tumor necrosis factor-α, TNF-α)和单核细胞趋化蛋白-1 (monocyte chemotactic protein 1, MCP-1)水平降低,并通过抑制NF-κB信号通路改善小鼠血管炎症。Zhang等[67]使用紫草羟基萘醌类混合物(hydroxynaphthoquinone mixture, HM)治疗葡聚糖硫酸钠(dextran sulfate sodium, DSS)诱导的溃疡性结肠炎,结果显示,HM能有效改善小鼠结肠炎临床症状和组织病理损伤。早在2012年,西班牙学者Andújar等[68]首次报道了紫草宁对DSS诱导的实验性结肠炎具有显著治疗作用,并证明紫草宁是通过阻断NF-κB和STAT3信号表达,以及它们的促炎反应,来调节结肠炎和与之相关的肿瘤发生。
4.3 抗菌作用研究
最初,人们发现紫草根部的粗提物具有抗菌活性,在过去五十年内,很多研究人员对紫草根部活性成分的抗菌活性做了更深入、系统的研究,并得出结论紫草宁及其衍生物具有广谱的抗菌活性。目前,紫草宁及其衍生物已被证明对革兰氏阳性菌有明显的抗菌活性,如金黄色葡萄球菌、肠球菌、枯草芽孢杆菌等,最小抑菌浓度(minimum inhibitory concentration, MIC)值大约在0.30~6.25 μg/mL之间。相反,它们对革兰氏阴性菌,如大肠杆菌、绿脓假单胞菌和黄色微球菌等没有明显的作用[69]。
早期文献报道紫草宁及其衍生物具有抑菌作用,但是近期的动力学研究说明紫草宁及其衍生物是具有杀菌作用的[70]。Shen等[71]的研究表明紫草宁(图5)可用于治疗对甲氧西林有抗性的金黄色葡萄球菌,MIC值为6.25 μg/mL。后期的研究发现紫草宁酯类衍生物对金黄色葡萄球菌有更好的活性,如甲基丁酰紫草宁(图5),其对金黄色葡萄球菌的MIC值为1.56 μg/mL。Kuo等[72]还报道了紫草宁以浓度依赖的方式抑制幽门螺旋杆菌的增殖。一些肠内细菌包括肺炎杆菌、沙门氏菌、金黄色葡萄球菌和大肠杆菌等体内的酶会表现N-乙酰基转移酶(N-acetyltransferase, NAT)活性,有助于化学致癌物质的代谢激活,Kuo等[72]研究人员还发现了紫草宁可以抑制NAT-介导的N-乙酰化,从而阻断NAT对化学致癌物质的激活。
4.4 抗病毒活性和其它药理功能
紫草宁及其衍生物抗病毒研究也由来已久。2003年,Chen等[73]的研究显示紫草宁可下调巨噬细胞表面一个HIV-1的主要共同受体趋化因子受体5 (chemokine receptor, CCR5)的表达从而抑制HIV病毒的复制。2017年,Zhang等[74,75]发现紫草宁衍生物PMM034可显著抑制横纹肌肉瘤(rhabdomyosarcoma, RD)细胞中促炎因子的表达水平,从而抑制引起手足口病的71型人肠病毒(human enterovirus 71 of hand, foot and mouth disease, EV71)的活性。此外,他们还发现PMM034可通过抑制神经氨酸苷酶有效抑制流感病毒H1N1的表达,且效果可媲美于阳性药奥司他韦[74,75]。

图5 具有抗炎、抗菌活性的代表性紫草宁及其衍生物
Shikonin (紫草宁),Acetylshikonin (乙酰紫草宁),2-Methylbutyryl shikonin (2-甲基丁酰紫草宁),β-hydroxyisovaleryl shikonin (β-羟基异戊酰紫草宁),CMEP-NQ (萘醌化合物)均为化合物在文献中的名称及编号。
Lee等[76,77]发现紫草宁可通过WNT/-catenin途径显著抑制3T3-L1细胞中脂肪的形成和积累,并由此推测其同样可以利用该通路对肥胖症及相关疾病起到治疗作用。Wang等[78]研究发现紫草宁可通过抗氧化作用对小鼠脑出血、再灌注损伤起到保护作用。从机制上来说,即紫草宁显著降低了神经系统缺陷评分、梗死面积、丙二醛(malondialdehyde, MDA)、羰基和活性氧的水平,减弱了神经元损伤,上调了超氧化物歧化酶(superoxide dismutase, SOD)、过氧化氢酶(catalase, CAT)、谷胱甘肽过氧化物酶(glutathioneperoxidase, GSH- px)的活性,降低了谷胱甘肽(glutathione, GSH)/谷胱甘肽二硫化合物(glutathione disulfide, GSSG)的比例。
5 结语与展望
紫草作为我国传统中药,国内外对其次生代谢产物—紫草素的生物合成与分子代谢研究日益精进。紫草素除了在药理作用方面显示良好的活性外,同时也是名贵的染料,作为天然食用色素或添加到化妆品中加以使用,基于此,当前面临着临床或生产需求量大与珍贵植物物种资源日益匮乏的矛盾。因此,亟需从不同角度入手提高紫草宁及其衍生物的产量,实现规模化生产紫草素:一方面,虽然紫草宁及其衍生物的合成途径已基本清楚,但涉及到控制催化GHQ-3ʺ-OH合成紫草宁过程以及紫草宁合成其一系列衍生物的关键酶基因尚不明确,已知的合成途径中关键基因的表达调控也有待探明,因此,有必要通过基因组学、转录组学、蛋白质组学和代谢组学等多组学研究相结合,明晰紫草宁及其衍生物合成调控通路中尚未发现的关键酶基因,继而通过基因工程或代谢工程手段,获得可高效生产紫草宁及其衍生物的高产转基因株系,用于生产实践;另一方面,可在明晰紫草宁及其衍生物生物合成调控途径的基础上,采用合成生物学的方法,通过改造工程菌株使其合成分泌紫草宁及衍生物;或者,还可通过增加高产紫草素等药用成分的紫草科药用紫草的广泛栽培,用以缓解野生植物种质资源与紫草素的供求矛盾。同时,国内外学者针对紫草素进行的抗肿瘤、抗炎、抗菌、抗病毒等药理研究,逐步挖掘了其潜在的分子作用机制。然而到目前为止,仍然没有找到明确的十分高效的作用靶点,以提高紫草素类天然产物对肿瘤的靶向性。此外,紫草素类天然产物抗细菌、真菌及病毒的确切分子机制也并不十分明确,这严重阻碍了其药用价值的开发。根据前人的研究,紫草素在抗炎、抗癌方向对PI3K/AKT/mTOR、MAPK、JAK/STAT、NF-κB通路的影响较为显著[79~84];在抗菌方面紫草素对生物膜的形成和成熟抑制、诱导群体感应分子法尼醇的产生、增加内源性活性氧(ROS)的产生、阻断组蛋白H3去乙酰化、导致内源性NO积累等都是研究的热点[85~88]。随着现代分子生物学、合成生物学及生物信息学的快速发展,相信明确紫草素的完整生物合成途径及其调控、挖掘紫草素的潜在作用靶点将不是难题;并根据可能的作用靶点,再进行深入的化学结构或生化功能等各种修饰,可望极大地促进中药植物天然产物生物合成的调控,有效地开发紫草素或其衍生物成为临床应用的植物源新药。
[1] Wang WC, Liu YL, Zhu GL, Lian YS, Wang JQ,Wang QR (eds). Angiospermae, Dicotyledoneae, Boraginaceae. Flora of China 64(2). Beijing: Science Press, 1989, 1–236.王文采、刘玉兰、朱格麟、廉永善、王镜泉、王庆瑞(编著). 被子植物门‧双子叶植物纲紫草科‧中国植物志(第六十四卷‧第二分册). 北京: 科学出版社, 1989, 1–236.
[2] Zhu GL, Harald R, Rudolf K. Boraginaceae. In: Flora of China, Vol.16, Science Press & Missouri Botanical Garden, 1995, 16: 329–427. http://www.iplant.cn/info/ Boraginaceae?t=foc.
[3] Cohen JI, Litt A, Davis JI. Comparative floral development in(Boraginaceae) and implications for the evolution and development of heterostyly., 2012, 99(5): 797–805.
[4] Chinese Pharmacopoeia Commission. 2000. Pharmacopoeia of the People's Republic of China (Part I). Beijing: Chemical Industry Press, 280.国家药典委员会. 中华人民共和国药典(2000版)(第一部). 北京: 化学工业出版社, 280.
[5] Zhou RH, Duan JA. Plant Chemotaxonomy. Shanghai: Shanghai Scientific & Technical Publishers, 2005, 309– 310.周荣汉, 段金廒. 植物化学分类学. 上海: 上海科学技术出版社, 2005, 309–310.
[6] Chinese Pharmacopoeia Commission. 2020. Pharmacopoeia of the People's Republic of China (Part I). Beijing: China Medical Science Press, 355–356.国家药典委员会. 中华人民共和国药典(2020版)(第一部). 北京: 中国医药科技出版社, 355–356.
[7] Sagratini G, Cristalli G, Giardinà D, Gioventù G, Maggi F, Ricciutelli M, Vittori S. Alkannin/shikonin mixture from roots of(L.) L.: Extraction method study and quantification., 2008, 31(6–7): 945– 952.
[8] Auber RP, Suttiyut T, McCoy RM, Ghaste M, Crook JW, Pendleton AL, Widhalm JR, Wisecaver JH. Hybrid de novo genome assembly of red gromwell () reveals evolutionary insight into shikonin biosynthesis., 2020, 7: 82.
[9] Assimopoulou AN, Sturm S, Stuppner H, Papageorgiou VP. Preparative isolation and purification of alkannin/shikonin derivatives from natural products by high-speed counter- current chromatography., 2009, 23(2): 182–198.
[10] Albreht A, Vovk I, Simonovska B, Srbinoska M. Identification of shikonin and its ester derivatives from the roots ofL., 2009, 1216(15): 3156–3162.
[11] Bergman ME, Davis B, Phillips MA. Medically useful plant terpenoids: biosynthesis, occurrence, and mechanism of action., 2019, 24(21): 3961.
[12] Eisenreich W, Bacher A, Arigoni D, Rohdich F. Biosynthesis of isoprenoidsthe non-mevalonate pathway., 2004, 61(12): 1401–1426.
[13] Miziorko HM. Enzymes of the mevalonate pathway of isoprenoid biosynthesis., 2011, 505(2): 131–143.
[14] Banerjee A, Sharkey TD. Methylerythritol 4-phosphate (MEP) pathway metabolic regulation., 2014, 31(8): 1043–1055.
[15] Gaisser S, Heide L. Inhibition and regulation of shikonin biosynthesis in suspension cultures of., 1996, 41(4): 1065–1072.
[16] Singh RS, Gara RK, Bhardwaj PK, Kaachra A, Malik S, Kumar R, Sharma M, Ahuja PS, Kumar S. Expression of 3-hydroxy-3-methylglutaryl-CoA reductase, p-hydroxybenzoate-m-geranyltransferase and genes of phenylpropanoid pathway exhibits positive correlation with shikonins content in arnebia [(Royle) Johnston]., 2010, 11: 88–98.
[17] Szkopinska A. Ubiquinone. Biosynthesis of quinone ring and its isoprenoid side chain. Intracellular localization., 2000, 47(2): 469–480.
[18] Padilla S, Jonassen T, Jiménez-Hidalgo MA, Fernández- Ayala DJM, López-Lluch G, Marbois B, Navas P, Clarke CF, Santos-Ocaña C. Demethoxy-Q, an intermediate of coenzyme Q biosynthesis, fails to support respiration inand lacks antioxidant activity., 2004, 279(25): 25995–26004.
[19] Sommer S, Köhle A, Yazaki K, Shimomura K, Bechthold A, Heide L. Genetic engineering of shikonin biosynthesis hairy root cultures oftransformed with the bacterial ubiC gene., 1999, 39(4): 683–693.
[20] Köhle A, Sommer S, Yazaki K, Ferrer A, Boronat A, Li SM, Heide L. High level expression of chorismate pyruvate- lyase (UbiC) and HMG-CoA reductase in hairy root cultures of., 2002, 43(8): 894–902.
[21] Takanashi K, Nakagawa Y, Aburaya S, Kaminade K, Aoki W, Saida-Munakata Y, Sugiyama A, Ueda M, Yazaki K. Comparative proteomic analysis ofreveals regulation of a variety of metabolic enzymes leading to comprehensive understanding of the shikonin biosynthetic pathway., 2019, 60(1): 19–28.
[22] Heide L, Nishioka N, Fukui H, Tabata M. Enzymatic regulation of shikonin biosynthesis incell cultures., 1989, 28(7): 1873–1877.
[23] Wang S, Wang RS, Liu T, Lv CG, Liang JW, Kang CZ, Zhou LY, Guo J, Cui GH, Zhang Y, Werck-Reichhart D, Guo LP, Huang LQ. CYP76B74 catalyzes the 3''-hydroxylation of geranylhydroquinone in shikonin biosynthesis., 2019, 179(2): 402–414.
[24] Ohara K, Muroya A, Fukushima N, Yazaki K. Functional characterization of LePGT1, a membrane-bound prenyltransferase involved in the geranylation of p-hydroxybenzoic acid., 2009, 421(2): 231–241.
[25] Song W, Zhuang YB, Liu T. Potential role of two cytochrome P450s obtained fromin catalyzing the oxidation of geranylhydroquinone during shikonin biosynthesis., 2020, 175: 112375.
[26] Oshikiri H, Watanabe B, Yamamoto H, Yazaki K, Takanashi K. Two BAHD acyltransferases catalyze the last step in the shikonin/alkannin biosynthetic pathway., 2020, 184(2): 753–761.
[27] Lange BM, Severin K, Bechthold A, Heide L. Regulatory role of microsomal 3-hydroxy-3-methylglutaryl-coenzyme A reductase for shikonin biosynthesis incell suspension cultures., 1998, 204(2): 234–241.
[28] Katsuyama Y, Matsuzawa M, Funa N, Horinouchi S. Production of curcuminoids bycarrying an artificial biosynthesis pathway., 2008, 154(Pt 9): 2620–2628.
[29] Sykłowska-Baranek K, Pietrosiuk A, Naliwajski MR, Kawiak A, Jeziorek M, Wyderska S, Lojkowska E, Chinou I. Effect of l-phenylalanine on PAL activity and production of naphthoquinone pigments in suspension cultures of(Royle) Johnst., 2012, 48(5): 555–564.
[30] Yamamura Y, Ogihara Y, Mizukami H. Cinnamic acid 4-hydroxylase from: cDNA cloning and gene expression., 2001, 20(7): 655–662.
[31] Yazaki K, Kunihisa M, Fujisaki T, Sato F. Geranyl diphosphate: 4-hydroxybenzoate geranyltransferase from. Cloning and characterization of a ket enzyme in shikonin biosynthesis., 2002, 277(8): 6240–6246.
[32] Yazaki K, Matsuoka H, Shimomura K, Bechthold A, Sato F. A novel dark-inducible protein, LeDI-2, and its involvement in root-specific secondary metabolism in., 2001, 125(4): 1831–1841.
[33] Pré M, Atallah M, Champion A, De Vos M, Pieterse CMJ, Memelink J. The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense., 2008, 147(3): 1347–1357.
[34] Zhu ZQ, An FY, Feng Y, Li PP, Li X, Mu A, Jiang ZQ, Kim JM, To TK, Li W, Zhang XY, Yu Q, Dong Z, Chen WQ, Seki M, Zhou JM, Guo HW. Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in., 2011, 108(30): 12539–12544.
[35] Zhang W, Zou A, Miao J, Yin Y, Tian R, Pang Y, Yang R, Qi J, Yang Y., a novel AP2/ERF family gene within the B3 subcluster, is down-regulated by light signals in., 2011, 13(2): 343–348.
[36] Fang RJ, Zou AL, Zhao H, Wu FY, Zhu Y, Zhao H, Liao YH, Tang RJ, Pang YJ, Yang RW, Wang XM, Qi JL, Lu GH, Yang YH. Transgenic studies reveal the positive role ofin regulating shikonin biosynthesis inhairy roots., 2016, 16(1): 121–132.
[37] Sauter M, Lorbiecke R, Ouyang B, Pochapsky TC, Rzewuski G. The immediate-early ethylene response geneencodes an acireductone dioxygenase involved in recycling of the ethylene precursor S-adenosylmethionine., 2005, 44(5): 718–729.
[38] Qi JL, Zhang WJ, Liu SH, Wang H, Sun DY, Xu GH, Shi MW, Liu Z, Zhang MS, Zhang HM, Yang YH. Expression analysis of light-regulated genes isolated from a full-length-enriched cDNA library ofcell cultures., 2008, 165(14): 1474–1482.
[39] Yazaki K, Bechthold A, Tabata M. Nucleotide sequence of a cDNA fromhomologous to PR-1 of parsley., 1995, 108(3): 1331–1332.
[40] Yamamura Y, Sahin FP, Nagatsu A, Mizukami H. Molecular cloning and characterization of a cDNA encoding a novel apoplastic protein preferentially expressed in a shikonin- producing callus strain of., 2003, 44(4): 437–446.
[41] Zhao H, Baloch SK, Kong LR, Zhang WJ, Zou AL, Wang XM, Qi JL, Yang YH. Molecular cloning, characterization, and expression analysis offrom., 2014, 58(3): 436–444.
[42] Zhao H, Chang QS, Zhang DX, Fang RJ, Zhao H, Wu FY, Wang XM, Lu GH, Qi JL, Yang YH. Overexpression ofenhances shikonin formation by up-regulating key shikonin biosynthesis-related genes in., 2015, 59(3): 429–435.
[43] Andújar I, Recio MC, Giner RM, Ríos JL. Traditional Chinese Medicine Remedy to Jury: The Pharmacological basis for the use of shikonin as an anticancer therapy., 2013, 20(23): 2892–2898.
[44] Lin HY, Li ZK, Bai LF, Baloch SK, Wang F, Qiu HY, Wang X, Qi JL, Yang RW, Wang XM, Yang YH. Synthesis of aryl dihydrothiazol acyl shikonin ester derivatives as anticancer agents through microtubule stabilization., 2015, 96(2): 93–106.
[45] Lu L, Qin AP, Huang HB, Zhou P, Zhang CY, Liu NN, Li SJ, Wen GM, Zhang CG, Dong WH, Wang XJ, Dou QP, Liu JB. Shikonin extracted from medicinal Chinese herbs exerts anti-inflammatory effectproteasome inhibition., 2011, 658(2–3): 242–247.
[46] Yang F, Chen Y, Duan WH, Zhang C, Zhu H, Ding J. SH-7, a new synthesized shikonin derivative, exerting its potent antitumor activities as a topoisomerase inhibitor., 2006, 119(5): 1184–1193.
[47] Ahn BZ, Baik KU, Kweon GR, Lim K, Hwang BD. Acylshikonin analogues: Synthesis and inhibition of DNA topoisomerase-I., 1995, 38(6): 1044–1047.
[48] Qiu HY, Zhu X, Luo YL, Lin HY, Tang CY, Qi JL, Pang YJ, Yang RW, Lu GH, Wang XM, Yang YH. Identification of new shikonin derivatives as antitumor agents targeting STAT3 SH2 domain., 2017, 7(1): 2863–2875.
[49] Acharya BR, Bhattacharyya S, Choudhury D, Chakrabarti G. The microtubule depolymerizing agent naphthazarin induces both apoptosis and autophagy in A549 lung cancer cells., 2011, 16(9): 924–939.
[50] Wang XM, Lin HY, Kong WY, Guo J, Shi J, Huang SC, Qi JL, Yang RW, Gu HW, Yang YH. Synthesis and biological evaluation of heterocyclic carboxylic acyl shikonin derivatives., 2014, 83(3): 334–343.
[51] Guo J, Chen XF, Liu J, Lin HY, Han HW, Liu HC, Huang SC, Shahla BK, Kulek A, Qi JL, Wang XM, Ling LJ, Yang YH. Novel shikonin derivatives targeting tubulin as anticancer agents., 2014, 84(5): 603–615.
[52] Baloch SK, Ling LJ, Qiu HY, Ma L, Lin HY, Huang SC, Qi JL, Wang XM, Lu GH, Yang YH. Synthesis and biological evaluation of novel shikonin ester derivatives as potential anti-cancer agents., 2014, 4(67): 35588–35596.
[53] Lin HY, Han HW, Bai LF, Qiu HY, Yin DZ, Qi JL, Wang XM, Gu HW, Yang YH. Design, synthesis and biological evaluation of shikonin thio-glycoside derivatives: new anti-tubulin agents., 2014, 4(91): 49796–49805.
[54] Sun WX, Han HW, Yang MK, Wen ZL, Wang YS, Fu JY, Lu YT, Wang MY, Bao JX, Lu GH, Qi JL, Wang XM, Lin HY, Yang YH. Design, synthesis and biological evaluation of benzoylacrylic acid shikonin ester derivatives as irreversible dual inhibitors of tubulin and EGFR., 2019, 27(23): 115153–115169.
[55] Huang ZS, Wu HQ, Duan ZF, Xie BF, Liu ZC, Feng GK, Gu LQ, Chan ASC, Li YM. Synthesis and cytotoxicity study of alkannin derivatives., 2004, 39(9): 755–764.
[56] Deng R, Tang J, Xie BF, Feng GK, Huang YH, Liu ZC, Zhu XF. SYUNZ-16, a newly synthesized alkannin derivative, induces tumor cells apoptosis and suppresses tumor growth through inhibition of PKB/AKT kinase activity and blockade of AKT/FOXO signal pathway., 2010, 127(1): 220–229.
[57] Zhang X, Cui JH, Zhou W, Li SS. Design, Synthesis and anticancer activity of shikonin and alkannin derivatives with different substituents on the naphthazarin scaffold., 2015, 31(3): 394–400.
[58] Wang RB, Zhou W, Meng QQ, Zhang X, Ding J, Xu Y, Song HL, Yang K, Cui JH, Li SS. Design, synthesis, and biological evaluation of shikonin and alkannin derivatives as potential anticancer agentsa prodrug approach., 2014, 9(12): 2798–2808.
[59] Huang G, Meng QQ, Zhou W, Zhang QJ, Dong JY, Li SS. Design and synthesis of biotinylated dimethylation of alkannin oxime derivatives., 2017, 28(2): 453–457.
[60] Chang MX, Wang HG, Niu JJ, Song Y, Zou ZH. Alkannin-induced oxidative DNA damage synergizes with PARP inhibition to cause cancer-specific cytotoxicity., 2020, 11: 610205–610218.
[61] Yang Y, Wang J, Yang Q, Wu SS, Yang ZG, Zhu HH, Zheng M, Liu WX, Wu W, He JL, Chen Z. Shikonin inhibits the lipopolysaccharide-induced release of HMGB1 in RAW264.7 cells via IFN and NF-κB signaling pathways., 2014, 19(1): 81–87.
[62] Komi Y, Suzuki Y, Shimamura M, Kajimoto S, Nakajo S, Masuda M, Shibuya M, Itabe H, Shimokado K, Oettgen P, Nakaya K, Kojima S. Mechanism of inhibition of tumor angiogenesis by beta-hydroxyisovalerylshikonin., 2009, 100(2): 269–277.
[63] Woo HJ, Jun DY, Lee JY, Park HS, Woo MH, Park SJ, Kim SC, Yang CH, Kim YH. Anti-inflammatory action of 2-carbomethoxy-2,3-epoxy-3-prenyl-1,4-naphthoquinone (CMEP-NQ) suppresses both the MyD88-dependent and TRIF-dependent pathways of TLR4 signaling in LPS-stimulated RAW264.7 cells., 2017, 205: 103–115.
[64] Fan XH, Cheng L, Yan AH. Ameliorative effect of acetylshikonin on ovalbumin (OVA)-induced allergic rhinitis in mice through the inhibition of Th2 cytokine production and mast cell histamine release., 2019, 127(10): 688–695.
[65] Zeng JC, Zhu BH, Su ML. Autophagy is involved in acetylshikonin ameliorating non-alcoholic steatohepatitis through AMPK/mTOR pathway., 2018, 503(3): 1645–1650.
[66] Cui LB, Yan Y, Zhang M, Wu JF, Tang XX, Yang J, Li LL, Yao K, Zou WG, Jiang CH. Acetylshikonin suppresses atherogenesis by attenuating vascular inflammation in apolipoprotein E-deficient mice., 2018, 11(3): 1882–1890.
[67] Zhang ZL, Fan HY, Yang MY, Zhang ZK, Liu K. Therapeutic effect of a hydroxynaphthoquinone fraction on dextran sulfate sodium-induced ulcerative colitis., 2014, 20(41): 15310–15318.
[68] Andújar I, Ríos JL, Giner RM, Cerdá JM, Recio MDC. Beneficial effect of shikonin on experimental colitis induced by dextran sulfate sodium in Balb/C mice., 2012, 38: 271606.
[69] Haghbeen K, Pourmolaei S, Mareftjo MJ, Mousavi A, Noghabi KA, Shirazi FH, Meshkat A. Detailed investigations on the solid cell culture and antimicrobial activities of the Iranian., 2011, 165852.
[70] Li HM, Tang YL, Zhang ZH, Liu CJ, Li HZ, Li RT, Xia XS. Compounds fromand their related anti-HCV and antibacterial activities., 2012, 78(1): 39–45.
[71] Shen CC, Syu WJ, Li SY, Lin CH, Lee GH, Sun CM. Antimicrobial activities of naphthazarins from., 2002, 65(12): 1857–1862.
[72] Kuo HM, Hsia TC, Chuang YC, Lu HF, Lin SY, Chung JG. Shikonin inhibits the growth and N-acetylation of 2-aminofluorene in Helicobacter pylori from ulcer patients., 2004, 24(3a): 1587–1592.
[73] Chen X, Yang L, Zhang N, Turpin JA, Buckheit RW, Osterling C, Oppenheim JJ, Howard OMZ. Shikonin, a component of Chinese herbal medicine, inhibits chemokinereceptor function and suppresses human immunodeficiency virus type 1., 2003, 47(9): 2810–2816.
[74] Zhang Y, Han H, Sun L, Qiu H, Lin H, Yu L, Zhu W, Qi J, Yang R, Pang Y, Wang X, Lu G, Yang Y. Antiviral activity of shikonin ester derivative PMM-034 against enterovirus 71., 2017, 50(10): e6586.
[75] Zhang YH, Han HW, Qiu HY, Lin HY, Yu LG, Zhu WZ, Qi JL, Yang RW, Pang YJ, Wang XM, Lu GH, Yang YH. Antiviral activity of a synthesized shikonin ester against influenza A (H1N1) virus and insights into its mechanism., 2017, 93: 636–645.
[76] Lee H, Bae S, Kim K, Kim W, Chung SI, Yang Y, Yoon Y. Shikonin inhibits adipogenesis by modulation of the WNT/beta-catenin pathway., 2011, 88(7–8): 294–301.
[77] Lee H, Kang R, Yoon Y. Shikonin inhibits fat accumulation in 3T3-L1 adipocytes., 2010, 24(3): 344–351.
[78] Wang ZH, Liu T, Gan L, Wang T, Yuan XA, Zhang B, Chen HY, Zheng QS. Shikonin protects mouse brain against cerebral ischemia/reperfusion injury through its antioxidant activity., 2010, 643(2–3): 211–217.
[79] Wang LN, Li ZZ, Zhang XJ, Wang S, Zhu CH, Miao JY, Chen LY, Cui LL, Qiao HM. Protective effect of shikonin in experimental ischemic stroke: attenuated TLR4, p-p38MAPK, NF-κB, TNF-α and MMP-9 expression, up-regulated Claudin-5 expression, ameliorated BBB permeability., 2014, 39(1): 97–106.
[80] Shan ZL, Zhong L, Xiao CL, Gan LG, Xu T, Song H, Yang R, Li L, Liu BZ. Shikonin suppresses proliferation and induces apoptosis in human leukemia NB4 cells through modulation of MAPKs and c-Myc., 2017, 16(3): 3055–3060.
[81] Lan WJ, Wan SB, Gu WQ, Wang HY, Zhou SW. Mechanisms behind the inhibition of lung adenocarcinoma cell by shikonin., 2014, 70(2): 1459–1467.
[82] Kim EK, Choi EJ. Compromised MAPK signaling in human diseases: an update., 2015, 89(6): 867–882.
[83] Fu DJ, Shang XF, Ni Z, Shi GG. Shikonin inhibits inflammation and chondrocyte apoptosis by regulation of the PI3K/Akt signaling pathway in a rat model of osteoarthritis., 2016, 12(4): 2735–2740.
[84] Fan C, Zhang XF, Upton Z. Anti-inflammatory effects of shikonin in human periodontal ligament cells., 2018, 56(1): 415–421.
[85] Yan Y, Tan F, Miao H, Wang H, Cao YY. Effect of shikonin againstbiofilms., 2019, 10: 1085–1095.
[86] Shishodia SK, Shankar J. Proteomic analysis revealed ROS-mediated growth inhibition ofby shikonin., 2020, 224: 103849–103860.
[87] Liao ZB, Zhu ZY, Li L, Wang L, Wan H, Jian YY, Cao YY. Metabonomics onindicate the excessive H3K56ac is involved in the antifungal activity of shikonin., 2019, 8(1): 1243–1253.
[88] Liao ZB, Yan Y, Dong HH, Zhu ZY, Jiang YY, Cao YY. Endogenous nitric oxide accumulation is involved in the antifungal activity of shikonin against., 2016, 5(8): e88.
Progress on biosynthesis and function of the natural products of Zi Cao as a traditional Chinese medicinal herb
Hongyan Lin, Xuan Wang, Cong He, Ziling Zhou, Minkai Yang, Zhongling Wen, Hongwei Han, Guihua Lu, Jinliang Qi, Yonghua Yang
Zi Cao is an important traditional medicinal plant resource in China. Shikonin and its derivatives, as the purple-red naphthoquinones among natural products of its roots, are commonly used clinically in the treatment of sores and skin inflammations. Over the past few decades, due to their highly effective multiple biological activities, pharmacological effects, good clinical efficacy and high utilization value, shikonin and its derivatives have attracted increasing attention of domestic and foreign researchers. For this reason, the wild plant germplasm resources have been suffering a grievous exploitation, leading to a serious threat to the habitat. With the development of the biosynthesis, molecular metabolism and biotechnology, as well as the continuous innovation of research methods on the biological activities and pharmacological effects of plant natural products, significant progress has been made in the research on the biosynthetic pathways and related regulatory genes of shikonin. The pharmacological action and its mechanism of shikonin have also been deeply elucidated, which greatly promoted the basic research and clinical application development of shikonin. In this review, we briefly introduce and analyze the classification of Zi Cao, structure and composition of natural shikonin and its biosynthesis pathway, functional genes related to the regulation of shikonin biosynthesis, and biological activities and pharmacological functions of shikonin. Finally, we address possible prospective for the trend on the future research and development of natural shikonin and its derivatives, hoping to provide a useful reference for the deep mining and development of medicinal natural products from important Chinese medicinal materials, and to promote the modern development of traditional Chinese medicine.
Zi Cao; shikonin; biosynthesis; gene regulation; pharmacological activity
2020-10-10;
2021-03-04
国家自然科学基金项目(编号:U1903201, 31670298, 31771413, 21702100, 21907051)和教育部创新团队项目(编号:IRT_14R27)资助[Supported by the National Natural Science Foundation of China (Nos. U1903201, 31670298, 31771413, 21702100, 21907051), and the Program for Changjiang Scholars and Innovative Research Team in University from the Ministry of Education of China (No. IRT_14R27)]
林红燕,博士,助理研究员,研究方向:药用植物天然产物化学和分子药理。E-mail: linhy@nju.edu.cn
王煊,博士研究生,研究方向:植物分子代谢。E-mail: dg1930043@smail.nju.edu.cn
林红燕和王煊并列第一作者。
杨永华,教授,博士生导师,研究方向:分子代谢与生物技术安全。E-mail: yangyh@nju.edu.cn
10.16288/j.yczz.20-341
2021/3/29 11:37:11
URI: https://kns.cnki.net/kcms/detail/11.1913.R.20210326.0956.002.html
(责任编委: 陈德富)
