新型喹唑啉衍生物的设计、合成及体外抗肿瘤活性
2021-05-06孙健康赵明霞李思辉姜俊兵
孙健康, 赵明霞, 李思辉, 高 丹, 姜俊兵*
(1. 山西农业大学 动物医学学院,山西 晋中 030600; 2. 山西工程技术学院 矿业系,山西 阳泉 045000)
随着环境恶化和人口老龄化等因素的影响,癌症发病率不断升高[1]。目前,虽然抗癌技术已经取得了一些突破,但癌症治疗仍然是一个重大挑战。化疗是治疗癌症的主要手段之一,但化疗药物存在选择性较差、不良反应较多,以及肿瘤细胞的耐药性增强等问题。因此,迫切需要开发更高效、更安全的抗肿瘤药物[2]。
表皮生长因子受体酪氨酸激酶(EGFR-TK)可通过引起受体的自磷酸化,而致使肿瘤细胞增殖和分化,在肿瘤发展的过程中起着至关重要的作用[3-6]。90%的肿瘤细胞表面均有表皮生长因子(EGFR)高表达,越来越多的研究人员将表皮生长因子作为肿瘤治疗的靶点[7]。
喹唑啉类化合物是重要的EGFR抑制剂。其中,PD153035[8](Chart 1)是最早发现的EGFR小分子抑制剂,其对EGFR的半数抑制浓度(IC50)为29±5.1 pM。此后,以喹唑啉为母环的EGFR小分子抑制剂,逐渐成为研究热点。特异性EGFR小分子抑制剂吉非替尼(Gefitinib)、厄洛替尼(Erlotinib)等含喹唑啉母环的抗肿瘤药物相继上市(Chart 1)。由此,喹唑啉类化合物越来越受到研究人员的重视,新合成的喹唑啉化合物表现出杀菌、杀虫、抗炎、抗病毒、抗高血压、抗结核、抗疟、抗肿瘤等活性[9-12]。
目前,对高活性的EGFR抑制剂喹唑啉类化合物的构效关系研究已经取得一定进展[13-15](Chart 2): (1)喹唑啉环的N-1, N-3分别以氢键与ATP结合,是抗肿瘤活性的重要结构单元(片段Ⅰ);若N-3被碳取代,喹唑啉类化合物抑制力将被削弱上百倍,N-1被取代,抑制力则降低至1/3700;(2)喹唑啉的2-位和8-位空间位阻比较大,很难引入取代基,而且5-位引入取代基也不利于提高活性;(3)4-位引入取代基(即片段Ⅱ)可提高抑制活性;(4)喹唑啉环中6,7-位引入活性基团可提高化合物的肿瘤抑制活性。由此可知,在喹唑啉类化合物的4,6,7-位引入活性基团,有望合成出高效抑制肿瘤活性的喹唑啉衍生物[16]。
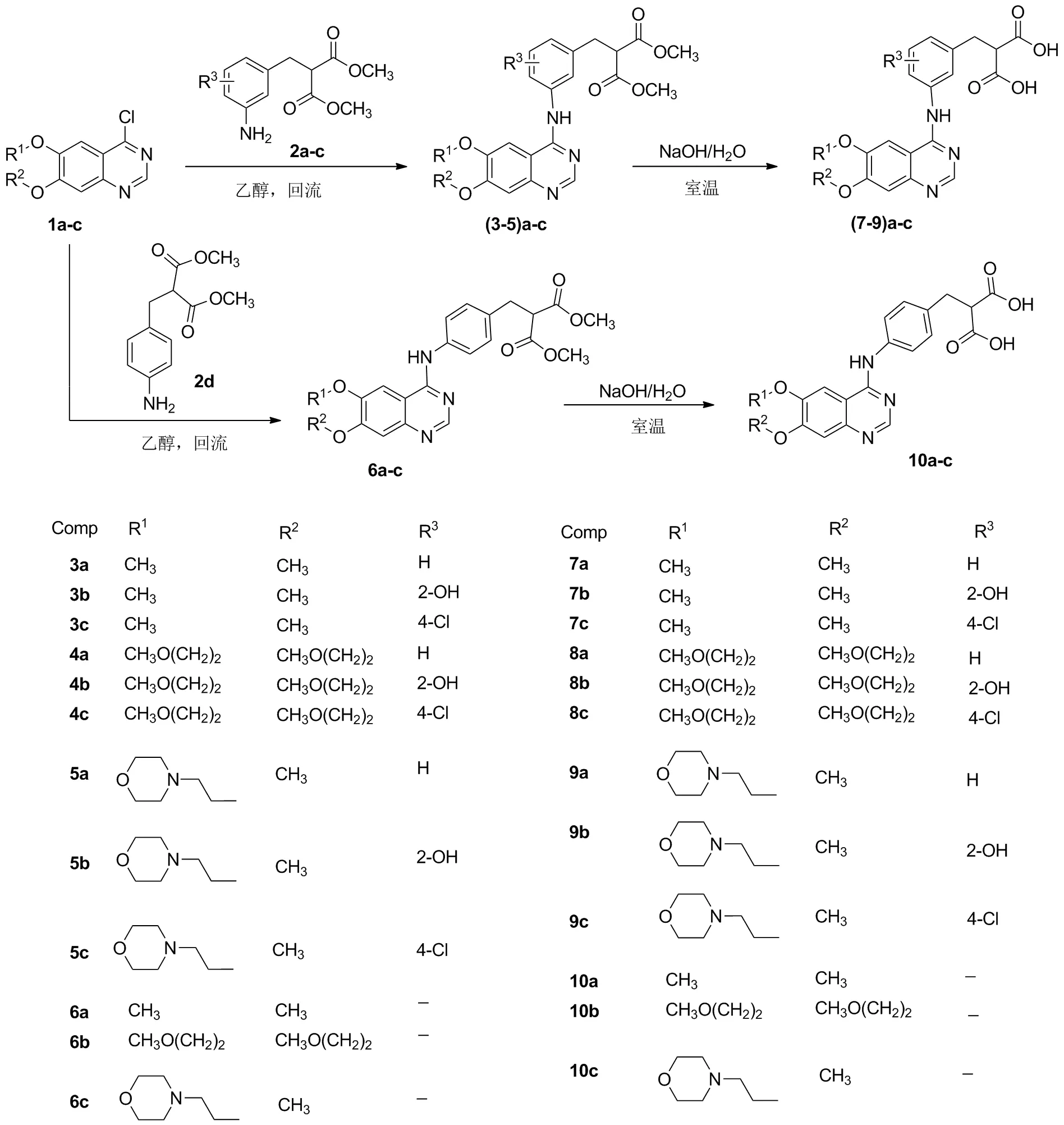
本文以喹唑啉衍生物PD153035,小分子抑制剂吉非替尼及厄洛替尼骨架为起始模型[17-18],在4-位引入间位或对位氨基苯甲基丙二酸二甲酯,然后经水解反应得到对应的丙二酸产物,设计并合成了24个喹唑啉类化合物(3a~10c, Scheme 1),其结构经1H NMR、13C NMR、 IR、 MS(ESI)和元素分析表征。采用MTT法筛选了喹唑啉类化合物对人盲肠腺癌细胞(HCT-8)、人肝癌细胞(HepG2)、人肺癌细胞(A549)和人高转移性肝癌细胞(MHCC-97H)的体外抗肿瘤活性。
1 实验部分
1.1 仪器与试剂
WRS-1B型数字熔点仪;Bruker AvanceⅢ HD 600 MHz 型核磁共振仪(DMSO-d6、 CDCl3或D2O为溶剂,TMS为内标);UPLC I-CLASS-XE-VOG2-XSQTOF型超高效液相-质谱联用仪。
4-氯-6,7-二甲氧基喹唑啉(1a), 4-氯-6,7-双(2-甲氧乙氧基)喹唑啉(1b), 4-(3-((4-氯-7-甲氧基喹唑啉-6-基)氧代)丙基)吗啉(1c),上海韶远科技有限公司;2-(3-氨基苯基)丙二酸二甲酯(2a), 2-(3-氨基-4-羟基苯基)丙二酸二甲酯(2b), 2-(5-氨基-2-氯苯基)丙二酸二甲酯(2c)和2-(4-氨基苯基)丙二酸二甲酯(2d)按文献[19-20]方法合成;人盲肠腺癌细胞(HCT-8),人肝癌细胞(HepG2),人肺癌细胞(A549),人高转移性肝癌细胞(MHCC-97H),美国细胞保存中心(ATCC),由本课题组传代培养;其余所用试剂均为分析纯。
1.2 合成
(1)3a~6c,7c的合成(以3a为例)
在25 mL圆形瓶中加入化合物1a448 mg(2 mmol),化合物2a711 mg(3 mmol)和乙醇15 mL,回流反应2 h(TLC检测)。冷却至室温,析出类白色沉淀,抽滤,滤饼用乙醇洗涤,真空干燥得类白色固体2-((3-(6,7-甲氧基喹唑啉-4-氨基)苯基)甲基)丙二酸二甲酯(3a),收率65%, m.p.184~185 ℃;1H NMR(400 MHz, DMSO-d6)δ: 8.81(s, 1H, -CH), 8.21(s, 1H, -NH), 7.53(t,J=7.80 Hz, 1H, ArH), 7.41(t,J=7.72 Hz, 1H, ArH), 7.32(s, 1H, -ArH), 7.18(d,J=7.48 Hz, 1H, ArH), 4.00(s, 6H, -CH3), 3.93(t,J=8.00 Hz, 1H, CH), 3.64(s, 6H, -CH3), 3.15(d,J=7.08 Hz, 2H, -CH2);13C NMR(100 MHz, DMSO-d6)δ: 168.71, 158.15, 156.22, 150.15, 148.53, 138.29, 136.90, 135.60, 128.68, 126.60, 125.07, 125.07, 123.32, 107.19, 104.08, 99.71, 56.95, 56.40, 52.52, 52.40, 33.96; MS(ESI)m/z: 426.1{[M+H]+}; Anal. calcd for C22H23N3O6: C 62.11, H 5.45, N 9.88, O 22.56; found C 62.23, H 5.72, N 9.93, O 22.71。
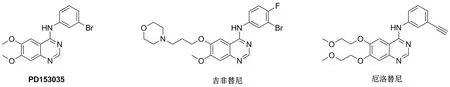
Chart 1
Scheme 1
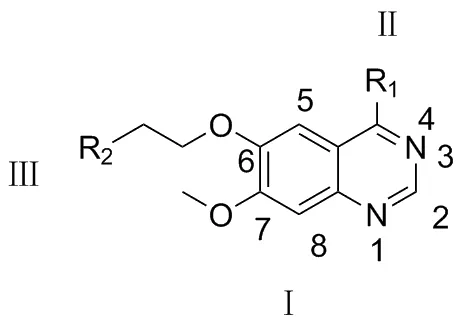
Chart 2
用类似方法合成3b~6c,7c。
2-((3-(6,7-甲氧基喹唑啉-4-氨基)-4-羟基苯基)甲基)丙二酸二甲酯(3b): 白色固体,收率63%, m.p.210~211 ℃;1H NMR(400 MHz, DMSO-d6)δ: 10.94(s, 1H, -OH), 9.78(s, 1H, -NH), 8.71(s, 1H, -ArH), 8.15(s, 1H, -ArH), 7.32(s, 1H, ArH), 7.14(s, 1H, ArH), 7.06(d,J=8.36 Hz, 1H, ArH), 6.93(d,J=8.32 Hz, 1H, ArH), 3.99(s, 6H, -CH3), 3.82(t,J=7.92 Hz, 1H, CH), 3.62(s, 6H, -CH3), 3.03(d,J=7.88 Hz, 2H, -CH2);13C NMR(100 MHz, CDCl3)δ: 174.06, 163.15, 161.38, 156.53, 155.30, 153.74, 151.63, 134.06, 133.58, 133.33, 128.66, 121.84, 112.12, 109.20, 106.29, 61.94, 61.64, 58.12, 57.57, 38.43; MS(ESI)m/z: 442.4 {[M+H]+}; Anal. calcd for C22H23N3O7: C 59.86, H 5.25, N 9.52, O 25.37; found C 59.93, H 5.70, N 9.93, O 25.43。
2-((2-氯-5-(6,7-甲氧基喹唑啉-4-氨基)苯基)甲基)丙二酸二甲酯(3c): 白色固体,收率65%, m.p.221~222 ℃;1H NMR(400 MHz, DMSO-d6)δ: 8.82(s, 1H, -CH), 8.21(s, 1H, -ArH), 7.63-7.68(m, 2H, ArH), 7.52(d,J=8.56 Hz, 1H, ArH), 7.33(s, 1H, ArH), 4.01(s, 6H, -CH3), 3.90(t,J=7.84 Hz, 1H, CH), 3.66(s, 6H, -CH3), 3.27(d,J=7.80 Hz, 2H, -CH2);13C NMR(100 MHz, CDCl3)δ: 173.95, 163.43, 161.52, 155.44, 153.82, 143.57, 142.17, 140.77, 133.94, 131.86, 130.32, 128.56, 112.46, 109.34, 105.03, 62.19, 61.67, 57.78, 57.65, 39.22; MS(ESI)m/z: 460.3 {[M+H]+}; Anal. calcd for C22H22N3O6Cl: C 57.46, H 4.82, Cl 7.71, N 9.14, O 20.87; found C 57.52, H 4.96, Cl 7.83, N 9.43, O 20.95。
2-((3-(6,7-二(2-甲氧乙氧基)喹唑啉-4-氨基)苯基)甲基)丙二酸二甲酯(4a): 白色固体,收率68%, m.p.193~194 ℃;1H NMR(400 MHz, DMSO-d6)δ: 8.74(s, 1H, -CH), 8.09(s, 1H, -ArH), 7.55(d,J=8.00 Hz, 1H, ArH), 7.51(s, 1H, -ArH), 7.40(t,J=7.8 Hz, 1H, ArH), 7.28(s, 1H, ArH), 7.16(d,J=7.36 Hz, 1H, ArH), 4.34(t,J=8.56 Hz, 4H, CH2), 3.91(t,J=7.96 Hz, 1H, CH), 3.78(t,J=7.96 Hz, 4H, CH2), 3.63(s, 6H, -CH3), 3.36(s, 6H, -CH3), 3.15(d,J=7.92 Hz, 2H, -CH2);13C NMR(100 MHz, DMSO-d6)δ: 168.71, 158.12, 155.55, 149.32, 148.56, 138.27, 136.89, 135.41, 128.66, 126.57, 125.02, 123.27, 107.21, 105.17, 100.67, 69.87, 69.74, 69.00, 68.72, 58.37, 58.32, 52.52, 52.40, 33.96; MS(ESI)m/z: 514.5 {[M+H]+}; Anal. calcd for C26H31N3O8: C 60.81, H 6.08, N 8.18, O 24.92; found C 60.98, H 6.37, N 8.83, O 25.12。
2-((3-(6,7-二(2-甲氧乙氧基)喹唑啉-4-氨基)-4-羟基苯基)甲基)丙二酸二甲酯(4b): 白色固体,收率66%, m.p.210~211 ℃;1H NMR(400 MHz, DMSO-d6)δ: 10.16(s, 1H, -OH), 8.16(s, 1H, -CH), 7.91(s, 1H, -NH), 7.28(s, 1H, -ArH), 6.89~6.99(m, 2H, -ArH), 4.40(s, 2H, CH2), 4.16(s, 2H, CH2), 3.67(s, 4H, CH2), 3.77(s, 6H, CH3), .361(t,J=7.72 Hz, 1H, CH), 3.43(s, 6H, -CH3), 3.10(d,J=7.40 Hz, 2H, -CH2);13C NMR(100 MHz, CDCl3)δ: 168.33, 154.97, 153.76, 150.90, 148.01, 147.16, 148.75, 128.55, 126.60, 125.58, 122.71, 118.65, 107.99, 102.15, 69.84, 69.38, 68.04, 67.36, 58.17, 58.11, 52.70, 51.57, 32.96; MS(ESI)m/z: 530.5 {[M+H]+}; Anal. calcd for C26H31N3O9: C 58.97, H 5.90, N 7.94, O 27.19; found C 59.32, H 6.22, N 8.13, O 27.45。
2-((5-(6,7-二(2-甲氧乙氧基)喹唑啉-4-氨基)-2-氯苯基)甲基)丙二酸二甲酯(4c): 白色固体,收率68%, m.p.236~237 ℃;1H NMR(400 MHz, DMSO-d6)δ: 8.56(s, 1H, -CH), 7.71(d,J=8.60 Hz, 1H, -ArH), 7.45(s, 1H, -ArH), 7.30(d,J=8.68 Hz, 1H, -ArH), 7.14~7.24(m, 4H, -ArH), 4.20~4.27(m, 4H, CH2), 3.83(t,J=7.68 Hz, 1H, CH), 3.80(t,J=9.24 Hz, 1H, CH2), 3.65(s, 6H, CH3), 3.41(s, 6H, -CH3), 3.28(d,J=7.72 Hz, 2H, -CH2);13C NMR(100 MHz, CDCl3)δ: 168.18, 155.24, 153.52, 152.43, 147.87, 146.40, 136.70, 134.62, 128.86, 127.70, 116.68, 114.07, 108.22, 107.71, 101.60, 69.91, 68.14, 58.24, 51.69, 50.13, 31.85; MS(ESI)m/z: 548.4 {[M+H]+}; Anal. calcd for C26H30N3O8Cl: C 56.99, H 5.52, Cl 6.47, N 7.67, O 23.36; found C 57.17, H 5.72, Cl 6.53, N 7.83, O 23.73。
2-((3-(7-甲氧基-6-(3-吗啉代丙基)喹唑啉-4-氨基)苯基)甲基)丙二酸二甲酯(5a): 淡黄色固体,收率68%, m.p.190~191 ℃;1H NMR(400 MHz, CDCl3)δ: 8.54(s, 1H, -CH), 8.01(s, 1H, -ArH), 7.71(t,J=8.40 Hz, 1H, -ArH), 7.29(m, 2H, -ArH), 7.00(s, 1H, -ArH), 4.44(t,J=6.6 Hz, 2H, CH2), 4.04(s, 4H, CH2), 3.92(s, 3H, CH2), 3.74(t,J=7.80 Hz, 1H, CH), 3.71(s, 6H, CH3), 3.25(d,J=7.72 Hz, 2H, -CH2), 3.10~3.20(m, 6H, -CH2), 2.34~2.41(m, 2H, -CH2);13C NMR(100 MHz, CDCl3)δ: 168.76, 159.65, 154.86, 151.50, 148.01, 143.42, 138.73, 137.88, 128.39, 124.43, 123.49, 121.55, 108.33, 104.86, 104.36, 66.92, 63.27, 56.02, 53.68, 52.66, 52.37, 51.16, 34.12, 22.91; MS(ESI)m/z: 426.98 {[M+H]+}; Anal. calcd for C28H34N4O7: C 62.44, H 6.36, N 10.40, O 20.79; found C 62.84, H 6.43, N 10.63, O 20.94。
2-((4羟基-3-(7-甲氧基-6-(3-吗啉代丙基)喹唑啉-4-氨基)苯基)甲基)丙二酸二甲酯(5b): 白色固体,收率65%, m.p.208~209 ℃;1H NMR(400 MHz, CDCl3)δ: 8.40(s, 1H, -CH), 7.77(s, 1H, -NH), 7.18(d,J=7.52 Hz, 1H, -ArH), 6.91~6.97(m, 2H, -ArH), 4.20(t,J=6.88 Hz, 2H, CH2), 3.94(s, 3H, CH3), 3.59~6.8(m, 11H, CH2and CH), 3.09(d,J=7.80 Hz, 2H, CH2), 2.53(d,J=6.88 Hz, 2H, CH2), 2.44(s, 4H, CH2), 2.08(t,J=6.84 Hz, 2H, -CH2);13C NMR(100 MHz, DMSO-d6)δ: 168.74, 168.49, 158.71, 157.46, 150.86, 140.22, 139.35, 138.37, 136.62, 128.82, 126.80, 124.58, 122.87, 118.20, 106.91, 101.31, 56.99, 52.52, 52.43, 33.92, 20.09; MS(ESI)m/z: 555.2 {[M+H]+}; Anal. calcd for C28H34N4O8: C 60.64, H 6.18, N 10.10, O 23.08; found C 60.87, H 6.32, N 10.33, O 23.43。
2-((2-氯-5-(7-甲氧基-6-(3-吗啉代丙基)喹唑啉-4-氨基)苯基)甲基)丙二酸二甲酯(5c): 白色固体,收率55%, m.p.236~237 ℃;1H NMR(400 MHz, CDCl3)δ: 8.59(s, 1H, -CH), 8.01(s, 1H, -NH), 7.87(s, 1H, -ArH), 7.79(d,J=8.64 Hz, 1H, -ArH), 7.31(d,J=8.64 Hz, 1H, -ArH), 7.26(s, 1H, -ArH), 4.41(t,J=6.72 Hz, 2H, -CH2), 4.04(s, 4H, -CH2), 3.96(s, 3H, -CH3), 3.92(t,J=7.76 Hz, 1H, -CH), 3.72(s, 6H, CH3), 3.34(d,J=7.72 Hz, 2H, CH2), 3.10~3.17(m, 6H, CH2), 2.33-2.41(m, 2H, CH2);13C NMR(100 MHz, CDCl3)δ: 174.01, 172.64, 163.78, 162.52, 156.34, 145.38, 143.30, 140.53, 140.42, 134.24, 121.44, 112.33, 101.04, 62.18, 57.78, 57.35, 39.85, 26.37; MS(ESI+)m/z: 573.5 {[M+H]+}; Anal. calcd for C28H33N4O7Cl: C 58.69, H 5.80, Cl 6.19, N 9.78, O 19.54; found C 58.87, H 5.92, Cl 6.53, N 9.97, O 19.97。
2-((4-(6,7-甲氧基喹唑啉-4-氨基)苯基)甲基)丙二酸二甲酯(6a): 淡黄色粉末,收率67%, m.p.231~232 ℃;1H NMR(400 MHz, DMSO-d6)δ: 11.22(s, 1H, -OH), 8.78(s, 1H, -CH), 8.27(s, 1H, -NH), 7.59(d,J=8.32 Hz, 2H, -ArH), 7.59(t,J=8.32 Hz, 2H, -ArH), 7.31-7.35(m, 3H, -ArH), 4.32~4.37(m, 4H, -CH2), 3.93(t,J=8.00 Hz, 1H, -CH), 3.78(s, 4H, CH2), 3.63(s, 6H, CH3), 3.14(d,J=7.88 Hz, 2H, CH2);13C NMR(100 MHz, DMSO-d6)δ: 166.76, 157.97, 155.47, 149.28, 148.44, 135.61, 135.41, 135.21, 128.84, 124.70, 107.22, 105.31, 100.57, 69.89, 69.73, 69.08, 68.69, 58.37, 58.31, 52.57, 52.33, 33.62; MS(ESI)m/z: 426.17 {[M+H]+}; Anal. calcd for C22H23N3O6: C 62.11, H 5.45, N 9.88, O 22.56; found C 62.43, H 5.87, N 9.93, O 22.71。
2-((4-(6,7-二(2-甲氧乙氧基)喹唑啉-4-氨基)苯基)甲基)丙二酸二甲酯(6b): 白色固体,收率67%, m.p.211~212 ℃;1H NMR(400 MHz, DMSO-d6)δ: 11.22(s, 1H, -OH), 8.78(s, 1H, -CH), 8.28(s, 1H, -NH), 7.59(d,J=8.12 Hz, 2H, -ArH), 7.33(d,J=8.08 Hz, 3H, -ArH), 7.31(s, 1H, -ArH), 4.32~4.37(m, 4H, -CH2), 3.93(t,J=8.00 Hz, 1H, -CH), 3.78(s, 4H, CH2), 3.64(s, 6H, CH3), 3.36(s, 6H, CH3), 3.14(d,J=7.88 Hz, 2H, CH2);13C NMR(100 MHz, DMSO-d6)δ: 168.76, 157.97, 155.47, 149.28, 148.44, 135.41, 128.84, 124.70, 107.22, 105.31, 100.57, 69.89, 69.73, 69.08, 68.69, 58.37, 58.31, 52.57, 52.33, 33.62; MS(ESI)m/z: 514.5{[M+H]+}; Anal. calcd for C26H31N3O8: C 60.81, H 6.08, N 8.18, O 24.92; found C 60.97, H 6.36, N 8.63, O 25.34。
2-((4-(7-甲氧基-6-(3-吗啉代丙基)喹唑啉-4-氨基)苯基)甲基)丙二酸二甲酯(6c): 白色固体,收率65%, m.p.190~191 ℃;1H NMR(400 MHz, CDCl3)δ: 8.64(s, 1H, -CH), 7.61(d,J=8.36 Hz, 2H, -ArH), 7.20~7.25(m, 4H, -ArH), 7.08(s, 1H, -ArH), 4.19(t,J=8.00 Hz, 1H, -CH), 3.99(s, 3H, CH3), 3.73(s, 10H, CH3and CH2), 3.67(t,J=8.00 Hz, 1H, -CH), 3.23(d,J=8.00 Hz, 2H, -CH2), 2.58 (t,J=8.00 Hz, 2H, -CH2), 2.49(s, 4H, CH2), 2.08~2.14(m, 2H, CH2);13C NMR(100 MHz, CDCl3)δ: 169.16, 156.51, 155.03, 153.59, 148.86, 147.34, 137.46, 133.55, 129.31, 122.09, 109.19, 107.73, 101.27, 67.52, 66.83, 56.06, 55.27, 53.64, 53.58, 52.53, 34.16, 26.07; MS(ESI)m/z: 539.6 {[M+H]+}; Anal. calcd for C28H34N4O7: C 62.44, H 6.36, N 10.40, O 20.79; found C 62.87, H 6.45, N 10.56, O 21.21。
(2)7a,7b,8a~10c的合成(以7a为例)
将化合物3a425 mg(1 mmol)加入8 mL乙醇中,搅拌使其溶解,滴加NaOH 160 mg(4 mmol)的水(2 mL)溶液,搅拌下反应至终点(TLC检测)。用1 mol/LHCl调至pH值中性,抽滤得白色固体2-((3-(6,7-甲氧基喹唑啉-4-氨基)苯基)甲基)丙二酸(7a),收率61%, m.p.195~196 ℃;1H NMR(400 MHz, DMSO-d6)δ: 9.57(s, 1H, -NH), 8.48(s, 1H, -CH), 7.87(s, 1H, -ArH), 7.73(d,J=7.96 Hz, 1H, ArH), 7.57(d,J=9.00 Hz, 1H, ArH), 7.31(t,J=7.84 Hz, 1H, ArH), 7.19(s, 1H, -ArH), 6.99(d,J=7.36 Hz, 1H, ArH), 3.95(s, 6H, -CH3), 3.58(t,J=7.68 Hz, 1H, CH), 3.07(d,J=7.56 Hz, 2H, -CH2);13C NMR(100 MHz, DMSO-d6)δ: 170.35, 156.47, 154.34, 152.43, 148.93, 145.81, 139.14, 138.92, 128.26, 123.92, 122.68, 120.76, 108.68, 106.42, 102.07, 56.24, 55.78, 53.20, 34.27; MS(ESI)m/z: 414.3 {[M+H]+}; Anal. calcd for C20H19N3O6: C 60.45, H 4.82, N 10.57, O 24.16; found C 60.63, H 4.97, N 10.75, O 24.65。
用类似的方法合成7b,8a~10c。
2-((3-(6,7-甲氧基喹唑啉-4-氨基)-4-羟基苯基)甲基)丙二酸(7b): 白色固体,收率69%, m.p.190~191 ℃;1H NMR(400 MHz, DMSO-d6)δ: 9.47(s, 1H, -OH), 8.38(s, 1H, -NH), 7.88(s, 1H, -CH), 7.22(s, 1H, -ArH), 7.17(s, 1H, -ArH), 6.96~6.99(m, 1H, ArH), 6.85(d,J=8.24 Hz, 1H, ArH), 3.94(s, 6H, -CH3), 3.49(t,J=7.64 Hz, 1H, CH), 2.98(d,J=7.60 Hz, 2H, -CH2);13C NMR(100 MHz, DMSO-d6)δ: 170.39, 157.29, 154.49, 151.97, 149.98, 148.93, 144.89, 129.20, 127.01, 126.97, 125.95, 116.96, 108.39, 106.06, 102.53, 56.19, 55.85, 53.45, 33.40; MS(ESI)m/z: 414.3 {[M+H]+}; Anal. calcd for C20H19N3O7: C 58.11, H 4.63, N 10.16, O 27.09; found C 58.42, H 4.96, N 10.43, O 27.47。
2-((2-氯-5-(6,7-甲氧基喹唑啉-4-氨基)苯基)甲基)丙二酸(7c): 淡黄色固体,收率68%, m.p.280~281 ℃;1H NMR(400 MHz, DMSO-d6)δ: 8.61(s, 1H, -NH), 7.97(s, 1H, -CH), 7.73~7.79(m, 2H, ArH), 7.48(d,J=8.68 Hz, 1H, ArH), 7.24(s, 1H, ArH), 3.98(s, 6H, -CH3), 3.65(t,J=7.68 Hz, 1H, CH), 3.19(d,J=6.68 Hz, 2H, -CH2);13C NMR(100 MHz, CDCl3)δ: 175.54, 161.70, 159.58, 157.81, 154.18, 151.39, 144.47, 144.18, 133.52, 129.11, 127.86, 125.95, 113.99, 111.91, 187.29, 61.48, 61.04, 58.46, 45.38, 45.18, 44.97, 44.76, 44.55, 44.34, 44.13, 39.52, 5.29; MS(ESI)m/z: 555.5 {[M+H]+}; Anal. calcd for C20H18N3O6Cl: C 55.63, H 4.20, Cl 8.21, N 9.73, O 22.23; found C 55.76, H 4.43, Cl 8.54, N 9.97, O 22.51。
2-((3-(6,7-二(2-甲氧乙氧基)喹唑啉-4-氨基)苯基)甲基)丙二酸(8a): 淡黄色固体,收率61%, m.p.217~218 ℃;1H NMR(400 MHz, DMSO-d6)δ: 8.64(s, 1H, -NH), 7.88(s, 1H, -CH), 7.72(d,J=7.08 Hz, 1H, -ArH), 7.58(d,J=9.20 Hz, 1H, ArH), 7.30(t,J=7.72 Hz, 1H, ArH), 7.21(s, 1H, ArH), 6.99(d,J=7.64 Hz, 1H, ArH), 4.28(m, 4H, CH2), 3.76(t,J=9.44 Hz, 4H, CH2), 3.57(t,J=7.68 Hz, 1H, CH), 3.37(s, 6H, -CH3), 3.08(d,J=7.64 Hz, 2H, -CH2);13C NMR(100 MHz, CDCl3)δ: 170.36, 156.52, 153.72, 152.44, 148.12, 145.67, 139.11, 138.92, 128.21, 123.92, 122.71, 120.75, 108.76, 107.39, 103.66, 70.10, 70.00, 68.48, 68.07, 58.33, 58.30, 53.13, 34.27; MS(ESI)m/z: 486.4 {[M+H]+}; Anal. calcd for C24H27N3O8: C 59.37, H 5.61, N 8.66, O 26.36; found C 59.91, H 5.72, N 8.94, O 26.73。
2-((3-(6,7-二(2-甲氧乙氧基)喹唑啉-4-氨基)-4-羟基苯基)甲基)丙二酸(8b): 淡黄色固体,收率66%, m.p.258~259 ℃;1H NMR(400 MHz, DMSO-d6)δ: 8.36(s, 1H, -NH), 7.89(s, 1H, -CH), 7.24(s, 1H, -ArH), 7.20(s, 1H, -ArH), 6.96-6.99(m, 1H, -ArH), 6.86(d,J=8.24 Hz, 1H, -ArH), 4.40(s, 4H, CH2), 3.76~3.78(m, 4H, CH2), 3.61(t,J=7.76 Hz, 1H, CH), 3.35(s, 6H, -CH3), 2.97(d,J=7.76 Hz, 2H, -CH2);13C NMR(100 MHz, DMSO-d6)δ: 170.45, 157.28, 153.86, 151.96, 149.95, 149.92, 148.11, 144.65, 129.22, 126.98, 125.90, 116.99, 108.43, 106.99, 103.90, 70.05, 70.00, 68.36, 68.11, 58.32, 58.31, 53.43, 33.42; MS(ESI)m/z: 502.4 {[M+H]+}; Anal. calcd for C24H27N3O9: C 57.48, H 5.43, N 8.38, O 28.71; found C 57.82, H 5.61, N 8.93, O 28.91。
2-((5-(6,7-二(2-甲氧乙氧基)喹唑啉-4-氨基)-2-氯苯基)甲基)丙二酸(8c): 白色固体,收率64%, m.p.265~266 ℃;1H NMR(400 MHz, DMSO-d6)δ: 8.55(s, 1H, -NH), 7.90(s, 1H, -CH), 7.73(d,J=9.08 Hz, 2H, -ArH), 7.48(d,J=8.36 Hz, 1H, -ArH), 7.24(s, 1H, -ArH), 4.31(m, 4H, CH2), 3.37(s, 6H, CH3), 3.19(s, 2H, -CH2);13C NMR(100 MHz, DMSO-d6)δ: 169.85, 156.57, 154.18, 151.60, 148.43, 137.85, 135.72, 129.11, 127.70, 124.80, 124.31, 122.67, 108.47, 106.15, 103.80, 70.04, 69.94, 68.59, 68.21, 58.34, 58.32, 51.35, 32.20; MS(ESI)m/z: 520.4 {[M+H]+}; Anal. calcd for C24H26N3O8Cl: C 55.44, H 5.04, Cl 6.82, N 8.08, O 24.62; found C 55.76, H 5.34, Cl 6.94, N 8.67, O 24.71。
2-((3-(7-甲氧基-6-(3-吗啉代丙基)喹唑啉-4-氨基)苯基)甲基)丙二酸(9a): 白色固体,收率68%, m.p.231~232 ℃;1H NMR(400 MHz, D2O)δ: 8.07(s, 1H, -CH), 7.29~7.33(m, 2H, -ArH), 7.27(s, 1H, -ArH), 7.19(s, 1H, -ArH), 7.06(d,J=6.96 Hz, 1H, -ArH), 7.02(s, 1H, -ArH), 6.73(s, 1H, -ArH), 3.91(t,J=5.88 Hz, 2H, -CH2), 3.75(s, 3H, CH3), 3.66(s, 4H, CH2), 3.34(t,J=7.96 Hz, 1H, CH), 2.99(t,J=7.96 Hz, 1H, CH), 2.42(s, 6H, CH3), 1.87~1.93(s, 2H, CH2);13C NMR(100 MHz, D2O)δ: 178.92, 156.45, 153.34, 151.95, 147.36, 1444.68, 141.77, 138.18, 128.94, 125.17, 123.66, 121.65, 108.34, 105.30, 101.15, 67.30, 66.20, 60.17, 55.61, 54.74, 52.53, 36.34, 25.04; MS(ESI)m/z: 511.5 {[M+H]+}; Anal. calcd for C26H30N4O7: C 61.17, H 5.92, N 10.97, O 21.94; found C 61.52, H 6.26, N 11.13, O 22.43。
2-((4羟基-3-(7-甲氧基-6-(3-吗啉代丙基)喹唑啉-4-氨基)苯基)甲基)丙二酸(9b): 淡黄色固体,收率57%, m.p.197~198 ℃;1H NMR(400 MHz, D2O)δ: 8.15(s, 1H, -CH), 7.43(s, 1H, -ArH), 7.31(s, 1H, -ArH), 6.89(s, 1H, -ArH), 6.78~6.84(m, 1H, -ArH), 6.54(d,J=8.28 Hz, 1H, -ArH), 3.98~4.06(m, 2H, -CH2), 3.63(s, 3H, CH3), 3.32(s, 4H, CH2), 3.29(t,J=7.72 Hz, 1H, CH), 2.86(d,J=7.72 Hz, 2H, CH2), 2.44(s, 6H, CH3), 1.88(t,J=8.56 Hz, 2H, CH2);13C NMR(100 MHz, D2O)δ: 179.54, 157.30, 156.49, 153.54, 152.64, 147.52, 144.61, 127.61, 125.86, 125.40, 123.90, 118.32, 108.91, 105.64, 101.74, 67.55, 66.17, 60.68, 55.75, 54.61, 52.39, 35.86, 24.91; MS(ESI)m/z: 527.6 {[M+H]+}; Anal. calcd for C26H30N4O8: C 59.31, H 5.74, N 10.64, O 24.31; found C 59.84, H 5.93, N 10.93, O 24.64。
2-((2-氯-5-(7-甲氧基-6-(3-吗啉代丙基)喹唑啉-4-氨基)苯基)甲基)丙二酸(9c): 棕色固体,收率69%, m.p.200~201 ℃;1H NMR(400 MHz, D2O)δ: 8.16(s, 1H, -CH), 7.44(s, 1H, -ArH), 7.31(s, 1H, -ArH), 6.89(s, 1H, -ArH), 6.78-6.89(m, 1H, -ArH), 6.55(d,J=8.28 Hz, 1H, -ArH), 4.04(s, 2H, CH2), 3.84(s, 3H, CH3), 3.64(s, 4H, -CH2), 3.31(t,J=7.72 Hz, 1H, -CH), 2.86(d,J=7.72 Hz, 2H, -CH2), 2.4(s, 6H, CH3), 1.88(t,J=8.56 Hz, 2H, -CH2);13C NMR(100 MHz, DMSO-d6)δ: 171.81, 156.24, 153.73, 152.08, 146.51, 145.70, 137.73, 133.82, 128.69, 121.76, 109.51, 106.84, 105.56, 55.82, 51.94, 33.88; MS(ESI)m/z: 573.5 {[M+H]+}; Anal. calcd for C26H29N4O7Cl: C 57.30, H 5.36, Cl 6.51, N 10.28, O 20.55; found C 57.83, H 5.42, Cl 6.54, N 10.47, O 20.91。
2-((4-(6,7-甲氧基喹唑啉-4-氨基)苯基)甲基)丙二酸(10a): 白色固体,收率60%, m.p.172~173 ℃;1H NMR(400 MHz, D2O)δ: 7.79(s, 1H, -CH), 7.11(d,J=7.44 Hz, 2H, -ArH), 7.05(d,J=8.20 Hz, 2H, -ArH), 6.61(s, 1H, -ArH), 6.38(s, 1H, -ArH), 3.47(s, 6H, CH3), 3.32(t,J=7.84 Hz, 1H, -CH), 2.81(d,J=7.92 Hz, 2H, -CH2);13C NMR(100 MHz, D2O)δ: 178.96,155.82, 152.87, 151.31, 147.39, 144.14, 137.70, 135.13, 128.96, 123.90, 107.48, 104.66, 99.42, 60.28, 55.36, 35.83; MS(ESI)m/z: 398.13 {[M+H]+}; Anal. calcd for C20H19N3O6: C 60.45, H 4.82, N 10.57, O 24.16; found C 60.84, H 4.98, N 10.93, O 24.53。
2-((4-(6,7-二(2-甲氧乙氧基)喹唑啉-4-氨基)苯基)甲基)丙二酸(10b): 黄色固体,收率68%, m.p.225~226 ℃;1H NMR(400 MHz, DMSO-d6)δ: 8.53(s, 1H, -CH), 7.92(s, 1H, -ArH), 7.63(d,J=8.32 Hz, 2H, -ArH), 7.27(d,J=8.36 Hz, 2H, -ArH), 7.24(s, 1H, -ArH), 4.29(s, 4H, CH2), 3.75~3.79(m, 4H, CH2), 3.62(t,J=7.68 Hz, 1H, -CH), 3.36(s, 6H, CH3), 3.06(d,J=6.48 Hz, 2H, -CH2);13C NMR(100 MHz, DMSO-d6)δ: 170.21, 156.80, 154.08, 151.80, 148.35, 137.59, 134.41, 128.72, 122.95, 108.39, 106.17, 103.87, 70.04, 69.97, 68.52, 58.33, 53.37, 33.73; MS(ESI)m/z: 486.4 {[M+H]+}; Anal. calcd for C24H27N3O8: C 59.37, H 5.61, N 8.66, O 26.36; found C 59.64, H 5.73, N 8.93, O 26.63。
2-((4-(7-甲氧基-6-(3-吗啉代丙基)喹唑啉-4-氨基)苯基)甲基)丙二酸(10c): 淡黄色固体,收率65%, m.p.190~191 ℃;1H NMR(400 MHz, D2O)δ: 8.07(s, 1H, -CH), 7.40(d,J=8.36 Hz, 2H, -ArH), 7.35(d,J=8.36 Hz, 2H, -ArH), 6.86(s, 1H, -ArH), 6.63(s, 1H, -ArH), 3.74~3.86(m, 9H, CH2and CH3), 3.47(t,J=8.00 Hz, 1H, -CH), 3.11(d,J=7.88 Hz, 2H, -CH2), 2.45~2.50(m, 6H, CH2), 1.93(t,J=8.00 Hz, 2H, -CH2);13C NMR(100 MHz, D2O)δ: 179.01, 156.28, 153.13, 151.78, 147.07, 144.37, 137.42, 136.27, 128.97, 123.69, 108.20, 105.06, 100.94, 67.13, 66.13, 60.30, 55.45, 54.62, 52.19, 35.87, 24.92; MS(ESI)m/z: 539.5 {[M+H]+}; Anal. calcd for C26H30N4O7: C 61.17, H 5.92, N 10.97, O 21.94; found C 61.45, H 6.32, N 11.33, O 22.41。
1.3 体外抗肿瘤活性测试
用MTT法测试了化合物的体外抗肿瘤活性[21]。分别取对数生长期的肿瘤细胞即人盲肠腺癌细胞(HCT-8)、人肝癌细胞(HepG2)、人肺癌细胞(A549)和人高转移性肝癌细胞(MHCC-97H),制成5×104个/mL的单细胞悬液,每孔加入100 μL,接种于96孔板中,置于37 ℃、 5%CO2、饱和湿度条件下培养24 h。分别给予不同浓度的药物处理(每个浓度设置5个平行复孔)。药物作用于细胞72 h后,每孔加入10 μL MTT(5 mg/mL),在37 ℃、 5%CO2条件下继续孵育4 h。向每孔加入100 μL裂解液(10%SDS+0.1%NH4Cl),避光孵育过夜。次日用酶标仪测定各孔的光密度值(OD值),并计算细胞增殖抑制率。细胞增殖抑制率(IC50)用SPSS16.0软件计算[22]。
2 结果与讨论
2.1 合成
以抗肿瘤小分子PD153035,吉非替尼及尼洛替尼前体骨架为原料,在其4-位引入间位或对位氨基苯甲基丙二酸二甲酯。在乙醇溶液中回流2~3 h可得到目标产物3a~6c。产物在氢氧化钠的甲醇溶液中水解得产物7a~10c。整个反应过程条件温和,操作简单,并且收率较高。
2.2 抗肿瘤活性
表1为目标化合物对多种肿瘤细胞的抑制活性。由表1可知,化合物5c对HCT-8、 A549和MHCC-97H均有较强的抑制作用,但是对HepG2细胞的IC50大于100 μmol/L,说明化合物5c对某些肿瘤细胞有选择抑制性。化合物4c、5b对HCT-8肿瘤细胞(4cIC50=48.265 μmol/L、5bIC50=32.497 μmol/L)、 A549肿瘤细胞(4cIC50=23.135 μmol/L、5bIC50=22.166 μmol/L)和MHCC-97H肿瘤细胞(4cIC50=5.051 μmol/L、5bIC50=10.075 μmol/L)也具有选择性抑制活性,化合物3a、3c、4a、5a对A549肿瘤细胞(3aIC50=38.289 μmol/L、3cIC50=43.250 μmol/L、4aIC50=25.473 μmol/L、5aIC50=16.890 μmol/L)和MHCC-97H肿瘤细胞(3aIC50=20.368 μmol/L、3cIC50=21.643 μmol/L、4aIC50=1.984 μmol/L、5aIC50=11.578 μmol/L)具有选择性抗肿瘤活性,化合物3b、4b、6a、6b、6c对MHCC-97H肿瘤细胞(3bIC50=6.790 μmol/L、4bIC50=12.561 μmol/L、6aIC50=36.275 μmol/L、6bIC50=28.420 μmol/L、6cIC50=22.932 μmol/L)具有选择性抗肿瘤活性。对化合物3a~ 6c和7a~10c进行构效关系分析可知,4-位为氨基苯甲基丙二酸二甲酯时,化合物的肿瘤抑制作用比其相应水解产物更强,比较化合物3a~5a和6a~6c可知,氨基在苯甲基丙二酸二甲酯间位时,肿瘤抑制活性明显比其在对位时更强。将目标化合物中丙二酸二甲酯水解成相应丙二酸后,化合物的抗肿瘤细胞活性消失。
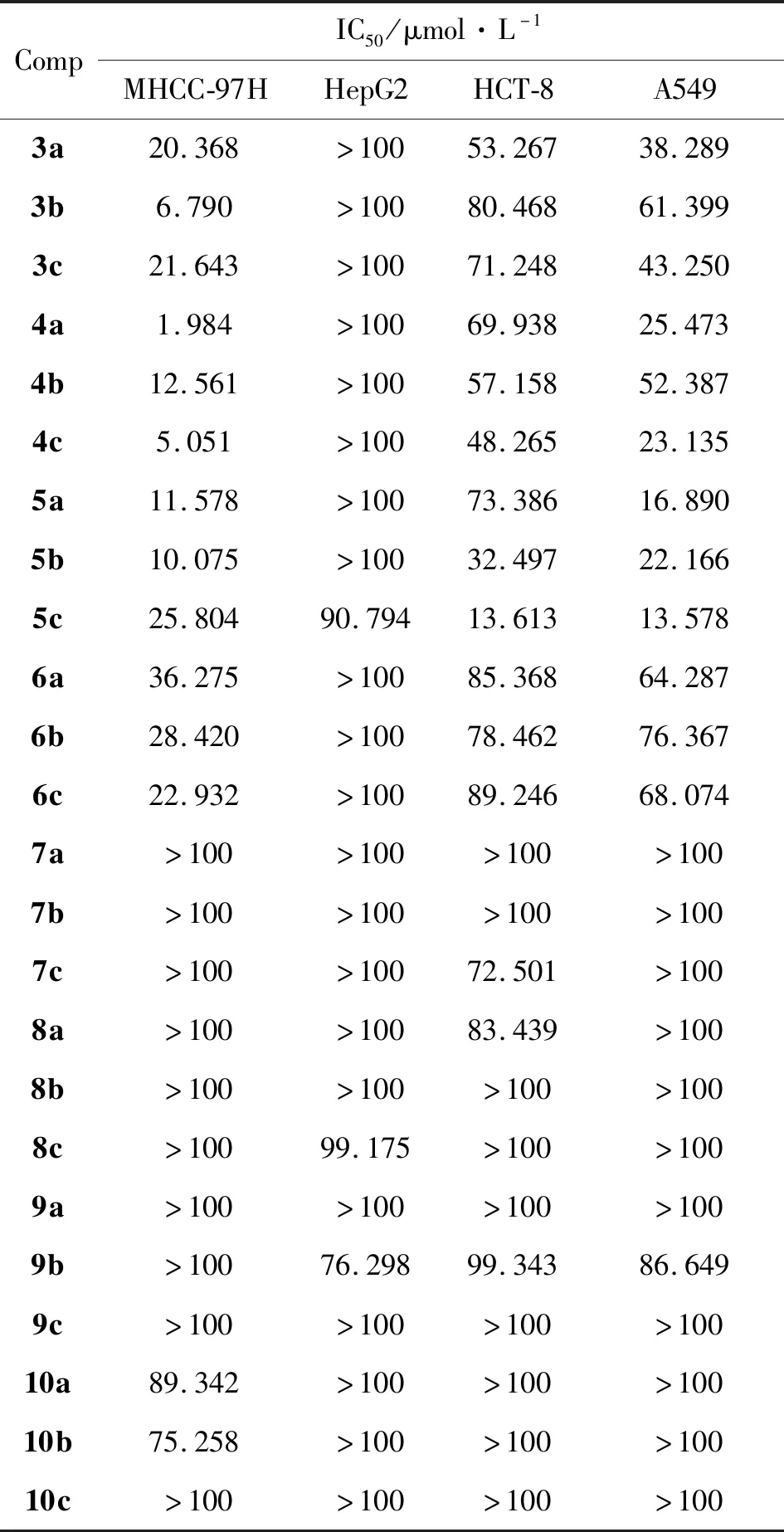
表1 化合物的体外抗肿瘤活性Table 1 In vitro antitumor activities of the compounds
设计并合成了24个新型喹唑啉衍生物(3a~10c),并选用人盲肠腺癌细胞(HCT-8)、人肝癌细胞(HepG2)、人肺癌细胞(A549)、人高转移性肝癌细胞(MHCC-97H)瘤株,考察了化合物的体外抗肿瘤活性。结果表明,化合物5c对HCT-8、 A549及MHCC-97H均有显著抑制作用,IC50值分别为13.613 μmol/L, 13.578 μmol/L, 25.804 μmol/L。构效分析结果表明,在喹唑啉衍生物的4-位引入间位氨基苯甲基丙二酸二甲酯,有望获得潜在的抗肿瘤药物前体。
