Stress response of Lymantria dispar asiatica (Lepidoptera:Erebidae) larvae and its gut microbiota to manganese ion
2021-04-30JianyongZengJiaxingGuoJianghongShiZhongbinShiGuocaiZhangJieZhang
Jianyong Zeng · Jiaxing Guo · Jianghong Shi ·Zhongbin Shi · Guocai Zhang · Jie Zhang
Abstract To study the Effect of manganese exposure on the herbivorous insect Lymantria dispar asiatica, fourthinstar larvae were fed a MnCl 2 -amended diet (LdMn) for 84 h (0.40 mmol MnCl 2 /g diet). Larvae were weighed before and after the diet administration to assess larval gain in mass under manganese exposure. The whole bodies of half of the survivors were ground in liquid nitrogen for measuring enzyme activities and total antioxidant capacity (T-AOC).The intestinal tracts of the remaining survivors were collected and immediately frozen in liquid nitrogen for 16S rDNA sequencing of gut microbiota. Larvae under manganese stress lost signif icant mass ( p < 0.05). The activities of digestive and antioxidant enzymes and T-AOC, but not trehalase and polyphenol oxidase, were signif icantly higher after Mn exposure, ( p < 0.05). A Venn diagram illustrated that the gut microbial OTU composition in the larvae also changed. Community pies and correlation heatmaps also showed diff erent relative abundances of gut microbes. In other words, species quantity and relative abundance of gut microbes agreed with PCoA visualization and indicated that the gut microbial community in L. dispar asiatica larvae diff ered signif icantly between control and LdMn. Functional classif ication also suggested that exposure to manganese stress signif icantly decreased gut microbial coenzyme transport and metabolism in L. dispar asiatica larvae. These results further our understanding about stress response of L.dispar asiatica larvae.
Keywords Insect · Manganese stress · Enzyme ·Antioxidation · Gut microbiota
Introduction
Manganese is essential for normal development and function of all animals and binds to and regulates many enzymes such as arginase, pyruvate carboxylase, and mitochondrial superoxide dismutase as a cofactor (Crossgrove and Zheng 2004; Lin et al. 2006; Murphy et al.2010). Manganese in trace amounts is essential for normal functioning of organisms and usually supplied by a typical diet (Takeda 2003). High environmental levels of manganese in humans, however, is toxic to the central nervous system in humans and can lead to the neurological syndrome manganism, with progressive, irreversible symptoms resembling those of Parkinson’s disease(Kawamura et al. 1941; Kondakis et al. 1989; Roth and Garrick 2003) and affect the intelligence and motor function of children and lead to hyperactivity (Wasserman et al. 2011; Carvalho et al. 2018). Work on rats showed that Mn can cross the blood brain barrier (Murphy et al.2010). On the other hand, in the model organismCaenorhabditis elegans, diet supplementation with Mn did not appear toxic; in fact, higher levels increased development and fertility of wild-type worms and increased the longevity of a short-lifespan mutant strain; the mechanism was hypothesized to be due a protective factor(s) or freeradical scavenging (Murphy et al. 2010).
The impacts of high levels of Mn on the physiology,biochemistry, and gut microbiota in insect herbivores,however, are not well understood. Nine aquatic insects were found to accumulate Mn at significantly different rates in laboratory studies, and those adsorbed or absorbed manganese partly be relieved during the molting process (Dittman and Buchwalter 2010). Manganese also negatively affects the foraging behavior ofApis mellifera(Hymenoptera: Apidae) through altering biogenic amine levels in the brain (Søvik et al. 2015), but artificial Mncontaminated diets (260-2600 mg Mn kg -1 diet) caused no apparent harm toMegaselia scalaris(Diptera: Phoridae) (Sorensen et al. 2010).
Lymantria dispar(gypsy moth), a rapidly spreading, worldwide pest that greatly impacts agroforestry(Vlahović et al. 2016) and feeds on more than 500 plant species from 73 families (Lazarević et al. 1998). BecauseL. disparlarvae have a short generation time and easily distinguished instars and rearing and collecting are relatively easy,L. disparlarvae are widely used as a model organism in entomological studies (Ilijin et al. 2015).
Digestive enzyme activities which affect nutrient acquisition by insect larvae are under neuroendocrine control and depend on diet composition and consumption(Waldbauer 1968; Perić-Mataruga et al. 2012; Lazarević and Janković Tomanić 2015). Antioxidant enzymes are important in scavenging the reactive oxygen species(ROS) (Felton and Summers 1995; Kim et al. 2018). Total antioxidant capacity (T-AOC) represents the ability of an organism to defend against the ROS damage (Mozaffari et al. 2018). Gut microbiota are widely found in the intestinal tract of the animals, including insects, and is significantly correlated with behavioral regulation (Qiao et al. 2019), metabolism (Ayayee et al. 2018), development (Chen et al. 2018), and immunity (Lee et al. 2017).
Herein, to better understand the stress response of an insect herbivore to Mn, we studied whether and how ingested Mn affectsL. dispar asiaticalarvae and its gut microbiota. Fourth-instarL. dispar asiaticalarvae were fed a MnCl2-amended diet for 84 h. Survivors were then analyzed to determine activities of digestive and antioxidant enzymes, T-AOC, and gut microbial 16S rDNA was sequenced to determine the community composition.
Materials and methods
L. dispar asiatica larvae rearing
TheL. dispar asiaticaeggs and diets, with 0.40 mmol g -1 MnCl 2 (LdMn) or with no MnCl 2 (control) were obtained from the Chinese Academy of Forestry and stored at 4 °C.Eggs were hatched and caterpillars reared as we did previously(Zeng et al. 2019) at 25 ± 1 °C, with 16 light/8 h dark, and 75%relative humidity.
Administration and sampling
We assessed the stress responses ofL. dispar asiaticalarvae and the gut microbiota to Mn ion at LC30(Guo et al. 2020),commonly used for toxicology studies (Rahmani and Bandani 2013; Toteja et al. 2017) and recommended as the mortality threshold for the use of pesticides in integrated pest management (Desneux et al. 2007; Mahdavi et al. 2011).For this experiment, randomly selected fourth-instar larvae were starved for 12 h, then weighed and fed with either the LdMn or control diet while checking for any dead larvae in the LdMn group until mortality reached 30% (after 84 h).Survivors in the LdMn and control groups were weighed to calculate mean mass gain and sampled for further analyses.Each diet group had 30 caterpillars, and the experiment was done three times.
Determinations of enzymatic activities and T-AOC
Eleven randomly selected 11 survivors (whole body) in each replicate were immediately ground in liquid nitrogen. Each powdered sample was then divided into 0.1 mg per sample tube and stored at − 80 °C until use. Protease and trehalase(THL) activities were determined using Folin’s reagent and 3,5-dinitrosalicylic acid (DNS)-reagent as described previously (Zhao et al. 2016). Kits (Nanjing Jiancheng Bioengineering Research Institute, China) were employed to determine the activities of α-Amylase (AMS), lipase (LPS),carboxylesterase (CarE), glutathione-S-transferase (GST),superoxide dismutase (SOD), peroxidase (POD), catalase(CAT), and polyphenol oxidase (PPO), and T-AOC. Protocols were carried out according to the user’s manuals. All user’s manuals are open access on the offi cial website of the manufacturer ( https://www.njjcb io.com/ ). Protein content was determined using the Bradford ( 1976) method.
Gut microbial 16S rDNA sequencing
The intestinal tracts of the remaining survivors were excised on a clean bench and immediately frozen in liquid nitrogen.These samples were sent to MajorBio, Shanghai, China on dry ice for 16S rDNA sequencing of gut microbes where DNA was extracted with E.Z.N.A. Tissue DNA Kit (Omega, USA) as per the manual. 16S rDNA (V3-V4) was amplif ied using primers 338F (5′-ACT CCT ACG GGA GGC AGC AG-3′) and 806R (5′-GGA CTA CHVGGG TWT CTAAT-3′) andTransStart FastPfu DNA Polymerase (TransGen, China). The amplif ication products were purif ied with an AxyPrep DNA Gel Extraction Kit(Axygen Biosciences, USA) and quantif ied using QuantiFluor-ST (Promega, USA) and the manufacturer’s protocols. Purif ied DNA was sequenced with TruSeqTM DNA Sample Prep Kit (Illumina, USA) according to the manufacturer’s protocols. Subsequent bioinformatic analyses were performed with microbial diversity analysis plugins v4.0 in I-Sanger Cloud software ( https://www.i-sange r.com/) (Ma et al. 2018b). Raw sequences were quality-f iltered by Trimmomatic and merged by FLASH using the following criteria: (1) reads were truncated at any site with an average quality score < 20 over a 50 bp sliding window; (2) primers were exactly matched allowing only two nucleotide mismatches, and reads containing ambiguous bases were removed; (3) long overlaps of greater than 10 bp were merged. Operational taxonomic units (OTUs) were clustered with 97% similarity cutoff using UPARSE, and chimeric sequences were identif ied and removed using UCHIME.Mothur was used to calculate the alpha diversity index of different samples. All other analysis and plotting were performed with R packages, including VennDiagram for Venn analysis,vegan for principal coordinate analysis (PCoA) and correlation analysis, PICRUSt for gut microbial function classif ication, and LEfSe for linear discriminant analysis Effect size(LEfSe). The version numbers and database links please refer analyzing software information of microbial diversity analysis plugins v4.0 in I-Sanger Cloud software ( https://cloud.major bio.com/repor t/analy sis_softw are/task_id/i-sange r_19827 7.html?v=20200 50811).
Statistical analyses
Software SPSS 21.0 (IBM, Armonk, NY, USA) was used for one way analysis of variance (ANOVA) with a post hoc Duncan test for enzyme activities, T-AOC, and weight gain;p< 0.05 was regarded as statistically signif icant. Partial least squares discriminant analyses (PLS-DA) of digestive and antioxidant enzyme activities was performed with R 3.6.0(R Foundation for Statistical Computing, Vienna, Austria).
Results
Physiological responses
While the THL activity between the LdMn and control groups did not diff er signif icantly (p> 0.05), the activity of all other tested digestive enzymes (protease, AMS, and LPS)increased after Mn exposure (Fig. 1 a-d), as did the activity of the all analyzed antioxidant enzymes in LdMn group,except PPO, was signif icantly higher (p< 0.05) than control group (Fig. 1 e-j). Furthermore, the PLS-DA visualizations of digestive and antioxidant enzymatic activities showed all conf idence ellipses in independent areas (Fig. 1 m, n). In other words, the PLS-DA results indicated that the digestive and antioxidant enzymatic activities were signif icantly aff ected by Mn exposure. Moreover, the T-AOC was also signif icantly increased by Mn exposure, but larval mass was signif icantly lower than the control (p< 0.05) (Fig. 1 k, l).
Gut microbial 16S rDNA sequencing information
The rarefaction curve and core analysis suggested a suffi -cient quantity of samples in the present study (Fig. 2). After quality-f iltering and removal of redundant sequences, the sequencing database (16S rDNA, V3-V4) was composed of 328,546 high-quality sequences. Next, Usearch clustered them into 184 operational taxonomic units (OTU), 171 species, 133 genera, 88 families, 54 orders, 30 classes, and 16 phyla.
Gut microbial community structure
A Venn diagram showed that 128 OTUs were unique to the LdMn group, 7 were unique to the control group, and the remaining 49 OTUs overlapped (Fig. 3 a). These results suggested that the Mn exposure signif icantly altered the OTU composition of gut microbiota in theL. dispar asiaticalarvae.
Among the phyla represented, Firmicutes and Proteobacteria were most found frequent in both the groups, together making up 99.93% and 99.67% of the gut microbiota in the control and LdMn group, respectively (Fig. 3 c).Lactobacillus,Weissella, andPseudomonas, represented the most abundant genera, together making up 99.15% and 98.79%of the gut microbiota in control and LdMn groups, respectively. Moreover, the proportion of these three genera differed signif icantly between the groups (p< 0.05). The gut microbiota in the control group was composed of 58.07%Lactobacillus, 27.10%Weissella, and 13.99%Pseudomonas,while the LdMn group consisted of 83.13%Lactobacillus,3.94%Weissella, and 11.73%Pseudomonas(Fig. 3 d). Thus,from the genus to phylum level, the relative abundance of the dominate gut microbes inL. dispar asiaticalarvae seemed to be aff ected by Mn exposure.

Fig. 1 Enyzmatic activity and change in mass in Lymantria dispar asiatica larvae after 84 h of feeding on artif icial diet (control) or diet amended with 0.40 mmol MnCl 2 g -1 diet. a Protease, b α-amylase(AMS), c trehalase (THL), d lipase (LPS), e glutathione- S-transferase(GST), f carboxylesterase (CarE), g superoxide dismutase (SOD), h peroxidase (POD), i catalase (CAT), j polyphenol oxidase (PPO), k total antioxidant capacity (T-AOC), l mass gain, partial least squares discrimination analysis (PLS-DA) visualization of m digestive- and n antioxidant-enzyme activities. Data are means ± standard errors.Analysis of variance (ANOVA) was followed by Duncan’s test(* p < 0.05)
Correlation heatmap suggested that the relative abundance of Bacteroidetes and Fusobacteria in the microbiota of the LdMn group diff ered signif icantly (p< 0.05) (Fig. 4 a).However, seven (35%) of the top 20 abundant genera diff ered significantly in abundance between control and LdMn groups (p< 0.05):Lactobacillus,Bacteroides,Prevotella_1,Romboutsia,Weissella,Acetobacter, and a no-rank genus(belongs to family Mitochondria) (Fig. 4 b). Furthermore, six OTUs of the top 30 abundant OTUs were observed maintaining signif icantly diff erent relative abundance between the control and LdMn groups (p< 0.05) (Fig. 4 c). These results also suggested that the relative abundance of gut microbes inL. dispar asiaticalarvae were aff ected by Mn exposure.
Thus, the number of species and relative abundance of gut microbes inL. dispar asiaticalarvae agreed with the PCoA visualization and indicated that the gut microbial community diff ered signif icantly between the control and LdMn groups(Fig. 3 b).
In the LEfSe analysis to discriminate microbes contributing to the different gut microbial community structure, the results showedAcetobacter malorum,Gluconobacter frateurii,Delftiasp.,Weissella paramesenteroides, and two unclassif ied bacteria in the control gut.Fusobacterium mortiferum,Bacteroidessp.,Prevotellasp.,Candidatus Alysiosphaerasp.,Bradyrhizobiumsp.,Lactobacillussp.,Enterococcus mundtii,Clostridiumsensu stricto sp.,Eubacterium rectale groupsp.,Ruminococcaceae_UCG_002sp.,Ruminococcaceae_NK4A214_groupsp.,Romboutsiasp.,Lachnospirasp.,Ruminococcussp.,Blautia producta, andLachnoclostridiumsp. were present in the LdMn group (Fig. 5).
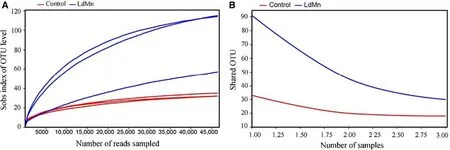
Fig. 2 Evaluation of sample adequacy for 16S rDNA sequencing of gut microbiota in in based on operational taxonomic unit level Lymantria dispar asiatica larvae after 84 h of feeding on artif icial diet (control) or diet amended with 0.40 mmol MnCl 2 g -1 diet. a Rarefaction curve with sobs index, b core analysis curve
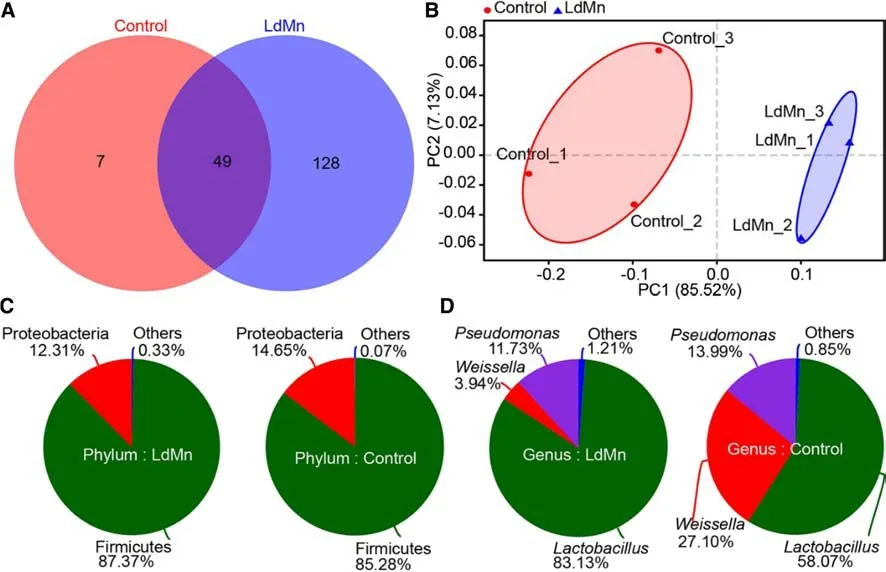
Fig. 3 Gut microbial community structure in Lymantria dispar asiatica larvae after 84 h of feeding on artif icial diet (control) or diet amended with 0.40 mmol MnCl 2 g -1 diet (LdMn). a Venn diagram for operational taxonomic units in control and LdMn, b PCoA plot with Bray-Curtis distance at OTU level. Community composition pie at c phylum level and d genus level
Orthologous groups of proteins (COG) database function classif ication

Fig. 4 Pearson correlation between administration and relative abundance of gut microbes in Lymantria dispar asiatica larvae after 84 h of feeding on artif icial diet(control) or diet amended with 0.40 mmol MnCl 2 g- 1 diet(LdMn). Correlation heatmap at a phylum level, b genus level, c phylum level. The top 10 most-abundant phyla, top 20 most-abundant genera, and top 30 most-abundant OTUs were used in respective Pearson correlation analyses (signif icance of correlation with administration: * p < 0.05; ** p < 0.01)
To study the function of altered gut microbes inL. dispar asiaticalarvae, sequences were used in a blast search of the COG database to predict function. Eight functional classes were upregulated by Mn exposure: amino acid transport and metabolism (function E), carbohydrate transport and metabolism (function G), transcription (function K), general function prediction only (function R), signal transduction mechanisms (function T), defense mechanisms (function V),extracellular structures (function W), cytoskeleton (function Z). The relative abundance of the remaining functions were downregulated by manganese exposure. Coenzyme transport and metabolism (function H) was the only function with a signif icant diff erence between the control and LdMn group(p< 0.05) (Fig. 6).
Discussion
Digestive enzymatic activity is regarded as an important physiological index for toxicity studies in insects (Filippov et al. 2013), owing to its signif icance in digestion and absorption (de Vries et al. 2012). Many plant compounds interfere with digestive processes and thus reduce or deter damage from herbivores insects (Rekha et al. 2004; Oliveira et al. 2007; Mehrabadi et al. 2012; Vazquez Flores et al.2018). Soybean trypsin inhibitor (STI) is a representative inhibitor that prevents the activity of all proteases inPlutellaxylostellaand leads to the delayed development (Zhao et al.2019).Enterolobium contortisiliquumprotease inhibitor(EcTI) is also reported as a potential pesticide inNasutitermes cornigerworkers and soldiers (Da Silva Ferreira et al. 2019). Similarly, heavy metals aluminium, chromium,copper, manganese, nickel and zinc were reported to be concentrated in the digestive glands ofAulacomya atra(Ruiz et al. 2018). Therefore, understanding how these heavy metals aff ect the digestive enzymes should help in developing a new strategy to manage pests (Rodrigues Macedo and Das Gracas Machado Freire 2011). In the present study, all the evaluated digestive enzymes were activated by Mn exposure,especially protease, AMS, and LPS. Our study agrees with an earlier study showing thatL. disparlarvae that fed on leaves ofPopulus alba berolinensiscontaminated with cadmium, zinc, and lead had higher levels of digestive enzymes(Jiang and Yan 2018). But calcium chloride, ethylenediaminetetraacetic acid (EDTA), and ethylene glycol tetraacetic acid (EGTA) inhibited trypsin activity inP. xylostella(Zhao et al. 2019), sanguinarine suppressed digestive enzymes inL.dispar(Zou et al. 2019), and a cadmium-supplemented diet decreased activities of α-glucosidase, trypsin and leucine aminopeptidase inL. dispar(Vlahović et al. 2015a, b). What factors (e.g., insect species, stressors, and doses) contributed to the contradicting results needs further investigation.In addition, our previous study revealed that Mn ion can inhibit the food consumption ofL. disparlarvae (Guo et al.2020), suggesting that the decreased larval mass might be due to inhibited food consumption. Moreover, larvae might increase activity of their digestive enzymes to obtain more nutrition and energy from the food digested when food consumption is low.
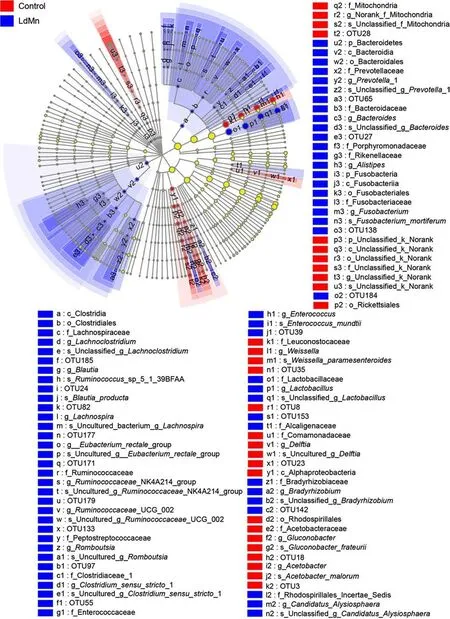
Fig. 5 Linear discriminant analysis Effect size (LEfSe) cladogram of gut microbiota in Lymantria dispar asiatica larvae after 84 h of feeding on artif icial diet (control) or diet amended with 0.40 mmol MnCl 2? diet (LdMn). The threshold of linear discriminant analysis (LDA)was set as 2. The cladogram was calculated using LEfSe with anallagainst-all comparison strategy. Nodes that are not yellow mean a signif icant contribution to grouping

Stressors can generate ROS, thus disturbing redox reactions and causing oxidative damage (Sies 1987; Ali et al.2017). Antioxidant enzymes play a critical role in ROS scavenging and are determiners of oxidative resistance (Krishnan and Kodrík 2006; Ma et al. 2018a; Paithankar et al. 2018).Prolonged or high concentration exposure of heavy metals can inhibit the antioxidant enzymes in microalga and rice seedlings in a time- and concentration-dependent manner(Mei et al. 2007; Srivastava and Dubey 2011). Evaluation of entomic antioxidants could lead to a strategy to prevent the development of resistance to pesticides. Additionally,the sensitivity of antioxidative enzymes to high levels of pollution suggest they might be good biomarkers (Gavrilović et al. 2017). In this study, T-AOC and levels of GST, CarE,SOD, POD, and CAT were signif icantly higher after Mn exposure. These results agree with an earlier study ofAiolopus thalassinusnymphs that had higher antioxidant enzyme activities when exposed to heavy metal pollution and showed(Yousef et al. 2019). Thus, these antioxidant enzymes could be primary players in defense against Mn exposure.
In 1885, the Austrian physician Theodor von Escherich identif ied theEscherichia colibacterium, and this was recognized as the beginning of the gut microbiota research(Anonymous 1985). However, due to limitations of culturing techniques, gut microbial research developed slowly until the advent of high-throughput sequencing (Cheung et al. 2019).The new technology has provided new insights into the complete community structure. In recent years, gut microbiota has become a focus of medical scientists and zoologists.Clinical researches showed us that many diseases or suboptimal health statuses such as obesity (Coelho et al. 2018),chronic constipation (Ohkusa et al. 2019), and non-alcoholic liver disease (Ding et al. 2019) are associated with the gut microbial community. Similarly, the gut microbiota in insects plays crucial roles, from mediating host metabolism and the immune system (Adams et al. 2011; Senderovich and Halpern 2013), even insecticide resistance (Xia et al.2018). Additionally, the gut microbiome is highly specif ic to species, instar stage, surrounding niche, among others (Chen et al. 2018). The present study showed that Mn exposure signif icantly altered the gut microbiota structure inL. dispar asiaticalarvae. However, whether the gut microbiota inL.dispar asiaticalarvae are involved in defense against the Mn stress needs further study. Additionally, manganese is absorbed by the intestinal canal, then accumulates in organs that are enriched with mitochondria such as liver, pancreas,and pituitary (Gavin et al. 1990; Jin et al. 2008; Deng et al.2013). The COG function classif ication showed that Mn stress signif icantly decreased gut microbial coenzyme transport and metabolism inL. dispar asiaticalarvae (p< 0.05);excess manganese might decrease coenzyme transport and metabolism through a negative feedback mechanism (Lerdau 2007; Yu et al. 2008). Whether this change in gut microbial function aff ects the actual function of the host also needs further investigation.
Conclusions
In conclusion, the present results showed thatL. dispar asiaticalarvae lost signif icant mass under Mn exposure(p< 0.05), T-AOC and digestive and antioxidant enzymes,except THL and PPO, signif icantly increased (p< 0.05).The exposure also altered the larval gut microbial community, and signif icantly aff ected the gut microbial coenzyme transport and metabolism in the larvae. These results may be useful for designing a new strategy to controlL. dispar asiaticalarvae.
Author’s ContributionsJZ provided the initial idea for the study,analyzed the data, and wrote the manuscript. JZ oversaw the project.JG, JS, and ZS assisted with the research. GZ and JZ directed the research. JZ and GZ funded the research. All authors have read and approved the f inal version of this manuscript.
Publisher’s NoteSpringer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affi liations.
杂志排行
Journal of Forestry Research的其它文章
- Sacred groves of India: repositories of a rich heritage and tools for biodiversity conservation
- Relationship between H 2 O 2 accumulation and NO synthesis during osmotic stress: promoted somatic embryogenesis of Fraxinus mandshurica
- Changes in leaf stomatal traits of diff erent aged temperate forest stands
- Somatic embryogenesis and plant regeneration in Betula platyphalla
- Hydrogen peroxide as a systemic messenger in the photosynthetic induction of mulberry leaves
- Production and quality of eucalyptus mini-cuttings using kaolin-based particle f ilms
