Changes in leaf stomatal traits of diff erent aged temperate forest stands
2021-04-30QianLiJihuaHouNianpengHeLiXuZihaoZhang
Qian Li · Jihua Hou · Nianpeng He · Li Xu ·Zihao Zhang
Abstract Stomata control carbon and water vapor exchange between the leaves and the atmosphere, thus inf luencing photosynthesis and transpiration. Combinations of forest patches with diff erent stand ages are common in nature, however, information of which stomatal traits vary among these stands and how, remains limited.Here, seven diff erent aged forest stands (6, 14, 25, 36, 45,55, and 100 years) were selected in typical temperate, mixed broadleaf-conifer forests of northeast China. Stomatal density, size and relative area of 624 species, including the same species in stands of diff erent ages were selected. Stomatal density, size and relative area were distributed log-normally,diff ering across all species and plant functional groups. Stomatal density ranged from 4.2 to 1276.7 stomata mm -2 , stomatal size ranged from 66.6 to 8315.7 μm 2 , and stomatal relative area 0.1-93.3%. There was a signif icant negative relationship between density and size at the species and functional group levels, while the relative stomatal area was positively correlated with density and size. Stomatal traits of dominant species were relatively stable across diff erent stand ages but were signif icantly diff erent for herbs. The results suggest that stomatal traits remain relatively stable for dominant species in natural forests and therefore, spatial variation in stomatal traits across forest patches does not need to be incorporated in future ecological models.
Keywords Forest restoration · Stomatal traits · Stand age · Plant functional groups · Variation
Introduction
Leaf stomata are composed of a pair of guard cells, the opening and closing of which are driven by moisture, temperature, light, and carbon dioxide (CO2) in the short term(Casson and Gray 2008; Lau and Bergmann 2012). Stomata allow plants to exchange gas with the external environment,controlling photosynthesis and transpiration (Martin and Glover 2007; Franks and Beerling 2009), in addition to inf luencing net primary productivity and water use effi ciency of the ecosystem (Kim and Lieth 2003; Miyashita et al. 2005).Therefore, ecological models and earth system models consider stomatal traits as important parameters for Effectively simulating carbon, water, and energy cycles (Kelliher et al.1995). Although the importance of stomata from the view of plant physiological ecology has been acknowledged, information about natural forests remains limited.
Plants respond quickly to short-term environmental changes by opening and closing their stomata, while morphological traits of stomata such as stomatal density (SD),stomatal size (SS), and stomatal relative area (SRA, %), are the result of long-term adaptations to the external environment. Several studies have shown how morphological traits vary for certain dominant species and common species in natural forest communities (Tian et al. 2016; Wang et al.2016; He et al. 2018; Liu et al. 2018, 2019). However, most studies have demonstrated these variations in controlled experiments, while focusing on the short-term behavior of stomatal opening and closing (Luomala et al. 2005; Fraser et al. 2009; Engineer et al. 2015). With increasing CO2concentrations, SD decreases, causing maximum stomatal conductance to decline and photosynthesis to increase(Hetherington and Woodward 2003). SD is generally negatively correlated with SS (Stenstrom et al. 2002; Martin and Glover 2007; He et al. 2018); however, this relationship is not suffi ciently compensatory to equalize SRA (Liu et al.2018 ). SRA represents an index of anatomical constraints on maximum stomatal gas exchange, where higher maximum stomatal gas exchange means higher productivity and competitiveness of plants (Bucher et al. 2018, 2019; Liu et al. 2018). Understanding how the morphological traits of leaf stomata vary could provide insights on the adaptation strategies of plants to changing external environments over the long term.
Natural forests are susceptible to natural and human disturbances (Xu et al. 2016) such as f ire (Wang et al. 2013b),pests (Kurz et al. 2008), weather, grazing pressures, and land-use changes. In other words, most forests in the world are recovering from past disturbances and generally consist of patches containing stands of diff erent ages (Fig. 1).Stand age is usually estimated as the time since the last major disturbance (Goulden et al. 2011; Pan et al. 2011;Poorter et al. 2016). How and/or which morphological stomatal traits (SD, SS, and SRA) vary among these patches remains unclear, even though such information is essential to optimize existing ecological models or to develop new ones.

Fig. 1 Schematic of forest patches with stands of diff erent ages. a Forest patches with stands of diff erent ages; b questions of scientif ic importance; c stomata of the broad-leaved species Acer tegmentosum Maxim.; d stomata of the coniferous species Pinus koraiensis Sieb. et Zucc
In this study, seven adjacent temperate forest stands with diff erent restoration times (6, 14, 25, 36, 45, 55, and 100 years) following selective cutting were selected. Three stomata morphological traits (SD, SS, and SRA) were measured in 624 plant species, including the same species in stands of diff erent ages. The main objectives were to:(1) explore the distribution frequency of stomatal traits of typical north-temperate, mixed broadleaf-conifer forests as a whole; (2) demonstrate the relationships among diff erent stomatal traits at the species level and for diff erent plant functional groups (PFGs); and, (3) reveal how stomatal traits change with stand age. By delineating how forest age inf luences stomatal traits, we expected to reveal the importance of using stomatal traits as the main parameters in ecological models to predict ecological functions so as to optimize models.
Materials and methods
Site description
The f ield investigation was conducted in the Jiaohe Forestry Experimental Bureau (43°57′ N, 127° 44′ E) in Jilin Province, northeastern China. The site contains typical temperate, mixed broadleaf-conifer forests and has a continental monsoon climate, with short, mild summers and long, cold winters. The hottest and coldest months are July (21.7 °C)and January (-18.6 °C), respectively, and the average annual temperature is 3.8 °C. The average annual precipitation is approximately 695.9 mm. The soil type is brown forest soil(Zhang et al. 2017) and the dominant tree species arePinus koraiensisSieb. et Zucc.,Acer monoMaxim.,Quercus mongolicaFisch. ex Ledeb. andFraxinus mandschuricaRupr. The dominant shrub species areCorylus mandshuricaMaxim. andRhamnus schneideriLévl. et Vant. The dominant herbaceous species areVitis amurensisRupr. andBrachybotrys paridiformisMaxim. ex Oliv.
Sampling and measurements
Field sampling
The f ield survey was conducted in August 2017. Due to long-term selective cutting as the main mode of forest management, patchy stands of diff erent ages have formed along a restoration gradient (Fig. 1). Seven adjacent temperate stands on similar topography with diff erent restoration ages(6, 14, 25, 36, 45, 55, and 100 years) were randomly selected after selective cutting.
Four 30 m × 40 m experimental plots were established in each stand, and two 5 m × 5 m quadrats and four 1 m × 1 m quadrats were located within each plot to measure shrub and herbaceous species (He et al. 2018; Liu et al. 2018). All plant species were present in the plots were collected. Latitude, longitude, and altitude and the composition of plant species were recorded for each plot. A total of 624 species of trees, shrubs, and herbs were collected (Table 1).
Measurement of stomatal traits
For each species within each plot, 20 or more mature leaves were collected from the top canopies of four healthy plants. The leaves were pooled, placed in sealed plastic bags, and immediately stored in a cooler box. Five to ten leaves from each sample were cut into 1.0 cm × 0.5 cm pieces and fixed in a 75% alcohol-formalin-glacial acetic acid-glycerin solution (90:5:5:5).Stomatal traits were observed using a scanning electron microscope (S-3400 N, Hitachi, Japan). Three pieces were selected from the pooled samples, and each photographed twice on the lower surface (Tian et al. 2016; Liu et al.2018, 2019). The number of pores (N) and the area of the image (S Photo ) were recorded to obtain stomatal density(SD, stomata mm -2 ). Five pores were randomly selected from each image to measure stomatal length (SL, μm)and hence, stomatal size (SS, μm 2 ). SD, SS, and SRA (%)were calculated as:
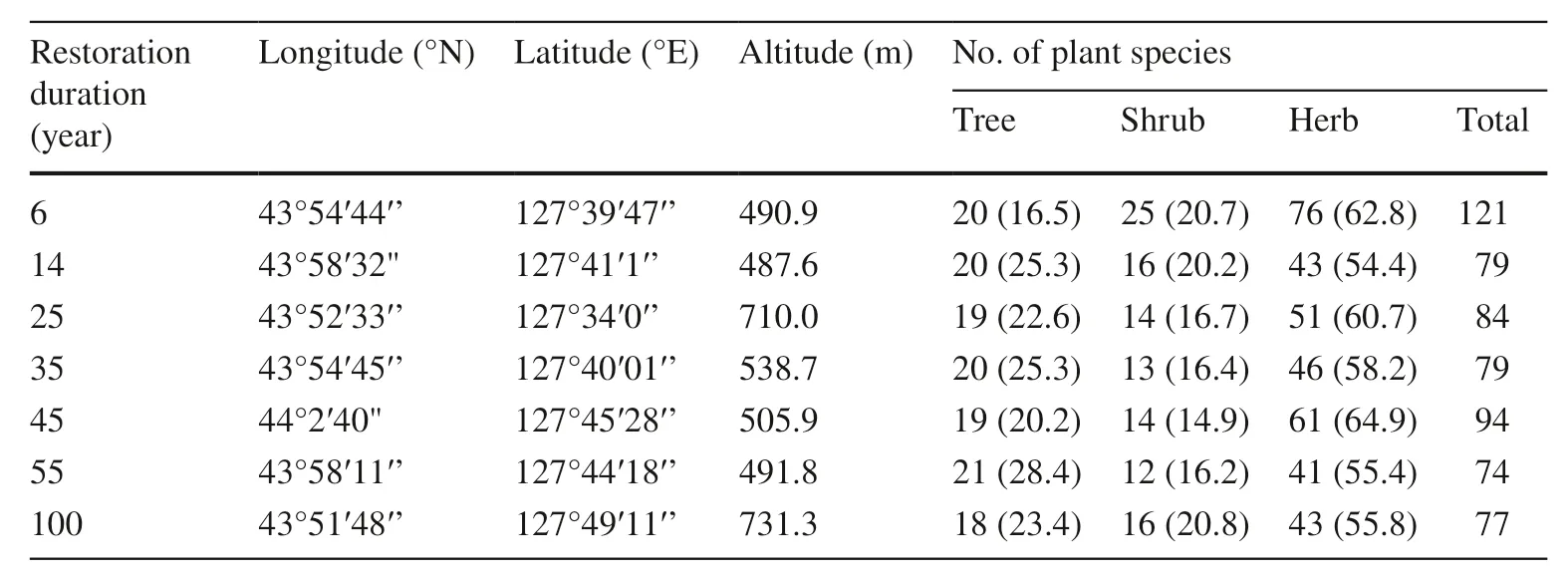
Table 1 Information on plots subjected to diff erent restoration periods after selective cutting

Dominant species
Height and diameter-at-breast-height (DBH) were recorded for each woody species with DBH ≥ 2 cm. Biomass was calculated using species-specific allometric regressions with DBH and height. Dominant tree species are defined as the minimum number of species that,combined, account for 85% of the total biomass (Grime 1998; Avolio et al. 2019). Dominant shrub species are defined as the minimum number of species that, combined, account for 55% of the shrub biomass, and dominant herb species are defined as the top two of the importance-value = (the relative cover + the relative height + the relative frequency)/3) (Zhang et al. 2015).
Data analysis
Stomatal traits were log transformed to obtain the approximate normality for the analysis of frequency distributions. The Pearson correlation coeffi cient was calculated for the various stomatal traits. One-way ANOVAs were performed to compare diff erences in stomatal traits among diff erent PFGs (trees,shrubs, herbs), as well as the variation in stomatal traits across stand ages. SD-SS relationships were tested using ordinary least squares (OLS) linear regressions, and diff erences in slope and intercept of SD-SS relationships among PFGs and stand ages were evaluated by standardized major axis (SMA) estimation using R-software. All data analyses and graphical presentations were performed using SPSS 13.0 (IBM Corp., Chicago,IL, USA) and SigmaPlot 10.5 software (Systat Software, Point Richmond, CA). Signif icance was set atP= 0.05.
Results
Changes in stomatal traits
Across all 624 sampled plant species, the SD, SS, and SRA were distributed log-normally. The mean values of SD, SS,and SRA were 178.7 stomata mm -2 , 1064.2 μm 2 , and 12.7%,respectively, ranging from 4.2 to 1276.7 stomata mm -2 , 66.6 to 8315.7 μm 2 , and 0.1 to 93.2% (Fig. 2). The stomatal traits of dominant species did not signif icantly diff er across diff erent aged stands (P> 0.05, Table 2).

Fig. 2 Frequency distributions of stomatal density ( a,SD), stomatal size ( b, SS), and stomatal relative area ( c, SRA)in temperate forests. N, number of species; Max, maximum;Min, minimum; CV, coeffi cient of variation. Data are the total of seven forests

Table 2 Variance of stomatal traits along the stand ages
SD, SS, and SRA diff ered signif icantly across diff erent PFGs (trees, shrubs, and herbs,P< 0.05; Fig. 3). Trees had higher SD, smaller SS, and larger SRA than shrubs and herbs. There were signif icant diff erences in stomatal traits between coniferous and broadleaf species (Fig. 3). Broadleaf trees had higher SD and smaller SS than coniferous species.The distributions of stomatal traits for the seven diff erent aged stands are shown in Fig. S1 and S2.
Changes in stomatal traits of diff erent aged stand
Stomatal traits were distributed log-normally in the seven diff erent aged stands (Fig. 4). SD and SRA were highest in 25-year-old stand and lowest in 36-year and 14-year-old stand, respectively. However, SS was largest in 55-year-old stand and smallest in the 14-year-old stand (Table S1). Coeff icients of variation for SD, SS, and SRA were largest in the 14-year-old stand (Fig. 4). The stomatal traits of the diff erent plant functional groups (PFGs) varied consistently for every stand age; however, trees tended to have higher SD, smaller SS, and larger SRA (Table S2).
Relationships among stomatal traits
Strong negative relationships between stomatal density (SD)and stomatal size (SS) were found across species (Fig. 5 a)and PFGs (Fig. 5 b, Table S3), whereas SS decreased linearly with increasing SD (after log transformation). After standardized major axis (SMA) tests (Tables S5 and S6),there was a signif icant diff erence in the slope among PFGs(P< 0.05) where the slope of herbs was steeper than for trees and shrubs. For each stand age, SS tended to decrease linearly with increasing stomatal density (Fig. 5 c, Table S4);however, the slopes diff ered signif icantly across stands of diff erent ages. SD and SS were positively correlated with SRA at the species and PFG levels, irrespective of stand age.
Relationship between stomatal traits and stand age

Fig. 3 Changes in leaf stomatal traits across diff erent plant functional groups. In each panel,data represent the mean ± 1 SE.Data with the same lowercase letters represent no signif icant diff erences at P = 0.05. Woody species were divided into coniferous and broadleaved plants.SD: Stomatal density, SS:Stomatal size, SRA: Stomatal relative area
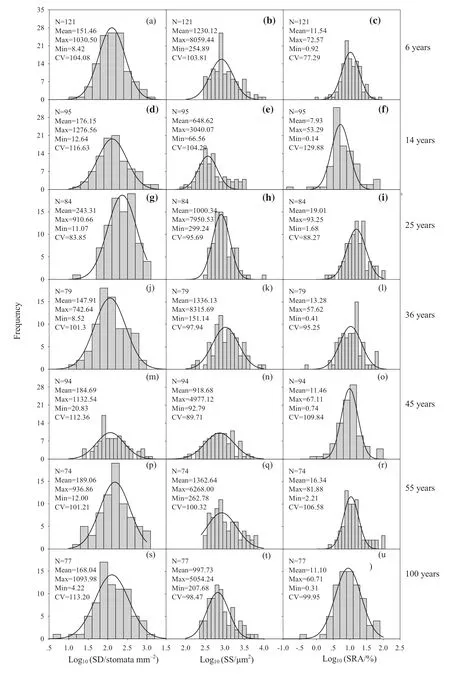
Fig. 4 Frequency distributions of stomatal density (SD), stomatal size (SS), and relative stomatal area (SRA) for temperate forests with diff erent stand ages
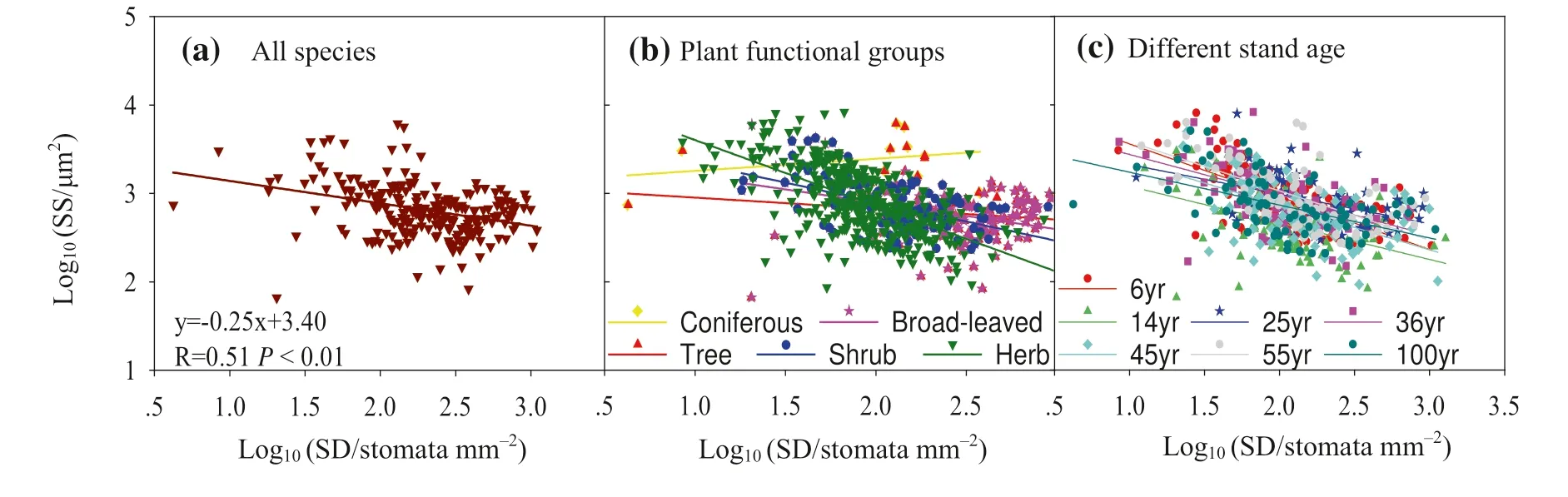
Fig. 5 Changes in the relationships between stomatal density (SD) and stomatal size (SS) at the species, plant functional group, and stand age levels
Stomatal traits signif icantly diff ered among diff erent aged stands at the species level (P< 0.05, Table 2) and between PFGs (Table S2). In the same plant functional groups, variations in stomatal traits diff ered between stand ages. For example, there was no signif icant diff erence in shrub SS(stomatal size) among stands, whereas SD (stomatal density)signif icantly diff ered (Table 2). Variation in stomatal traits was irregular with increasing stand age (P> 0.05). Importantly, the stomatal traits of dominant species did not diff er signif icantly across stand age (P> 0.05, Table 2).
Discussion
Variation in stomatal traits
Stomatal density, size and relative area for 624 plant species were used to quantify the distribution frequency of stomatal traits in these temperate forests, conf irming and extending existing knowledge of the distribution of stomatal traits established by Hetherington and Woodward ( 2003). Previous reports on the stomatal traits of 90 woody and semi-woody plants showed that stomatal relative area ranged from 2.2 to 42.0%, while this study also provided information on herbaceous plants in which SRA ranged from 0.1 to 93.2%.This large variation in SRA among species could strongly inf luence the maximum stomatal conductance of plants,ref lecting changes to maximum gas exchange under diff erent environmental conditions.
Consistent with previous studies, stomatal traits diff ered signif icantly across plant functional groups (Fraser et al.2009). This study showed that trees had the highest stomatal density and relative area, followed by shrubs and herbs,while the opposite pattern was found for stomatal size. These patterns ref lect the diff erent strategies of plant functional groups to adapt to their environment (Wang et al. 2016).For example, the larger stomatal relative size of trees allows them to adapt effi ciently to dry environmental conditions.This combination of smaller stomatal size and greater stomatal density allows tree species to achieve maximum gas exchange when the environment is suitable (McDowell et al.2008; Drake et al. 2013; Krober et al. 2015). In contrast,large stomata are essential for herbs for optimal light capture when it is limited. Diff erences in stomatal traits between coniferous and broadleaf species ref lect diff erences in vein structure (Lammertsma et al. 2011; Zhang et al. 2012). Stomatal size and relative area showed similar trends, being lower in broadleaf species and higher in coniferous plants,with the opposite pattern for stomatal density (Lammertsma et al. 2011). As coniferous species are often found in harsh,arid, or cold environments, lower stomatal density is benef icial to help maintain high water use effi ciency (Yoo et al.2010). The relative stomatal area of coniferous species is greater than for broadleaf species; consequently, the photosynthetic capacity of coniferous species per unit area is greater than for broadleaf species (Tian et al. 2016). Therefore, coniferous species likely have higher growth potential in our study area.
Trade-off between stomatal traits
Stomatal morphological traits can inf luence the balance of CO 2 uptake for photosynthesis against water loss by adjusting stomatal opening and closing. To some extent, stomatal relative area (SRA) is an index of how maximum stomatal gas exchange is constrained by anatomy (Tian et al. 2016;Bucher et al. 2018, 2019; Liu et al. 2018). This phenomenon is related to stomatal density and size which determine maximum anatomical stomatal conductance (Bucher et al.2016; Sack and Buckley 2016), inf luencing the photosynthesis and transpiration of plants (Sack and Buckley 2016).Plants adapt to their environment by altering stomatal conductance, consequently stomatal relative area varies across environments (Fanourakis et al. 2015). For instance, under drought conditions, decreasing stomatal relative area minimizes water loss and improves water use effi ciency, allowing plants to endure drought for longer periods (Drake et al.
2009; Franks and Beerling 2009; Taylor et al. 2012; Franks et al. 2015). Higher stomatal relative area should benef it species exposed to low concentrations of CO2, high irradiance and insuffi cient nutrients for higher productivity, than plants with smaller stomatal relative area (Franks and Beerling 2009; Taylor et al. 2012; Wang et al. 2016). Stomatal density and size allows researchers to estimate the theoretical, maximum anatomical stomatal conductance, indicating the extent to which plants are adapted to a given environment (Franks and Beerling 2009; Franks et al. 2009). Previous studies have shown that increasing stomatal density and decreasing stomatal size under water def icits help plants to adapt to drought conditions (Martinez et al. 2007). Through a trade-off between stomatal density and size, the relative stomatal area may be kept within an appropriate range to maximize ecological functions (Lawson and McElwain 2016). However, if the relative stomatal area increases due to increased stomatal density and constant stomatal size, or increased density and size, there would insuffi cient space to accommodate stomata, which in turn, would impact the other functions of leaves (Franks et al. 2009).
This study demonstrates a signif icant, negative correlation between stomatal density and size, here size decreased linearly with increasing density (Fig. 5). This supports Frank et al. (2009) studies on multiple species at various geological scales. This negative relationship between stomatal density and size represents a long-term adaptation (Hunt et al. 2003;Mott et al. 2008; Drake et al. 2013) that might be explained by physical and energetic constraints (Franks et al. 2009).The physical constraints mainly relate to the spatial limitations of embedding stomata with suffi cient density and size into the leaf epidermis to optimize stomatal conductance.The energetic constraints relate to investment and return in terms of stomatal conductance, and thus photosynthesis and water use effi ciency. The trade-off between stomatal traits might be an important strategy for adapting to the environment, independent of plant functional groups or stand age.
Stable stomatal traits of dominant species with stand age
The stomatal traits of the dominant species remained relatively stable across stands of diff erent ages (Table 2),whereas the total stomatal traits (of all species) diff ered signif icantly between stand ages (Table 2). Changes in stomatal traits are mainly controlled by the external environment such as temperature, light intensity, CO 2 levels, and water supply (Casson and Gray 2008; Lau and Bergmann 2012;He et al. 2018). Consequently, plants can adapt to a given environment by adjusting their stomatal traits (Zhu et al.2011; Wang et al. 2013a; Carlson et al. 2016). The stomatal traits of herbs were far more labile to change than those of trees and shrubs, with trees and shrubs being less aff ected by the environment relative to herbs. Due to the inf luence of the herb component, total stomatal traits of all species differed signif icantly between diff erent stand ages. However, in each stand, species in the upper layers, occupying favorable niches in terms of nutrient acquisition and water absorption.When the environment does not change drastically, dominant species retain their existing stomatal traits, exhibiting no variation (Delgado et al. 2011).
Conclusions
This study demonstrates how stomatal traits are distributed in typical temperate forests with diff erent stand ages. Stomatal traits varied signif icantly across species and plant functional groups (Tables 2 and S2). This variation ref lects the diff erent strategies of plant functional groups to adapt to their environment. Stomatal traits of entire species were signif icantly diff erent across diff erent aged stands, whereas stomatal traits of dominant species remained relatively stable(Table 2). These f indings indicate that variations of stomatal traits due to changing environments diff ered for all species,including dominant species. Considering the importance of dominant species in forests and the associated small variations in stomatal traits across forest patches, variations in stomatal traits between stands of diff erent ages should not be incorporated in future ecological models.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creat iveco mmons.org/licen ses/by/4.0/.
杂志排行
Journal of Forestry Research的其它文章
- Sacred groves of India: repositories of a rich heritage and tools for biodiversity conservation
- Relationship between H 2 O 2 accumulation and NO synthesis during osmotic stress: promoted somatic embryogenesis of Fraxinus mandshurica
- Somatic embryogenesis and plant regeneration in Betula platyphalla
- Hydrogen peroxide as a systemic messenger in the photosynthetic induction of mulberry leaves
- Production and quality of eucalyptus mini-cuttings using kaolin-based particle f ilms
- A regeneration system using cotyledons and cotyledonary node explants of Toona ciliata
