Relationship between H 2 O 2 accumulation and NO synthesis during osmotic stress: promoted somatic embryogenesis of Fraxinus mandshurica
2021-04-30LingYangHanyangGuoYingyingLiuDongyanZhangHongnanLiuHailongShen
Ling Yang · Hanyang Guo · Yingying Liu ·Dongyan Zhang · Hongnan Liu · Hailong Shen
Abstract Osmotic stress promotes somatic embryogenesis of Fraxinus mandshurica, which leads to accumulation of reactive oxygen species (ROS). The single pieces of cotyledons of F. mandshurica were used as explants to induce somatic embryogenesis in osmotic-stress medium. Furthermore, the hydrogen peroxide (H 2 O 2 ) content of explanted cells was varied by adding exogenous H 2 O 2 or catalase solution to assess the Effects of the exogenous H 2 O 2 on somatic embryogenesis, intracellular H 2 O 2 accumulation, and the relationship between signaling mediated by ROS or reactive nitrogen species. The results revealed that exogenous H 2 O 2 (100-300 μmol L -1 ) increased the number of somatic embryos. On 60th day of exogenous H 2 O 2 (200 μmol L -1 )treatment, the number of somatic embryos of explants treated, which was 136.54%, was higher than the control.Moreover, exogenous H 2 O 2 (100 μmol L -1 ) signif icantly increased the intracellular H 2 O 2 content and enhanced the activities of superoxidase dismutase and peroxidase. Finally,exogenous H 2 O 2 (100 μmol L -1 ) activated the intracellular non-enzymatic pathway for nitric oxide (NO) synthesis.The somatic embryogenesis in broadleaf trees increases with the change of endogenic ROS content, and depends on the upregulation of antioxidant enzymes. Both H 2 O 2 and NO, as signaling molecules, were found to be involved in the process of somatic embryogenesis in broadleaf trees. In the process of exogenous H 2 O 2 promoting somatic embryogenesis,NO synthesis depended on non-enzymatic reactions. These results provide a scientif ic basis for resolving the mechanism by which ROS levels are regulated during somatic embryogenesis of broadleaf trees and establish a reasonable and effi cient technology system for regulating somatic embryogenesis of trees.
Keywords Fraxinus mandshurica · Somatic
Introduction
Reactive oxygen species (ROS) and reactive nitrogen species are two important regulators of plant development (germination, f lowering, and senescence) which are used as second messengers together with traditional plant hormones. ROS play key roles in numerous plant-cell signaling pathways(Veselin et al. 2015; Jo et al. 2014; Swanson and Gilroy 2010), and ROS concentration and subcellular distribution can aff ect plant-cell fate (Vanková 2013; Veselin et al. 2015).Treatment of explants with an appropriate concentration of hydrogen peroxide (H2O2) leads to induction of somatic embryogenesis (Cheng et al. 2015), a process that is sensitive to the intracellular concentrations of H2O2and antioxidant enzymes (Zhou et al. 2014; Saeed and Shahzad 2015;Zhou et al. 2016). For example, somatic embryogenesis results in the release of H2O2by cells of cotton (Gossypiumspp.). Many studies have shown that nitric oxide (NO) is an important redox signaling molecule and toxic molecule in organisms, and it is also a reactive nitrogen species involved in many physiological processes of plants (Wilson et al.2008). However, the mechanisms underlying H2O2 and NO actions during plant development remain unclear.
The Manchurian ash (Fraxinus mandshurica) is the most important tree in component of the cold-temperate forest ecosystem of northeastern China, and this tree has important wood-utilizing value, horticultural ornamental value and ecological value (Kong et al. 2012). Immature and mature zygotic cotyledons ofF. mandshuricacan serve as explants that, when cultured on MS½ (Murashige and Skoog, all elements are half in the medium), can induce somatic embryogenesis. However, this method of producing somatic embryos produces only a small number of embryos;moreover, the resultant embryos are often unstable, and therefore the proliferation effi ciency is low and the embryos are diffi cult to root (Kong et al. 2012; Yang et al. 2013).These problems severely constrain the application of somatic embryogenesis techniques for the propagation and genetic transformation ofF. mandshurica. Therefore, the regulation by which somatic embryogenesis is an important research aim forF. mandshurica, toward the goal of producing synchronized and high-quality somatic embryos. We previously reported that osmotic stress-induced somatic embryogenesis inF. mandshuricacauses a burst of ROS production;specif ically, H2O2accumulates to a high level in explanted cells (Yang et al. 2019). However, for somatic embryogenesis ofF. mandshurica, the relationship between H2O2 (that is, with respect to its function as a signaling molecule) and reactive nitrogen species as well as its mechanism of action are unclear.
Our goal was to clarify two issues: (1) whether the function of exogenous H2O2in somatic embryogenesis is within a hormetic framework. (2) The function and relationship between H2O2and NO in somatic embryogenesis. In our study, single pieces of cotyledons were used as explants to induce somatic embryogenesis when cultured on osmoticstress medium supplemented with high concentration of sucrose. Moreover, H2O2 was added to explant cultures to assess the accumulation of intracellular H2O2 as well as its Effects on osmotic stress-promoted somatic embryogenesis and the relationship between ROS and reactive nitrogen species signals. The f indings reveal a role for H2O2and NO in osmotic stress-induced somatic embryogenesis of broadleaf trees and their relationship with ROS accumulation and antioxidant defense responses, thereby providing a scientif ic basis for resolving the mechanism by which ROS are regulated during somatic embryogenesis of broadleaf trees and establishing a reasonable and effi cient technology system for regulating somatic embryogenesis of trees.
Materials and methods
Materials
In mid-October 2015, mature seeds ofF. mandshuricawere collected from 10 healthy 60-year trees, the mother trees were growing on the campus of Northeast Forestry University in Harbin City, Heilongjiang Province, P.R. China(126° 37′ 55″ E, 45° 43′ 16″ N).
Pretreatment of materials
Materials were pretreated according to Yang et al. ( 2013).The collected seeds were mixed and peeled, rinsed under running water for 3 d, and soaked in 75% (v/v) ethanol solution for 30 s, then soaked in 5% (v/v) sodium hypochlorite solution for 15 min. Finally, the seeds were rinsed with sterile distilled water f ive times on a sterile surface. At the time of inoculation, zygotic embryos were extracted from the sterilized seeds on a sterile surface, and the excised cotyledon explants were placed on somatic-embryo (SE) induction medium with the adaxial surface of cotyledons attached to the solid medium.
Somatic-embryo induction
Somatic-embryo induction medium was prepared according to Yang et al. ( 2013). The MS½ (all components of MS medium are half) medium was supplemented with 5 mg L -1 NAA, 2 mg L -1 BA, 400 mg L -1 hydrolyzed casein, 75 g L -1 sucrose, and 6.5 g L -1 agar powder (Beijing Aobox Biotechnology Co., Ltd., Beijing, China), and adjusted to pH 5.8. Thirty explants were cultivated in per 90-mm dish, with 100 replicates per treatment. Explants transferred to a fresh medium after 30-d culture. Culture conditions were as follows: 23-25 °C in the dark, and 60-70% humidity.
Methods for treating cotyledons with H2O2or CAT have been described by Sun et al. ( 2012). The f inal concentration of H2O2in the culture medium was 100, 200 or 300 μmol L -1 , and the f inal CAT concentration was 5, 50,500 or 5000 U mL -1 , respectively. Culture medium that had not been supplemented with H2O2or CAT solution served as the control. Somatic embryogenesis was assessed on the 30th, 45th, and 60th days.
The various stages of SE ontogeny were photographed using a stereomicroscope (SZX7-3732, Olympus, Tokyo,Japan) and a digital camera (Moticam 3000C; Motic ChinaGroup Co. Ltd., Tianjin, China). Histological analyses were carried out according to Yang et al. ( 2013). Images were acquired with a photomicroscope (BX51; Olympus) associated with the Moticam 3000C.
Analysis of H 2 O 2 metabolism and NO synthesis
H2O2(100 μmol L -1 , f inal concentration) or CAT (5 U mL -1 )was added to the SE induction medium. On days 3, 7, 9, 12,15 and 19 after addition of H2O2 or CAT, for the cultures on each medium, 10 samples were taken at each time point for the analysis of a physiological index. The experiments were repeated 3 times.Intracellular H2O2content was measured according to Gniazdowska et al. ( 2010), and intracellular NO content was assayed according to Libourel et al. ( 2006). Superoxidase dismutase (SOD) activity was assayed according to Yang and Shen ( 2011), and peroxidase (POD) activity was assayed according to Lu et al. ( 2009). Nitric oxide synthase (NOS)activity was assayed with a NOS assay kit (NOS A014-2,Nanjing Jiancheng Biotechnology Institute, Nanjing, China).For enzyme extraction, 0.25 g samples of representative culture in a pre-cooled mortar (30 min, − 20 °C) were immersed in 4 mL of 0.1 mol L -1 sodium phosphate buff er (pH 7.4) and ground to yield a homogenate on ice. After transfer to a centrifuge tube, each sample was centrifuged at 7000×gfor 15 min at 4 °C, and the resulting supernatant was considered the enzyme solution. NOS activity was determined according to the instructions supplied with the kit, and optical density was measured at 530 nm relative to that measured with 0.1 mol L-1sodium phosphate buff er.Nitrate reductase (NR) activity was assayed with a kit (NR A096, Nanjing Jiancheng Bioengineering Institute). For enzyme extraction (per kit instructions), adding ninefold the volume of homogenate medium (solution for NR extract) in a ratio of weight (g): volume (mL) = 1: 9 and mechanically homogenize in an ice-water bath to make 10% homogenate.The homogenate was centrifuged at 6000×gfor 10 min at 4 °C, and the supernatant was sampled. NR activity was determined according to the instructions supplied with the kit, and optical density was measured at 540 nm relative to that measured with solution for NR extract.
Data analysis
Excel (2010, Microsoft) software was used for data processing. SPSS (v21.0, SPSS Inc.) was used for one-way analysis of variance, Duncan multiple comparison (Duncan, α = 0.05), and correlation analysis. Sigmaplot (v12.5,SYSTAT) software was used for mapping of the somatic embryogenesis percentage (SEp, %), number of SEs per explant (SEn), SEs synchronlzation percentage (SEg, %),SE malformation percentage (SEm, %), H2O2content,SOD activity, POD activity, NO content, NOS and NR activities. Somatic embryogenesis was calculated according to the following formulas:
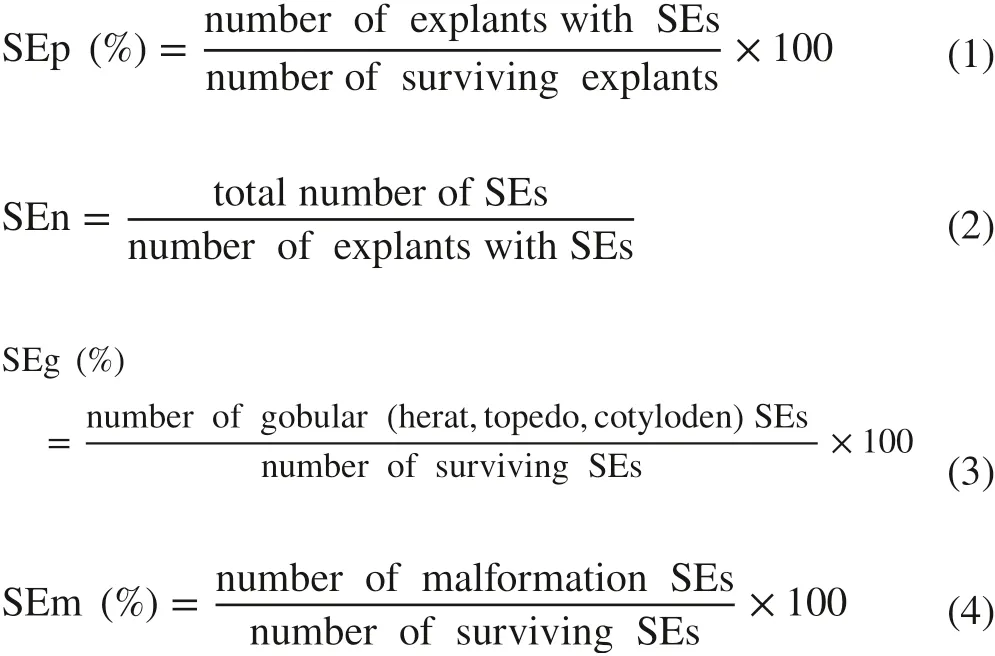
Results
Effects of exogenous H 2 O 2 on somatic embryogenesis of F. mandshurica
Exogenous addition of H2O2promoted somatic embryogenesis ofF. mandshurica(Fig. 1). On the 30th day, somatic embryogenesis of explants treated with 100-300 μmol L -1 H2O2peaked about 40% higher than the control (Fig. 2 a). Exogenous H2O2produced a signif icant Effect on the number of somatic embryos (Fig. 2 b).In Fig. 2 b, a hormetic biphasic concentration-response relationship was clear. On the 60th day, the number of somatic embryos treated with 200 μmol L -1 H2O2reached a maximum of 17.4 per explant (136.55% higher than the control). The extracellular H2O2concentration did not signif icantly aff ect the percentage of synchronized somatic embryos or of malformed embryos (Fig. 2 c, d). The percentage of malformed somatic embryos from explants treated with exogenous 100 μmol L -1 H2O2was the lowest,at 3.44% (34.85% lower than the control).
Exogenous CAT did not significantly affect somatic embryogenesis ofF. mandshurica(Fig. 3). The low concentration (5 U mL -1 ) CAT could promote embryogenesis,but the increase was not signif icant (66.3%, which was only 38.2% higher than that of the control; Fig. 3 a). The higher concentration (50 U mL -1 ) CAT treatment increased the number of somatic embryos (7.31 per explant, 58.7% higher than in the control) but also increased the percentage of malformed embryos (9.49%, 7.72% higher than in the control)(Fig. 3 b, d); moreover, CAT delayed the development of the embryos (Fig. 3 c). On the 60th day, most of the somatic embryos were still in the heart-shaped, embryo stage.

Fig. 1 Effect of exogenous H 2 O 2 or CAT on somatic embryogenesis of F. mandshurica at 60-d culture. a Histology of somatic embryo of controls (CK, without addition of H 2 O 2 or CAT; arrows). Somatic embryos (SEs) were at the globular stage. b Histology of SEs that had been treated with 200 μmol L -1 H 2 O 2 (arrows). SEs were at the torpedo stage. c Histology of SEs that had been treated 5 U mL -1 CAT(arrows). SEs were at the cotyledonary stage. White bar = 1.25 mm.Black bar = 300 μm
Effects of exogenous H 2 O 2 on endogenous H 2 O 2 accumulation and metabolism
Addition of exogenous H2O2or CAT had a signif icant Effect on intracellular H2O2content (Fig. 4 a). In the initial period of culture (3-9 days), exogenous H2O2 promoted the accumulation of intracellular H2O2 , and exogenous CAT had the opposite Effect. In the late period of culture (15-19 days),the results were opposite. In the absence of any treatment(control), the intracellular H2O2content peaked on the 13rd day (1.23 μmol g -1 ) and then decreased slightly. The treatment of exogenous H2O2shortened the time at which intracellular H2O2peaked (the H2O2content was highest on the 7th day, but it was not signif icantly diff erent from that of the control), after which H2O2content decreased (Fig. 4 a). The treatment of exogenous CAT delayed the time of reaching the peak of intracellular H2O2accumulation. On the 15th day, intracellular H2O2peaked at 1.83 μmol g-1, which was 81.0% higher than in the control, after which it decreased.
Exogenous H2O2 or CAT enhanced both SOD activity (Fig. 4 b) and POD activity (Fig. 4 c). Upon treatment with H2O2, both the SOD and POD activities in the cells peaked on the 19th day (1214.17 U g -1 FW h -1 and 1994.66 U g -1 min -1 , respectively; FW, fresh weight).Upon treatment with CAT, the SOD activity peaked on day 15 (1122.64 U g -1 FW h -1 , 78.7% higher than in the control), and POD activity peaked on the 19th day(1692.81 U g -1 min -1 , 65.7% higher than in the control).
Effects of exogenous H 2 O 2 on NO synthesis
Exogenous addition of H2O2 or CAT signif icantly aff ected NO content, the NO: H2O2ratio, NOS activity, and NR activity in explants (Fig. 5). The results for NO content are shown in Fig. 5 a. In the control, the intracellular NO content peaked on the 15th day (0.35 μmol g -1 ) decreased thereafter.Compared with the control, exogenous H2O2signif icantly increased the content of intracellular NO, and exogenous CAT increased the intracellular NO content over the short term (7-13 days), although the NO content decreased with prolonged culture. On the 3rd day, the NO: H2O2ratio in H2O2-treated cells peaked at 10.71 (149.72% higher than that of the control). Subsequently, the intracellular NO: H2O2 ratio decreased. In the CAT-treated cells, the intracellular NO: H2O2ratio peaked on the 7th day at 13.05, and then decreased (Fig. 5 b). Exogenous H2O2or CAT increased the intracellular NOS activity in the f irst 7 days of culture compared with controls (Fig. 5 c). Exogenous H2O2or CAT increased the intracellular NR activity (higher than that of the control) in the f irst 9 days of culture, after which NR activity declined (lower than that of the control; Fig. 5 d).
Discussion
Effects of exogenous H 2 O 2 on somatic embryogenesis of F. mandshurica
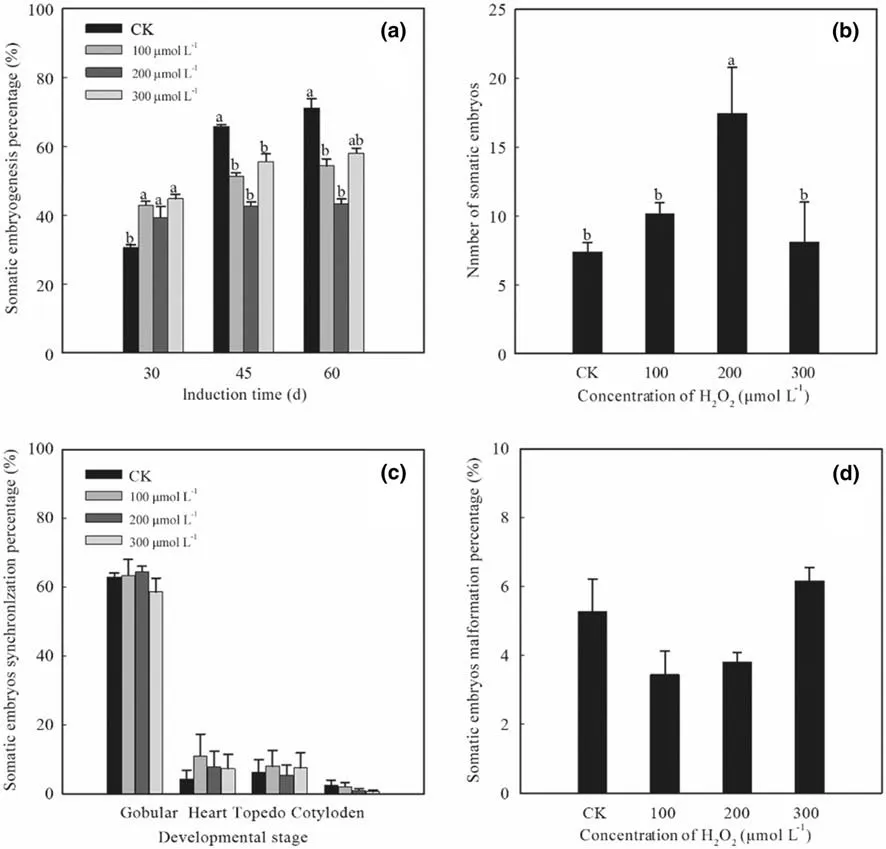
Fig. 2 Effect of exogenous H 2 O 2 on somatic embryogenesis of F.mandshurica. a Prevalence of somatic embryogenesis (percentage) at 30, 45, and 60-d culture; b number of somatic embryos at 60-d culture; c percentage of synchronized somatic embryos at 60-d culture;d percentage of malformed somatic embryos at 60-d culture. CK,control without addition of H 2 O 2 or CAT. Diff erent lowercase letters indicate a signif icant diff erence at the p = 0.05 level, and identical letters indicate no signif icant diff erence
H2O2plays an important role in the response of plants to both biological and abiotic stresses as well as during somatic embryogenesis (Jo et al. 2014; Guler and Pehlivan 2016),and H2O2is a key signaling molecule between oxidative stress and plant regeneration (Libik et al. 2005). In our study, treatment ofF. mandshuricacotyledon explants with 100-300 μmol L -1 H2O2promoted somatic embryogenesis within 30 d, although the ability of H2O2to promote somatic embryogenesis lessened over time. This result was in agreement with the hormesis concept with now a known decrease of the stimulation magnitude over time (after 30 d), but still maintained at biologically signif icant levels (Agathokleous et al. 2019 ). Similar results were obtained from experiments withLarix leptolepis(Zhang et al. 2010),Lycium barbarum(Cui et al. 2002), andGossypiumspp (Zhou et al. 2016).Zhou et al. ( 2016) reported that addition exogenous H2O2 activated the expression of specif ic genes (AUX/IAAs,SAURlike genes,GhABCB19,GhLAX1,GhPILS2,GhAUX1andGhPIN1), which resulted in a change in intracellular indole acetic acid (IAA) content that promoted somatic embryogenesis and development inGossypiumspp.
Effects of exogenous H 2O 2 on intracellular endogenous H 2O 2 accumulation and metabolism
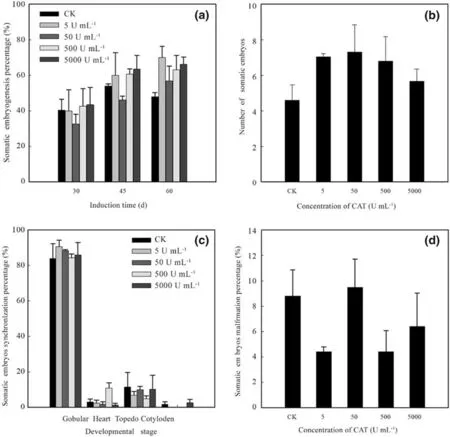
Fig. 3 Effect of exogenous CAT on somatic embryogenesis of F.mandshurica. a Prevalence of somatic embryogenesis (percentage) at 30, 45, and 60-d culture. b Number of somatic embryos at 60-d culture. c Percentage of synchronized somatic embryos at 60-d culture.d Percentage of malformed somatic embryos at 60-d culture. CK,control without addition of H 2 O 2 or CAT. Diff erent lowercase letters indicate a signif icant diff erence at the p = 0.05 level, and identical letters indicate no signif icant diff erence
Under ex vivo conditions, both the resistance to stress of plants and their antioxidant responses depend on the amount of ROS produced (Aragón et al. 2010). In recent years, many scholars have focused their research on the role of ROS and their antioxidant responses in the diff erentiation and development of plant cells (Baťková et al.2008; Blazquez et al. 2009; Barba-Espin et al. 2010). Our study demonstrated that, during somatic embryogenesis ofF. mandshurica, the peak of ROS accumulation in the control was on the 13rd day of culture (By this time, somatic embryos were generated on explants; data not shown). We found that exogenous H2O2promoted somatic embryogenesis, with H2O2content peaking on the 7th day of culture.Moreover, exogenous CAT delayed somatic embryogenesis and decreased the intracellular H2O2content, with H2O2content peaking on the 15th day. The intracellular concentration of H2O2is dynamic, and an increase in H2O2content results in an increase in SOD and POD activities to maintain normal physiological and biochemical reactions in cells (Shohael et al. 2007; Li et al. 2011; Hasanuzzaman et al. 2017). In our study, treatment of explants with H2O2or CAT increased both the SOD activity and POD activity, and there was a signif icant positive correlation between H2O2content and POD activity in response to exogenous CAT. Therefore, H2O2accumulation during somatic embryogenesis ofF. mandshuricais the result of a synergistic Effect of the antioxidant enzyme system.
Effects of exogenous H 2 O 2 on NO synthesis

Fig. 4 Effect of exogenous H 2 O 2 or CAT on intracellular H 2 O 2 accumulation and metabolism during somatic embryogenesis of F. mandshurica. a H 2 O 2 content; b SOD activity; c POD activity. Diff erent lowercase letters indicate a signif icant diff erence at the p = 0.05 level,and identical letters indicate no signif icant diff erence
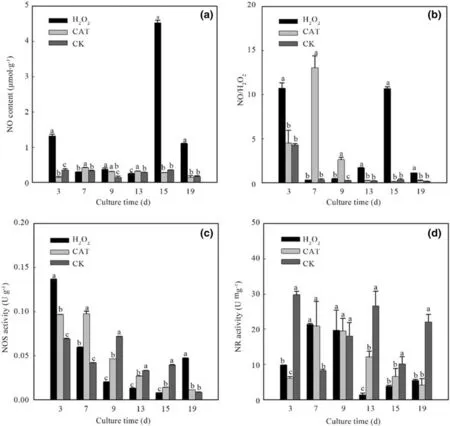
Fig. 5 Effect of exogenous H 2 O 2 or CAT on intracellular NO synthesis. a NO content; b NO: H 2 O 2 ratio; c NOS activity; d NR activity.CK, control without addition of H 2 O 2 or CAT. Diff erent lowercase letters indicate a signif icant diff erence at the p = 0.05 level, and identical letters indicate no signif icant diff erence
NO can serve as an internal or external messenger to regulate developmental and biological processes in plants,including root development, seed germination, senescence, respiration, cell death, disease resistance, hormone response, and response to stress (Marcos et al. 2016; Cheng et al. 2015). In our study withF. mandshurica, we found that exogenous H2O2promoted intracellular NO synthesis.Over the short term (7-13 days), intracellular NO content increased in response to exogenous CAT; over longer periods of culture, intracellular NO content decreased. However,there was no signif icant correlation between H2O2and NO content in explants during somatic embryogenesis, regardless of treatment (exogenous H2O2or CAT). In this scenario,we speculate that H2O2regulates NO synthesis in explants during somatic embryogenesis ofF. mandshuricathrough other, indirect signals. Similarly, inArabidopsis, abscisic acid and brassinosteroids were found to stimulate NO production in guard cells depending on the concentration of H2O2(Bright et al. 2006; Shi et al. 2015). Our study also revealed a very signif icant negative correlation between the intracellular NO:H2O2ratio and intracellular SOD and POD activities under osmotic-stress conditions, and similar results were obtained for experiments done with exogenous CAT.In H2O2-treated explants, however, there was no correlation between the NO:H2O2ratio and the activities of antioxidant enzymes. Therefore, exogenous H2O2likely decreases the intracellular NO:H2O2 ratio to a point below which antioxidant enzymes cannot be activated (Pokora et al. 2017).
There are three major pathways for NO synthesis in plant cells: (1) the NO synthase pathway; (2) NR pathway;(3) non-enzymatic pathway (Gupta et al. 2010; Igamberdiev et al. 2010; Moreau et al. 2010; Cueto et al. 1996).In our present study, exogenous addition of H2O2or CAT signif icantly aff ected NOS activity in explant cells ofF.mandshurica. Exogenous H2O2or CAT increased both the intracellular NOS activity and NR activity in the early stage of culture. Although there was no signif icant correlation between NO content and NOS activity or NR activity in H2O2 -treated explants, the NO content in response to exogenous CAT signif icantly correlated with NR activity(Tables S1, S2 and S3). We speculate that exogenous H2O2can activate non-enzymatic processes of NO synthesis during somatic embryogenesis ofF.mandshurica.
Conclusion
Exogenous H2O2could Effectively increase the number of osmotic stress-induced somatic embryos inF. mandshuricaand could promote the release of intracellular H2O2 and NO and enhance the activities of antioxidant enzymes (SOD,POD and CAT) within a hormetic framework. Exogenous H2O2promoted NO synthesis in explants via non-enzymatic reaction pathways. Therefore, we believe that both H2O2and NO, as signaling molecules, are involved in the process of somatic embryogenesis in broadleaf trees. This study provides a basis for further understanding the role of H2O2in stress-induced somatic embryogenesis of broadleaf trees and the relationship between H2O2, NO, and antioxidant enzymes.
杂志排行
Journal of Forestry Research的其它文章
- Sacred groves of India: repositories of a rich heritage and tools for biodiversity conservation
- Changes in leaf stomatal traits of diff erent aged temperate forest stands
- Somatic embryogenesis and plant regeneration in Betula platyphalla
- Hydrogen peroxide as a systemic messenger in the photosynthetic induction of mulberry leaves
- Production and quality of eucalyptus mini-cuttings using kaolin-based particle f ilms
- A regeneration system using cotyledons and cotyledonary node explants of Toona ciliata
