Hydrogen peroxide as a systemic messenger in the photosynthetic induction of mulberry leaves
2021-04-30WeiLiGengChenYujiaoFangTaoWangYixiongWuYueWuXinmengLiuBaiwenJiang
Wei Li · Geng Chen · Yujiao Fang · Tao Wang ·Yixiong Wu · Yue Wu · Xinmeng Liu · Baiwen Jiang
Abstract Mulberry ( Morus alba L.) seedlings were used to test hydrogen peroxide (H 2 O 2 ) as a potential systemic messenger in photosynthetic induction. The upper leaf of mulberry was dark-adapted for 45 min and then illuminated with photosynthetically active radiation (PAR) of 1000 μmol m −2 s −1 . Photosynthetic induction and H 2 O 2 content in the lower leaf was measured. The results show that pre-illumination of the upper leaf promoted photosynthetic induction and increased endogenous H 2 O 2 in the lower leaf.Without pre-illuminating upper leaf, exogenous H 2 O 2 treatment on the lower leaf promoted photosynthetic induction.The application of diphenyleneiodonium and trichloroacetic acid on petioles of the upper leaf inhibited H 2 O 2 increase in the lower leaf, indicating that H 2 O 2 transport was from upper leaves to lower leaves through the phloem. The results show that H 2 O 2 might serve as a signal messenger to promote rapid induction of photosynthesis of leaves in lower parts of the canopy and enable plants to use light energy more effi ciently.
Keywords Hydrogen peroxide · Photosynthetic induction · Mulberry seedlings · Systematic signal ·Passway
Introduction
For plants grown under low light environments of an understory, light f lecks are primary sources of energy (Iio et al.2009; Fernández-Marín et al. 2018). Approximately 30-60%of the daily carbon gain by understory plants is contributed by sunf lecks (Koizumi and Oshima 1993; Kaiser et al. 2018).Enhancing sunf leck utilization is important to the survival of understory plants (Pearcy 1990). However, sunf lecks change with movement in the canopy and with clouds and sun, and most sunflecks are short and unpredictable (Naumburg and Ellsworth 2000; Zhang et al. 2016; Deans et al. 2019).The rising phase of a response curve of photosynthetic rate to light is def ined as photosynthetic induction (Deans et al. 2019). Urban et al. ( 2007) reported that understory plants displayed faster photosynthetic induction responses to sunf lecks than gap or open-grown plants in general. In this way, understory plants can achieve more carbon gain(Pearcy et al. 1997; Bai et al. 2008). Faster photosynthetic induction is required for Effective utilization of f luctuating irradiance (Kaiser et al. 2018) and is critical for CO2gain for understory plants (Leakey et al. 2005; Vialet-Chabrand et al. 2017).
Environmental stimuli directly aff ect one part of a plant but specialized signals carry the message through the plant to its proper destination (Thomas et al. 2004). This communication within a plant, called systemic signaling, has been well-documented in the role it plays (Lake et al. 2001;Heil and Ton 2008; Shah 2009; Szechyńska-Hebda et al.2010; Gaupels et al. 2012). Lake et al. ( 2001) reported that the light intensities and CO 2 concentrations surrounding mature leaves control the stomatal density of new leaves.Yano and Terashima ( 2001) noted that the light environment around mature leaves of lamb’s quarters (Chenopodium albumL.) regulate the diff erentiation of new leaves in terms of thickness. Recently, Hou et al. ( 2015) found that pre-illuminating upper leaves ofRumexK-1 promoted photosynthetic induction of lower leaves. In this manner, lower leaves ofRumexK-1 can have rapid photosynthetic induction and absorb more CO2. However, intra-plant communication requires signal messengers. Guo et al. ( 2016) reported that illuminating the shoot apex of tomato promoted systemic induction of photosynthesis mediated by IAA (indole acetic acid) signaling, triggering hydrogen peroxide (H2O2 ) accumulation. H2O2is considered a signaling messenger involved in plant response to stress (Karpinski et al. 1999; Hung and Kao 2004; Larkindale and Huang 2004; Melillo et al. 2006).Karpinski ( 1999) reported that H2O2induced an increase of photoprotection inArabidopsisunder high light stress. It has also been demonstrated that H2O2is an important component of the long-distance signals required to respond to CO2and ozone (Foyer et al. 1997).
The objective of this study was to determine if systemic signaling is involved in photosynthetic induction, with a focus on H2O2 as a potential signal messenger. Mulberry seedlings were used; the species is an economically important crop widely planted in China (Liu et al. 2019). Photosynthetic induction and H2O2levels in lower leaves with and without pre-illuminating upper leaves were analyzed and in leaves with or without H2O2or water. To determine whether H2O2is transported from upper to lower leaves and the pathway, diphenyleneiodonium (DPI) and trichloroacetic acid(TCA) were used on petioles of upper leaves.
Materials and methods
This research was carried out at the Northeast Agricultural University, Harbin, China. Mulberry seedlings were planted in 30 m × 40 cm pots, one plant per pot, f illed with black soil,and placed in a greenhouse with photosynthetically active radiation (PAR) of 650-800 μmol m −2 s −1 on a clear day at noon. Air temperatures f luctuated from 19 to 32 °C and relative humidity was 60%. Water and nutrients were supplied to avoid drought and nutrients stress. Seedlings with six functional leaves were used, and moved to the laboratory and kept in the dark for 12 h.
Experimental design
In four treatments, the lower leaves were marked as target leaves. All leaves were shaded by opaque cloth before the experiment.
In treatment 1, only the upper leaf was illuminated with PAR of 1000 μmol m −2 s −1 for 45 min. A plant growth lamp(ECO-014, Guangzhou, China) provided the light source to pre-illuminate the upper leaf in all treatments. After preillumination, H2O2concentration and photosynthetic induction of the lower leaf was measured.
In treatment 2, the lower leaf was uniformly sprayed with 20 mL of 10 mM H2O2or with water. Stem bases were covered with plastic f ilm to prevent the sprayed water from falling onto the soil. After 90 min, H2O2 concentration and photosynthetic induction of the lower leaf was measured.
In treatment 3, to inhibit the transport of H2O2from the upper leaf to the lower leaf, 0.2 ml of 50 μM diphenyleneiodonium (DPI) chloride was applied to petioles of the upper leaf for 15 min. DPI acts as a ROS (reactive oxygen species)inhibitor (Miller et al. 2009; Suzuki et al. 2013; Devireddy et al. 2018). The upper leaf was then illuminated with PAR of 1000 μmol m −2 s −1 for 45 min. After illumination, H2O2concentration and photosynthetic induction of the lower leaf was measured.
The application of trichloroacetic acid (TCA) blocks material transport from one set of tissues to others by killing phloem cells (Yano and Terashima 2001). In treatment 4,chemical girding of the petiole of the upper leaf was carried out with 0.5 ml of 10% TCA for 12 h before illumination,which blocked signal messenger transport. The upper leaf was illuminated with PAR of 1000 μmol m −2 s −1 for 45 min and then H2O2concentration and photosynthetic induction of the lower leaf were measured. Lower leaf without upper leaf illumination was marked as CK.
Photosynthetic induction
Net photosynthetic rate (Pn) and photosynthetic photon f lux density (PPFD) were measured using a portable photosynthesis system (LI-6400, LI-COR, Lincoln, NE,USA). Photosynthetic induction measurements were performed at 1000 μmol m −2 s −1 PPFD with a 350 μl L−1CO2concentration and recorded every 30 s. The leaf chamber was 25 °C and a relative humidity of 60%. Data was collected automatically with the photosynthesis detection system. Four plants were used in each treatment. The net photosynthetic rate (Pn) was standardized as follows: standardized Pn = photosynthetic rate/maximum photosynthetic rate.
Detection of H 2 O 2 in mulberry leaves
To quantify H2O2 levels, 0.5 g of leaves were ground in liquid nitrogen and extracted with 5 mL of 5% (w/v) trichloroacetic acid. The precipitate was removed by centrifugation,and the clear extract analyzed for H2O2content (Zhang et al.2014; Cheng et al. 2016). A 0.4 mL of 20% titanic tetrachloride was added to 3 mL of the supernatant extract and 0.6 mL of NH4OH for precipitating the peroxide-titanium complex was added. The mixture was centrifuged at 10,000 rpm for 5 min. The precipitate was collected, solubilized in 2 mL of 2 M H2SO4. Hydrogen peroxide content was measured by the absorbance change at 415 nm against the corresponding reagent blank. A standard curve made from known H2O2concentrations was used calculate the concentration of H2O2 in the solution.
Relative water content (RWC)
The relative water content of lower leaves in each treatment was calculated using the following equation:RWC = (FW − DW)/(SW − DW). FW is the fresh weight,DW is the dry weight that was measured by desiccating leaves for 12 h at 85 °C, and SW is the saturated fresh weight that was measured after submerging leaves under water for 2 h.
Statistical analysis
Tukey’s test was used to analyze diff erences between the treatments in SPSS (10.0 for Windows). Diff erent letters indicate signif icant diff erences at a 5% level.
Results
Photosynthetic induction and H 2 O 2 content of target leaves after upper leaf illumination
Pre-illumination of the upper leaf for 45 min with PAR of 1000 μmol m −2 s −1 promoted photosynthetic induction in the lower leaf (Fig. 1 b). The time required to reach both 50% (T50) and 90% (T90) photosynthetic rates in lower leaf was signif icantly lower after upper leaf were illuminated(Fig. 1 c). The promotion of photosynthetic induction was accompanied by the accumulation of H2O2(Fig. 1 d). There were no signif icant diff erences between the lower leaf with upper leaf illumination and CK in RWC (Fig. 1 e).
Photosynthetic induction and H 2 O 2 content of target leaves after treatment with H 2 O 2
Exogenous H2O2 promoted photosynthetic induction in the lower leaf without pre-illuminating upper leaf (Fig. 2 b). The T50 and T90 in the lower leaf decreased signif icantly after treatment with exogenously applied H2O2(Fig. 2 c). H2O2content increased as well (Fig. 2 d). This suggests that hydrogen peroxide might be a single messenger in photosynthetic induction.
Effects of diphenyleneiodonium chloride (DPI)on photosynthetic induction and H 2 O 2 contents of target leaves
DPI prevented the promotion of photosynthetic induction in the lower leaf with upper leaf illumination (Fig. 3 b, c). The application of DPI to the petiole of the upper leaf caused the inhibition of H2O2levels in the lower leaf even if the upper leaf was illuminated under 1000 μmol m −2 s −1 PPFD(Fig. 3 d).
Photosynthetic induction and H 2 O 2 content of target leaves after girdling upper leaf petioles
Treatment with TCA stopped photosynthetic induction in the lower leaf as promoted by the illumination of upper leaf(Fig. 4 b). The T50 and T90 in the lower leaf with upper leaf illumination was signif icantly higher than that of the TCA treatment (Fig. 4 c). Girding the petiole of upper leaf with TCA inhibited H2O2levels of the lower leaf, even when the upper leaves were illuminated by 1000 μmol m −2 s −1 PPFD(Fig. 4 d). TCA may kill phloem cells to block material transmission, suggesting that H2O2might be transported through phloem.
Discussion

Fig. 1 Effect on photosynthetic induction and H 2O 2 content of the lower leaf after the upper leaf was illuminated.a The sketch map for experimental design. b Time course of photosynthetic rate in the lower leaf after the upper leaf was illuminated with PAR of 1000 μmol m− 2 s− 1 for 45 min.Each curve was the mean of three replicates. c The time taken to reach 50% or 90% of the maximum photosynthetic rate, labeled respectively as T50 and T90. The values in plot c were obtained from four plants.d Change in H 2O 2 content in lower leaf. The values in plot d were obtained from four plants.e The relative water content(RWC) in lower leaf in 2 h. The values in plot e were obtained from four plants. For plots c-e,the values were presented as means ± SE. Diff erent letters indicate a signif icant diff erence at the 0.05 level. HL represents the upper leaf was illuminated with PAR of 1000 μmol m− 2 s− 1 for 45 min. CK represents lower leaf without upper leaf illumination
This study shows that systemic signal in photosynthetic induction exists in mulberry and is important for light effi ciently of leaves grown in the understory and inside crowns of the canopy. Light f lecks are the main source of energy for leaves at diff erent spatial locations, ranging from the understory to the canopy (Iio et al. 2009; Fernández-Marín et al. 2018). In a dynamic light environment, photosynthetic utilization of sunf lecks requires a rapid physiological response and therefore this is critical for understory plants which must compete and survive in a forest community (Valladares et al. 1997; Tomimatsu and Tang 2012;Deans et al. 2019). Heightened Rubisco content and activity, the f irst major enzyme involved in carbon f ixation, as well as an increase in stomatal conductance during dark adaptation are indicators of rapid photosynthetic induction(Pearcy 1994; Carmo-silva and Salvucci 2013). The results in this study indicate that pre-illuminating upper leaf of mulberry promoted photosynthetic induction in the target or lower leaves (Fig. 1 a), and took less time in the process of photosynthetic induction (Fig. 1 b). This result concurs with studies by Hou et al. ( 2015) and Guo et al. ( 2016) in which a systemic signal from one part of a plant to another promoted photosynthetic induction in lower leaves. What is the molecular signal messenger that promotes photosynthetic induction in lower leaves? These results indicate that exogenous H2O2is the answer (Fig. 2 b). Pre-illuminating upper leaf cloud promote photosynthetic induction and accumulation of H2O2in the lower leaf (Fig. 1 c); the exogenous H2O2pre-treatment showed the same result (Fig. 2 b, c). Thus, our results indicate that H2O2acts as a signal messenger from upper leaf to lower leaf in photosynthetic induction. The difference in H2O2content between the two treatments was not caused by water content in leaves (Figs. 1 e, 2 e).
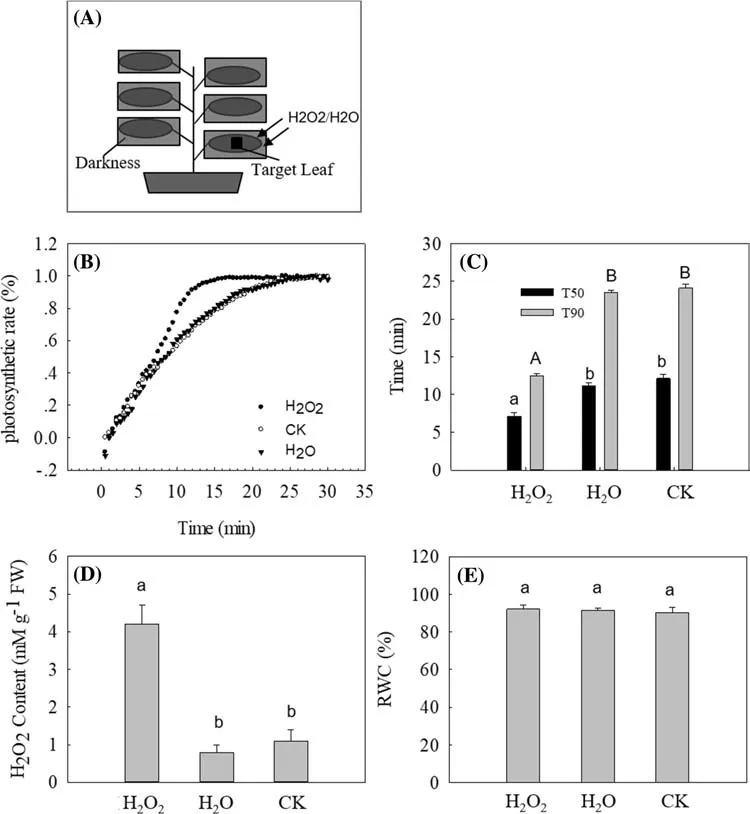
Fig. 2 Effect on photosynthetic induction and H 2O 2 content of the lower leaf after the lower leaf treated with H 2O 2. a The sketch map for experimental design. b Time course of photosynthetic rate in the lower leaf after the lower leaf treated with 10 mM H 2O 2 for 90 min.Each curve was the mean of three replicates. c The time taken to reach 50% or 90% of the maximum photosynthetic rate, labeled respectively as T50 and T90. The values in plot c were obtained from four plants.d Change in H 2O 2 content in the lower leaf. The values in plot d were obtained from four plants. e The relative water content (RWC) in the lower leaf in 2 h. The values in plot e were obtained from four plants. For plots c-e, the values were presented as means ± SE. Diff erent letters indicate a signif icant difference at the 0.05 level. H 2O 2 and H 2O represent the lower leaf treated with 10 mM H 2O 2 for 90 min
Guo et al. ( 2016) indicated that indoleacetic acid (IAA)synthesized in the upper leaves functioned as a systemic signal leading to Effects on the induction of CO 2 assimilation.Previous studies have reported that H2O2 plays a signaling role in plants (Neill et al. 2002). The many characteristics of H2O2match key features of signaling molecules, including a long lifespan, the ability to cross biological membranes,to diff use rapidly from cell to cell and to travel far from its origin (Cheng and Song 2006). Compelling evidence shows that H2O2plays a key role in the activation of cyclic electron f low around photosystem I through modulation of the activity of the NADPH-plastoquinone reductase complex (Strand et al. 2015). In this way, H2O2can increase CO2assimilation(Jiang et al. 2012).

Fig. 3 Photosynthetic induction and H 2O 2 content of the lower leaf after upper leaf was illuminated with PAR of 1000 μmol m− 2 s− 1 for 45 min,before illumination the petiole of the upper leaf was treated with DPI for 15 min. a The sketch map for experimental design. b Time course of photosynthetic rate in the lower leaf. Each curve was the mean of three replicates. c The time taken to reach 50% or 90% of the maximum photosynthetic rate, labeled respectively as T50 and T90. The values in plot c were obtained from four plants.d Change in H 2O 2 content in the lower leaf. The values in plot d were obtained from four plants. e The relative water content (RWC) in the lower leaf in 2 h. The values in plot e were obtained from four plants. For plots c-e, the values were presented as means ± SE. Diff erent letters indicate a signif icant diff erence at the 0.05 level. DPI represents the petiole of the upper leaf was treated with DPI before illumination
To test whether H2O2is transported from upper to lower leaf in mulberry, DPI was applied on petioles of upper leaf.Devireddy et al. ( 2018) indicated that H2O2as a signal messenger transported from local leaves to systemic leaves aff ected stomatal closure by using DPI as a reactive oxygen species inhibitor. Our results indicate that the accumulation of H2O2in upper leaf occurred after illumination (see supporting information Fig. S1). The application of DPI prevented the accumulation of H2O2in lower leaf even when upper leaf was pre-illuminated and also did not promote photosynthetic induction (Fig. 3 b). It was indicated that H2O2was involved in systemic signaling that promotes photosynthetic induction from upper leaf to lower leaf.
To determine the H2O2transfer pathway from upper to lower leaves, trichloroacetic acid was used to block the transmission of H2O2 through the phloem as TCA killed phloem cells (Fig. 4 d). At the same time, TCA applied on petioles of upper leaves also prevented the promotion of photosynthetic rates in lower leaves (Fig. 4 b). However, the xylem transports materials only from lower to upper plant parts,indicating that H2O2transmission is not via the xylem (Morris et al. 2018).

Fig. 4 Photosynthetic induction and H 2O 2 content of the lower leaf after upper leaf was illuminated with PAR of 1000 μmol m− 2 s− 1 for 45 min,the petiole of the upper leaf was treated with TCA for 12 h before illumination. a The sketch map for experimental design. b Time course of photosynthetic rate in the lower leaf. Each curve was the mean of three replicates. c The time taken to reach 50% or 90% of the maximum photosynthetic rate, labeled respectively as T50 and T90. The values in plot c were obtained from four plants.d Change in H 2O 2 content in lower leaf. The values in plot d were obtained from four plants.e The relative water content(RWC) in lower leaf in 2 h. The values in plot e were obtained from four plants. For plots c-e,the values were presented as means ± SE. Diff erent letters indicate a signif icant diff erence at the 0.05 level. TCA represents the petiole of the upper leaf was treated with TCA before illumination
In conclusion, pre-illuminating upper leaves promoted photosynthetic induction and the accumulation of H2O2in lower leaves. Hydrogen peroxide is transported via the phloem and has a key role in the promotion of photosynthetic induction in lower leaves.
Acknowledgements The authors would like to thank Professor Elizabeth Tokarz at Yale University for her assistance with English language and grammatical editing of the manuscript. The authors also wish to thank Guangyu Sun, Huihui Zhang and Xiuli Zhang for providing technical support.
Publisher’s NoteSpringer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affi liations.
杂志排行
Journal of Forestry Research的其它文章
- Sacred groves of India: repositories of a rich heritage and tools for biodiversity conservation
- Relationship between H 2 O 2 accumulation and NO synthesis during osmotic stress: promoted somatic embryogenesis of Fraxinus mandshurica
- Changes in leaf stomatal traits of diff erent aged temperate forest stands
- Somatic embryogenesis and plant regeneration in Betula platyphalla
- Production and quality of eucalyptus mini-cuttings using kaolin-based particle f ilms
- A regeneration system using cotyledons and cotyledonary node explants of Toona ciliata
