A new species of Zanclognatha Lederer and three new records of the families Erebidae and Noctuidae (Lepidoptera, Noctuoidea)from Korea, with notes on larval feeding habits
2021-04-30KangWonLeeJoonMoKooJaeDongKimHuiLinHanKyuTekPark
Kang-Won Lee · Joon-Mo Koo · Jae-Dong Kim ·Hui-Lin Han · Kyu-Tek Park
Abstract A new species of Zanclognatha Lederer, 1857 of the family Erebidae, Z. querciella sp.n., one species of Family Erebidea, Asota egens (Walker, 1854) and two species of Family Noctuidae: Meganephria retinea Gyulai and Ronkay,1999 and Amphipyra subrigua Bremer and Grey, 1853, are reported for the f irst time from Korea. In addition, larval feeding habits with their host plants for three species are newly recognized from Korea. Illustration of adults, larvae for Zanclognatha querciella and Meganephria retinea, and the genitalia of male or female for all species are provided.
Keywords Host plants · New species · New records ·Korean peninsula · Taxonomy
Introduction
From a result of monitoring surveys on the biodiversity for moths and their host plants of larvae in Korea over the last several years, a new species ofZanclognathaLederer:Z.querciellasp.n. is described, one species of Family Erebidae and two species of Family Noctuidae are newly discovered in Korea. In addition, larval host plants ofZanclognatha querciellasp.n. andMeganephria retineaGyulai and Ronkay were investigated. Host plants for larvae of all known Lepidoptera species in Korea have been poorly documented and mostly dependent on data from outside the country.However, over the last several years, larval host plants of Lepidoptera in Korea have been studied and three comprehensive volumes published (Lee 2016, 2017, 2019).
This study describes a new species of Erebidae and reports three species, one of Family Erebidae and two FamilyNoctuidae from Korea.
Materials and methods
For a survey of larval feeding habits of Lepidoptera, larvae feeding on host plants and debris were collected from various areas in Korea. The collected larvae were reared by supplying fresh material of their host plants until they pupate under natural conditions in a specially designed glass house of the Holoce Ecosystem Conservation Research Institution(HECRI) in Heongseong, Kangweon Province. The date of adult emergence were recorded along with the collection date of the larvae, if available. For identif ication of the species, genitalia of the moths were dissected and examined, stained with chlorazol-black and mounted on slides in Euparal mounting medium. All materials are deposited in HECRI.
Results
Description of new species
Zanclognatha querciellaHan and Lee, sp.n. (Figs. 1, 5,7, 13)
Systematic accounts: Family Erebidae; Subfamily Hermininae.
Types.Holotype: male, Hampyoung Ecological Park,Hampyoung, Jeonnam Prov., 22 viii ~ 4 x 2016, leg. KW Lee, gen. slide no. HL19-15/Park. Paratypes: 5 males, 4 females, same date; gen. slide no. HL19-15/Park (male),HL19-17/Park (female), HL19-29/Park (female), HL19-30/Park (female), in HECRI.
Diagnosis.The species is similar toZanclocnatha cuvilinea(Wileman and South, 1917) in the superf icial and genital characters, and hardly distinguished from each other.The male genitalia are very similar to those ofZ. cuvilinea(Fig. 6), but can be distinguished by the following: terminal spine-like process of apical process of left cucullus longer,about 1/3 length of costal process, whereas inZ. cuvilinea,about 1/4 of the apical process; terminal process of right process much shorter than that ofZ. cuvilinea; juxta more narrowly elongated than that ofZ. cuvilinea; aedeagus with a pair of cornuti, while three cornuti inZ. cuvilinea. The female genitalia can be easily distinguished from those ofZ.cuilinea(Fig. 8), with more distinct diagnostic characters as followings: ductus bursae more densely wrinkled, and with strongly sclerotized plates, whereas inZ. cuvilinea, nearly not as wrinkled and sclerotized plates absent; also corpus bursae more densely wrinkled posteriorly than the latter.

Figs. 1-4 Adults. 1. Zanclognatha querciella sp.n.; 2. Asota egens(Walker, 1854); 3. Meganephria retinea Gyulai and Ronkay, 1999; 4.Amphipyra subrigua Bremer and Grey, 1853. ( Scale bar 10 mm)
Description Adult(Fig. 1). Wingspan 18-22 mm. Head:Dorsal surface pale brownish orange. Antenna shorter than forewing; in male with flagellum brownish orange dorsally, ciliate, uniquely broadened at 2/5; in female normally slender. Labial palpus with second segment thick, strongly bent, brownish orange on outer surface; 3rd segment slightly shorter than 2nd, strongly upturned, more or less f lattened with rough hair-like scales along posterior margin; apex acute. Thorax: tegula and thorax grayish brown on dorsal surface; abdomen grayish brown. Fore-leg tibia with orangewhite hair-like scales dorsally and with short, blackish scale-tuft basally; the f irst tarsus large, uniquely broadened.Forewing pale brownish orange or pale orange; basal line indistinct; antemedial line dark brown, narrow, slightly convex on upper cell; postmedial line strongly convex beyond cell; very indistinct, obscure halo band; subterminal line well-developed, dark brown, slightly incurved, arising from near apex to prior to tornus; a distinct dark-brown line welldeveloped along termen; orbicular spot small, dark brown,often indistinct; reniform spot dark brown; fringe concolorous with ground color, with narrow orange white basal line.Hindwing broader than forewing; ground color same as forewing; median line indistinct; postmedial line dark brown,slightly bent on 2A.
Male genitalia(Fig. 5). Uncus stout, strongly bent at base; median expansion on anterior margin triangular,located beyond middle; apical spine small, heavily sclerotized. Tegumen as long as uncus. Valva extremely broad basally, trifurcated, asymmetrical; left valva longer than right; cucullus membranous, slender; costal process of left valva heavily sclerotized, narrow, curved outwardly, with apical spine-like process about half length of basal part;that of right with shorter apical spine-like process; sacculus broad, asymmetrical, left apical process heavily sclerotized,strongly incurved medially; right one much shorter, directed posteriorly. Juxta weakly sclerotized, narrowly elongated posteriorly in triangle, with two carina at both sides, globular basally. Saccus U-shaped. Aedeagus extremely stout, as long as valva, narrowed at base, slightly bent beyond middle,with a large sac with numerous spinules in vesica; cornuti consist of a pair of heavily sclerotized bars arising from a sclerotized basal plate.
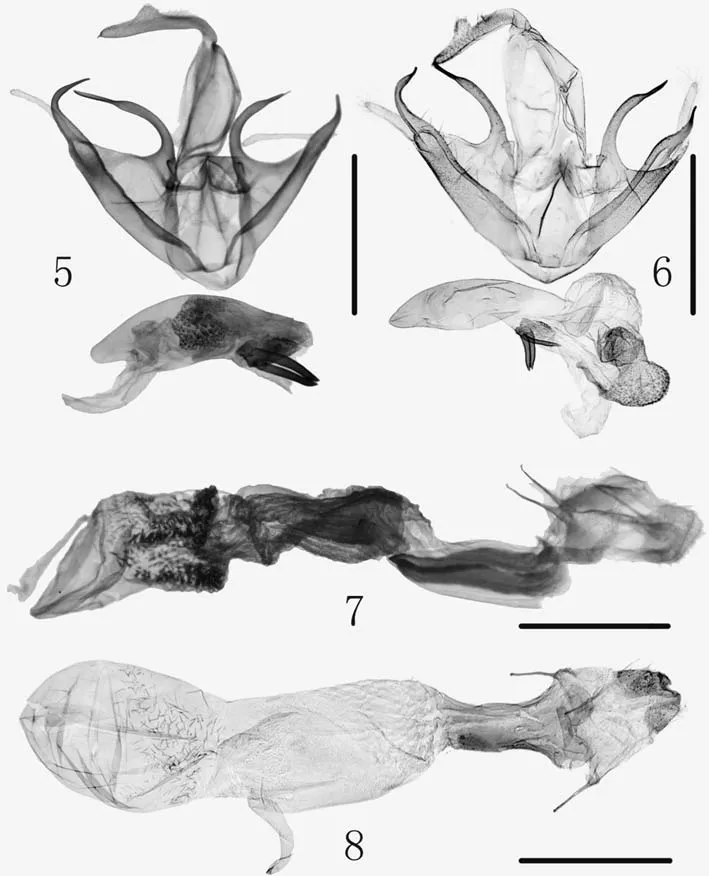
Figs. 5-8 Genitalia. 5. Zanclognatha querciella sp.n.,male, holotype, gen. slide no.HL19-15/Park; 6. Z. cuvilinea(Wileman and South, 1917),male (after Kononenko and Han 2007); 7. Z. querciella sp.n.,female, paratype, gen. slide no.HL19-29/Park; 8. Z. cuvilinea(Wileman and South, 1917),female (after Kononenko and Han 2007). ( Scale bar 1.0 mm)
Female genitalia(Fig. 7). Papillae anales trapezoidal,with dense hairs. Apopyses anteriores slender, as long as posteriores. Antrum weakly sclerotized, elongated in V-shape, slightly shorter than ductus bursae. Ductus bursae membranous, broad, wrinkled, with two sclerotized plates at both sides posteriorly; ductus seminalis narrow,arising from before middle. Corpus bursae ovate, membranous, heavily wrinkled in posterior half; signum absent.
Larva (Fig. 13). Body is yellowish brown, partly mixed with red. An inverted Y-shaped line extended downward from the top of the head and covered in brown spots. Larvae have four pairs of abdominal prolegs from third to sixth (A3-A6). A3 and A4 prolegs under developed are relatively smaller than A5 and A6. The body length of mature larva is 15-18 mm.
Biology. Eleven larvae feeding on dried leaves ofQuercusspecies and Oriental Cherry in Hampyoung on 21 vii 2016 (HERCI Breeding Number: 16-Noc-93), and reared feeding on dried leaves of the host plants under room temperature in a specially designed glass house(22 × 21 cm). Adults emerged from 22 viii to 4 × 2016 (Lee 2016, 2017, 2019).
DistributionKorea.
EtymologyThe species name is derived from the genus name of the host plant,Quercusspp.
Description of new records
Asota egens(Walker, 1854) (Figs. 2, 9)
Hypsa egensWalker, 1854.List Spec. Lep. Ins. Coll. Brit.Mus.2: 453. TL: India.
Hypsa nebulosiButler, 1875.Trans. Ent. Soc. Lond.1876: 322.
Asota egens; Holloway, 1976: 5; Inoue, 1982: Part I: 659,Part II: 342; Bayarsaikhan et al. 2016: 228.
Systematic accounts: Family Erebidae; Subfamily Aganainae.
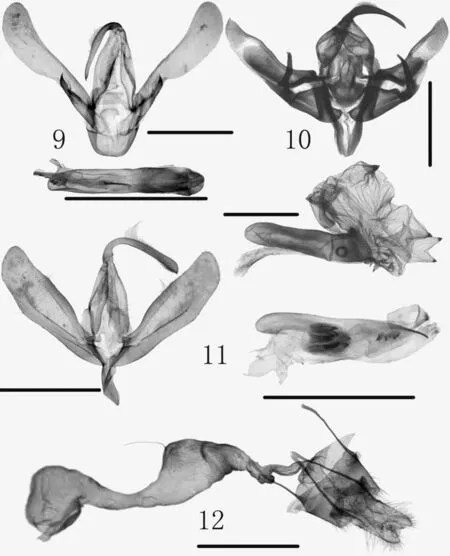
Figs. 9-12 Genitalia. 9. Asota egens (Walker, 1854), male,gen. slide no. HL19-33/Park;10. Meganephria retinea Gyulai and Ronkay, 1999, male, gen.slide no. HL19-14/Park; 11.Amphipyra subrigua Bremer and Grey, 1853, male, gen. slide no. HL19-22/Park; 12. ditto,female, gen. slide no. HL19-23/Park. ( Scale bar 1.0 mm)
Diagnosis(Fig. 2). The species is characterized by yellowish ground color of the forewing, with dark spots near base of the wing. Wingspan 51 mm. A male was found in Korea. The male genitalia show some diff erences from those of Cambodian species illustrated by Bayarsaikhan et al.( 2016: Fig. 6C), with the uncus much longer and the phallus with a single cornutus, whereas two cornuti in the latter.
Male genitalia(Fig. 9). Characterized by the slender uncus strongly bent anteriorly; valva with broadly elongate cucullus; aedeagus with short, spine-like cornutus.
Material examined1 male, Haderi, Gapcheon, Heongseung, Gangwon Prov., 37° 31′ N 128° 11′ E, 26 ii 2012, leg.KW Lee, gen. slide no. HL19-33/Park.
DistributionKorea (new record), China (Southwest, Taiwan), Japan, N. India, also known in New Guinea.
Meganephria retineaGyulai and Ronkay, 1999 (Figs. 3,10, 14)
Meganephria retineaGyulai and Ronkay, 1999.Esperian,Band.7: 706, Figs. 16, 17, 20, 28-29. TL: Mongolia.
Systematic accounts: Family Noctuidae; Subfamily Amphipyrini; Tribe Psaphidini.
Diagnosis(Fig. 3). The species is similar toM. tancreiGraeser, 1889 which is known in Korea, China (Northeast)and Russian Far East, but it can be distinguished by the darker ground color of the forewing with f iner, more reticulate scaling, crosslines more sinuous, the subterminal line with stronger dark def inition; the male genitalia with valva shorter, narrower, with f iner, less straight harpe, the extension of the right sacculus stronger, larger, the basal cornuti in vesical smaller, the large bulbed cornuti more conical(Gyulai and Ronkay 1999). Wingspan 49 mm.

Figs. 13-14 Larvae feeding on hosts. 13. Zanclognatha querciella sp.n., host is Oriental Cherry; 14. Meganephria retinea Gyulai and Ronkay, 1999, host is Ulmus davidiana var. japonica (Rehder). ( Scale bar 2.0 mm)
Male genitalia(Fig. 1 0). Uncus long, directed downward. Valva elongated, with sclerotized band along costa;process of sacculus long, about 2/3 length of uncus.Aedeagus as long as valva, nearly straight; cornuti consists of more than four conic spines.
Material examined1 male, Haderi, Gapcheon, Heongseung, Gangwon Prov. emerged on 4 xi 2017, gen. slide no. HL19-14/Park.
Larva(Fig. 14). Head pale yellowish brown with blackish transverse line posteriorly; abdomen yellowish brown with a dark-brown central stripe on dorsal surface.Larvae have 4 pairs of abdominal prolegs from third to sixth segments (A3-A6); A8 dorsal strongly humped (Lee 2016, 2017, 2019). The body length of mature larva is 38-40 mm.
BiologyA larva was collected from leaves ofUlmus davidiana planchvar.japonica(Rehder) Nakai on 7 v 2017(HERI Breeding Number: 17-Noc-31). The larva was reared,feeding on leaves of the host plant under room temperature in a specially designed glass cage (22 × 21 cm). It pupated on 2 June, and emerged on 4 xi 2017.
Distribution Korea(new record), NE. China, Mongolia.
Amphipyra subriguaBremer and Grey, 1853 (Figs. 4,11, 12)
Amphipyra subriguaBremer and Grey, 1853.Schemett.N. China: 17. TL: China.
Amphipyra subrigua: Sugi, 1982, Part 1: 770, Part 2: 371.
Systematic accounts: Family Noctuidae; Subfamily Amphipyrini; Tribe Amphipyrini.
Diagnosis(Fig. 4). The species is one of the smallest of the genus with wingspan 36-38 mm. The male genitalia are characterized by the slender uncus expanded apically and phallus with an apical needle-like spine.
Male genitalia(Fig. 11). Uncus long, strongly bent downward from near base, slightly dilated apically, with a minute spine at apex. Valva slightly expanded medially, with sclerotized band to 2/3 length of costa; sacculus broadly developed; saccus V-shaped. Aedeagus stout, shorter than valva, nearly straight; dorsal margin with narrowly produced apical process; cornuti consists of three small sclerites near apex and a large sac containing three short plates.
Female genitalia(Fig. 12). Antrum narrow, membranous. Ductus bursae broad medially; ductus seminalis narrow, arising from middle, connecting to corpus bursae with narrowed anterior part. Corpus bursae short; signum absent.
Material examined1 male, 1 female, Gomyeong-ri, Jaecheon, Chungbug Prov., 18 vi 2016, leg. KW Lee, gen. slide no. HL19-22/Park (female), HL19-23/Park (male).
Biology(Fig. 14). Last instar of larvae were collected fromBuxus microphyllavar.koreanaNakai on 20 iv 2016 and pupated before taking photos of the larvae. They emerged 18 vi 2016.
DistributionKorea (new record), China, Japan.
Discussion
To date, except for major pests, there is little information on the host plants of Noctuoidea. The hosts ofZanclognathaLederer, 1857 are mainly fresh leaves ofCryptomeria,Tsuga,Pinus,Abies(Z. griselda); fresh leaves ofAbies f irmaSieboldetZuccarini (Z. lilacina); dead leaves of dicotyledonous plants (Z. lunalis,Z. yakushimalis,Z. yaeyamalis,Z. tarsipennalis,Z. subgriselda,Z. sugii); fresh leaves of grass,Festuca arundinaceaSchreber, Gramineae, andCarex incisaBoott andCarexspp., Cyperaceae (Kogi, 1985) (Z.fumosa,Z. oblique) (Owada 1987). TheMeganephiraHübner, [1821]1816:M. bimaculosaonUlmus minorMiller,Prunus spinosaL.,P. domesticaL.,Amygdalus persicaL.,Cerasus,Crataegus,Pyrus,Quercus;M. kononenkoiandM. tancreionQuercus mongolicaFischerexLedebour andQ. dentateThunberg (Kononenko 2016). In this paper, the f irst host (Ulmus davidianavar.japonica(Rehder) Nakai)ofMeganephria retineaGyulai and Ronkay, 1999 in Korea was identif ied;Quercussp. and oriental cherry are hosts to the new speciesZ. querciellaHan and Lee, sp.n., thirteen species of the genusZanclognathaare known in the Korean Peninsula (Kononenko and Han 2007). A new species was found by raising larvae onQuercussp. and oriental cherry in 2016.
AsotaHübner, [1819] is an Oriental genus belonging to the subfamily Aganainae of Erebidae which comprises more than 100 species worldwide (Zahiri et al. 2012). The subfamily Aganainae has often been treated as the Family Hypsidae (Inoue 1982) or the subfamily Aganainae of Noctuidae, but it has now been widely accepted as a subfamily of Erebidae (Fibiger and Lafontaine 2005; Zahiri et al.2012; Bayarsaikhan et al. 2016). The genus is widely distributed in Southern and South-East Asia, including India,Thailand, Cambodia, China and Japan. The genusAsotahas been introduced for the f irst time from Korea byA. egens(Walker, 1854). There is a possibility that the species temporary migrated from overseas.
In the Korean Peninsula, six species belong to the genusMeganephriaHübner, [1821]1816, and eight species ofAmphipyraOchsenheimer, 1816 are known (Kononenko et al. 1998; Kononenko and Han 2007). Two species of Noctuidae were found:Meganephria retineaGyulai and Ronkay,1999 andAmphipyra subriguaBremer and Grey, 1853 are reported for the f irst time from Korea during this biodiversity investigation.
Publisher’s NoteSpringer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affi liations.
杂志排行
Journal of Forestry Research的其它文章
- Sacred groves of India: repositories of a rich heritage and tools for biodiversity conservation
- Relationship between H 2 O 2 accumulation and NO synthesis during osmotic stress: promoted somatic embryogenesis of Fraxinus mandshurica
- Changes in leaf stomatal traits of diff erent aged temperate forest stands
- Somatic embryogenesis and plant regeneration in Betula platyphalla
- Hydrogen peroxide as a systemic messenger in the photosynthetic induction of mulberry leaves
- Production and quality of eucalyptus mini-cuttings using kaolin-based particle f ilms
