Diversity of mycorrhizal fungi and soil indicative species in coastal plantations of northeast Brazil
2021-04-30OlmarBallerWeberMariatiaBarrosodaSilvaCristianeFigueiradaSilvaDivaCorreiaDeborahdosSantosGarrutiMarcelaClaudiaPagano
Olmar Baller Weber · Maria Cátia Barroso da Silva · Cristiane Figueira da Silva ·Diva Correia · Deborah dos Santos Garruti · Marcela Claudia Pagano
Abstract The aim of this work was to evaluate arbuscular mycorrhizal (AM) fungi as soil indicators and the mycorrhization of native and exotic tree species planted in the Acaraú basin, a transition area from the coast to the Brazilian semiarid region. Plots with 6-year-old trees of four native and three non-native species as well as one non-forested area were evaluated in terms of the diversity of AM fungi in the mycorrhizosphere and the root colonization by AM and ectomycorrhizal (EcM) fungi. Twenty-four AM fungi were identif ied; Claroideoglomus etunicatum, Glomus sinuosum, Paraglomus albidum, Acaulospora laevis, and Acaulospora brasiliensis were abundant in the forest soil. Diversity, dominance, evenness and richness indices of AM fungi were higher in plots with native trees. All root samples were colonized by AM fungi and only Anadenanthera colubrina,Acacia mangium, Casuarina equisetifolia and Eucalyptus urophylla formed associations with EcM fungi. Acaulospora morphotypes served as soil indicators for coverings with the native species Astronium fraxinifolium and Colubrina glandulosa. Exotic species may favor the proliferation of rarer AM fungi. These fungi-plant relationships may be important in the management of forest systems, and the evidence with mycorrhizal associations allows the inclusion of Brazilian species in tropical reforestation.
Keywords Acacia mangium · Arbuscular mycorrhizal fungi · Brazilian species · Casuarina equisetifolia ·Ectomycorrhizal fungi · Eucalyptus urophylla
Introduction
Tropical species are an important agribusiness in Brazil(Moreira et al. 2017) with large areas withEucalyptusspp. (5.7 million ha),Pinusspp. (1.6 million ha) and other species (0.59 million ha) for the production of timber and cellulose (IBÁ 2017), in addition to vast natural forests which contain a great diversity of plants and tree species (Beech et al. 2 017). Plantations are mainly concentrated in the Atlantic Forest and Cerrado biomes (IBÁ 2017). An intensif ciation of forestry activities would be desirable in vulnerable semiarid areas which are found in nine Brazilian states and cover more than 10% of the country. While globally dry forests occupy the largest expanses of land (Bastin et al. 2017), they are very vulnerable to degradation. In the Brazilian semiarid region, there is anthropogenic pressure to convert forested areas into productive agro-systems(Silva et al. 2018). On coastal plateaus with sandy soils which are highly vulnerable to degradation (Mota and Valadares 2011), more conservative practices should be adopted in agricultural and aff orestation systems. The planting of species such as the pink lapacho (Handroanthus impetiginosus(Mart. ex DC.) Mattos (Fonseca Filho et al. 2017), vilca (Anadenanthera colubrina(Vell.) Brenan (Monteiro et al. 2006) and other tree species (Silva et al. 2018) are recommended, given the specif ic objectives of the plantations (Chazdon et al. 2016).
At a regional level in semi-arid areas, there are some reforestation initiatives in Bahia (IBÁ 2017), although there is little information about forest management and the impact of these initiatives on soils. Forests aff ect soil properties (Aiala-Orozco et al. 2018), particularly biological components (Chandra et al. 2016) as edaphic organisms inf luence biogeochemical cycles of essential plant nutrients.To improve the understanding of the multi-functionality of agroforestry systems, symbioses with mycorrhizal fungi have been identif ied as vital (Smith and Read 2008), particularly on sites where crop productivity is limited by available phosphorus (Liu et al. 2018; Pedone-Bonf im et al. 2018).
Arbuscular mycorrhizal (AM) fungi are biotrophic symbionts associated with approximately 70% of vascular plants,while ectomycorrhizal (EcM) fungi form symbioses with forest species (Brundrett and Tedersoo 2018). Both AM and EcM types of symbiosis are associated withEucalyptusspp.(Mello et al. 2006; Chen et al. 2007; Campos et al. 2011),Acaciaspp. (Aggangan et al. 2010),Casuarinaspp. (Diagne et al. 2013) and other genera and families of trees (Brundrett and Tedersoo 2018). AM symbioses are related the types of plant species in the Atlantic Forest (Zangaro et al. 2013) and the Caatinga vegetation types (Pagano et al. 2013; Souza and Freitas 2017; Pedone-Bonf im et al. 2018; Sousa et al.2018). However, the diversity of fungi and symbioses of AM in plantations in the region, especially on arenosols or sandy-textured soils of the coastal tablelands is unknown.
The objective of this study was to evaluate the spore communities of AM fungi as indicators of forest soil fertility, and to determine the intensity of root colonization by AM and EcM fungi with native and exotic tree species planted in the Acaraú basin, a transition zone between the coast and semiarid region. We hypothesized that tree species naturally form symbiosis with AM fungal species that diff er in sporulation, resulting in variations in the occurrence and frequency of propagules detected in the rhizosphere which allows for the detection of AM fungi indicative of diff erent vegetative covers. However, some forest species also form EcM associations which may interfere with other plant-mycorrhizal interactions in tropical coastal forest ecosystems.
Material and methods
Plantation sites
This study was carried out in plantations on the coastal basin of Acaraú, a transition area from the coastal dune vegetation to the semi-arid region in Ceará, Brazil(3°6′638″S, 40°4′4.66″W). The climate is classif ied as Aw(tropical, with a dry winter) (Alvares et al. 2014), in which annual precipitation is almost 900 mm, with the most intense rainfall between February and June, and relative humidity 70%; evaporation can be as high as 1600 mm annually. The soil was classif ied as Dystrophic Sandy Gray Argisol and the upper horizons indicate a sandy texture with pH 5.5 and low levels of nutrients per kg (0.5 m molcK; 15.1 m molcCa;6.3 m mol c Mg) (Weber et al. 2019) in addition to the low P content (9.12 mg kg −1 ). Sandy Argisols are common on the coastal tablelands of the northeast region (Bezerra et al.2015), are vulnerable to degradation (Mota and Valadares 2011), but they can be used for agriculture with conservation practices, including reforestation.
In plantations on the Acaraú basin (3.6 ha), plots were selected and planted with 6-year-old trees of four native species: (1)Anadenanthera colubrina(Vellozo) Brenan (vilca),(2)Astronium fraxinifoliumSchott. Ex Spreng. (kingwood),(3)Handroanthus impetiginosus(Mart. ex DC) Mattos (pink lapacho), (4)Colubrina glandulosaPerkins (sobrasil). An additional three plots were planted with exotic or introduced species: (5)Acacia mangiumWilld., (6)Casuarina equisetifoliaL., and (7)Eucalyptus urophyllaS. T. Blake (clone GG 702). There was one non-forested plot. In the 270 m 2 plots,there were 28-30 trees with a 3-m spacing between rows and 2 m between plants in the same row. Fertilization at the base of the seedlings was carried at the time of planting with 120 gof NPK (10:28:20) and 30 g per plant of fritted trace elements BR 12 (3.2% S, 1.8% B, 0.8% Cu, 2.0% Mn,0.1% Mo, 9% Zn). At 6 months old, the seedlings were given an NPK supplement (50 g plant −1 ) to promote establishment. Herbaceous vegetation was left on the plots and in the rainy season, a grass cover from the generaPaspalumandPanicum(Poaceae) were present on the non-forested area. In areas protected by tree canopies, herbaceous species of to the generaCommelina(Commelinaceae),Momordica(Cucurbitaceae),Emilia(Asteraceae), among others, were common. This herbaceous stratum was similar to ones in areas of natural vegetation, and was left in place to mitigate the impact of rainfall maintain soil properties.
Soil and root samples
Field sampling took place in four periods, August and November 2016, February and May 2017. From each plot,four composite samples (one per period) were collected,each consisting of 12-15 samples taken from the soil up to 10 cm deep, keeping a minimum 1-m distance from the base of the trees. The samples were passed through a 2-mm sieve to remove roots, larger organic fragments, and mineral aggregates. The sieved samples were kept under refrigeration at 4 °C until analysis.
Fine roots were collected from the f ield from six to eight trees in each plot. For this collection, roots were followed from the base of the trunks to avoid collecting roots from invasive species. The roots were rinsed under tap water,separated, and placed in containers with a 60% commercial alcohol and 5% acetic acid solution to maintain root integrity.
Density of spores and identif ication of soil arbuscular mycorrhizal fungi
The spores of the mycorrhizal fungi were extracted from 50 g of soil using the wet-sieving and decanting technique,followed by centrifugation at 2000 RPM with spore suspension in 50% sucrose gradient (Brundrett et al. 1996). Spores were examined under a stereomicroscope (50× to 200×) and separated according to color, size, shape and the characteristics of their surface (Sieverding 1991). Morphological characteristics were available on the INVAM (International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi) located at https://invam.wvu.edu/ and descriptions of these species. Spores from various fungus morphotypes were placed on glass slides using PVLG (polyvinyl alcohollactic acid-glycerol) and PVLG + melzer reagent as f ixatives in order to facilitate identif ication. This enabled calculation of the relative abundance (percentage of spores from fungal morphotypes in samples) as well as the relative frequency(percentage of samples from diff erent covers in which fungal morphotypes were present).
Diversity of arbuscular mycorrhizal fungi in soil and the mycorrhizal colonization of trees
Morphotypes and the relative abundance (P i ) of AM fungi enabled Shannon and Weiner’s indexes of diversity (H′),Simpson dominance (Si), evenness (J′) and richness (Ri) to be calculated, accordingto Mirzaei and Moradi ( 2017) and Magurran ( 2004). The formulae used were: H′ = − ∑(Piln Pi), Si = 1 − ∑(Pi2); J′ = H′lnS −1 and Ri = S(N −1/2 ), where N represents the number of spores and S the number of AM fungi per 50 g of soil.
Root colonization was evaluated after small roots had been placed in a 10% KOH solution (Phillips and Hayman 1970) and stained with blue aniline in lactoglycerol (875 mL of lactic acid, 63 mL of glycerin, 0.5 g of dye and 62 mL of distilled water). Segments of 1-mm thick stained roots were evaluated by the grid line intersect method according to Giovannetti and Mosse ( 1980) and Brundrett et al. ( 1996).Approximately 60 cm of f ine roots were examined under a stereomicroscope at 200×; the presence of hyphae, vesicles and other internal root structures characteristic of AM fungi were conf irmed under an optical microscope up to 400×. In other stained roots, morphological changes were noted by the presence of mantle and formation of root tips typical of ectomycorrhizae (Brundrett et al. 1996).
Analysis of mycorrhizal attributes
To obtain a better understanding of the mycorrhizal attributes under examination, an agglomerative hierarchical clustering analysis (AHC) was carried out according to the dissimilarities among the vegetation covers. The means of mycorrhizal attributes from the four samples were used to evaluate the vegetative cover. An abnormal distribution of the data (not shown) was observed, and therefore the Spearman dissimilarity matrix was used as an agglomeration technique of unweighted pair-groups (Unweighted Pair-Group Average, UPGMA), with centralized and reduced data. Principal component analysis (PCA) used was Spearman′s, enabling the identif ication of atypical attributes through average values from the soil analysis, and the separation of vegetation cover among the principal components. Calculations and graphics were generated by the XLSTAT © program version 2016.1 (Addinsoft Inc., Brooklyn, NY, USA).
An analysis of AM fungi species indicators (IndVal)of the soil was carried out (Dufrêne and Leandre 1997) to detect possible linkages between soil fungi and vegetation cover. IndVal values were measured, as was the signif icance(P) in the Monte Carlo test. Indival values ≥ 0.25 (Silva et al.2017) andP≤ 0.1 values were adopted for the indication of AM fungi in vegetation cover.
Results
Twenty-four species of AM fungi distributed over seven families and 10 genera were identif ied (Table 1). The diversity was slightly higher in areas with native species: 17 morphotypes withA. fraxinifolium, 18 withA. colubrinaandC.glandulosa, and 20 withH. impetiginosus, compared to areas with exotic species (15 morphotypes withC. equisetifoliaand 16 withA. mangiumandE. urophylla) or the unforested area (16 fungal morphotypes). These morphotypes were related to AM fungi and some new species could not be excluded. For some soil samples, it was diffi cult to separate the fungal spores, especially among the generaAcaulosporaandGlomusbased on their color, shape, size, surface and presence of self-sustaining hyphae. However, the description of fungal populations up to the level of genus may be suffi cient for the rapid monitoring of soil AM fungi. In a complementary way, multiphasic analysis may be considered when specif ic fungus-plant relationships are assessed.
In this study, a variation in the relative abundance of AM fungi was evident throughout the diff erent vegetation covers(Table 1), as well as the relative frequency of the morphotypes in the topsoil. In terms of frequency, the followingwere prominent:Claroideoglomus etunicatum>Aculospora laevis=Acaulospora brasiliensis>Paraglomus albidum>Acaulosporasp.3 >Glomus sinuosum>Scutellospora calospora.Rare fungi related toA. excavatawere observed in the plot planted withE. urophylla, andGlomus aff . fasciculatumwas detected in the soil under theC. glandulosaandA. colubrinacanopies.

Table 1 Relative abundance and frequency (Freq) of groups of arbuscular mycorrhizal (AM) fungi in soil under forests and non-forested areas
Diff erent spore populations and ecological indices of AM fungi were detected among the diff erent vegetation covers(Table 2), as well as variations in the intensity of root colonization by AM and EcM fungi. Higher values of H′ (1.94)and Si (0.81) occurred in plots withA. fraxinifoliumandC.Glandulosa(H′ = 1.92; Si = 0.83), while these indices were particularly low in the area planted withC. equisetifolia(H′ = 1.41; Si = 0.65), an exotic species that substantially reduced AM fungi sporulation in the root zone. Root colonization by AM fungi was evident in all species (Table 3),in particular by the presence of fungal mycelium as well as other fungal structures (vesicles and arbuscules) found in the f ine roots. Howewer, the intensity of colonization was higher in the roots of native species, reaching 57% inH. impetiginosus.The AM symbiosis may help to explain the adaptation and performance of native species to edaphic and climatic conditions in the study area. In turn, the colonization of rootsby EcM fungi was greater in exotic species, up to 29.9% inE. urophylla. Additionally, reproductive structures typical of EcM fungi or fruiting bodies were also noted under canopies ofA. colubrinaand exotic species.
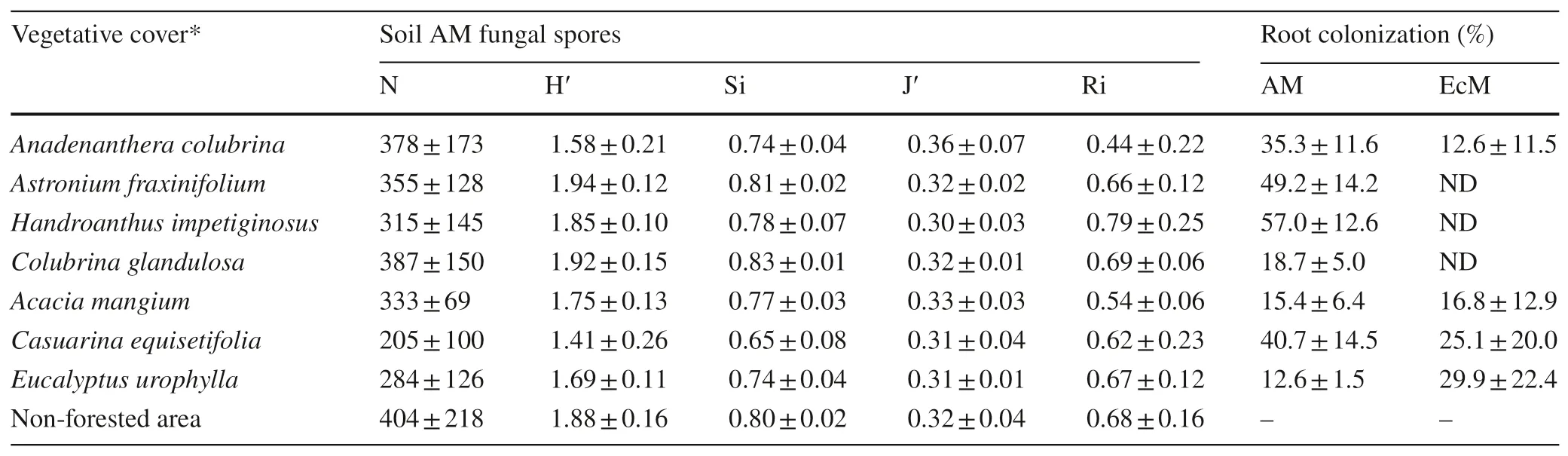
Table 2 Mean values with standard deviations of spore populations (N) and ecological indices of arbuscular mycorrhizal (AM) fungi and root colonization by AM and ectomycorrhizal (EcM) fungi in forested and non-forested areas

Table 3 Spearman’s correlation matrix between the mycorrhizal attributes assessed in forested and non-forested (NF) areas
The ecological indices of AM fungi and the levels of AM and EcM colonization (Table 2) made it possible to carry out hierarchical cluster analysis (HCA) (Fig. 1) and principal component analysis (PCA) (Fig. 2). Three groups were formed, species in cluster 2 were the most similar (node level 0.268),A. colubrina(native) andA. mangium, both belonging to theFabaceae(Mimosoideae) family,and able to establish AM and EcM symbioses in addition to nitrogen-f ixing bacteria in root nodules (not measured). Cluster 1 group ( node level 0.357) includedE. urophylla(Myrtaceae)andC. equisetifolia(Casuarinaceae) (Fig. 1), which showed high levels of root colonization by EcM soil fungi. Cluster 3 species (node level 0.415) includedA. fraxinifoliumandH. impetiginosusandC. glandulosa,which were similar to the non-forested area.
In Fig. 2, the f irst two PCA components represent 80.8%of the information and can explain most of the total variance, showing a distinction between plantations, forming groups like the clusters obtained in the HCA. The F1 component separated the native species except forA. colubrinathat was colonized by EcM fungi, from the exotic species,placing them on the right side of the graph. There was no clear diff erence between the non-forested site and plots withC. glandulosa,H. impetiginosusandA. fraxinifolium(cluster 3), particularly in relation to the population of fungal spores (N) and the H′ and Si indices. The F2 component separated the plantations regarding J′ versus R i with species from cluster 2 (A. colubrinaandAcacia mangium),showing higher values for J′ and lower values for Rithan species from cluster 1 (E. urophyllaandC. equisetifolia).Root colonization by AM fungi did not contribute signif icantly to diff erentiate the plots.
There was a positive correlation between AM spore populations and the value Si, the index of which was also correlated with H′ (Table 3). In turn, richness of AM fungi was inversely correlated with the evenness of the soil fungi. A signif icant inverse correlation also ocurred between the indices H′ and Si in AM fungi and radicular colonization by EcM soil fungi. This last plant-mycorrhizal ratio was undetected in three of four forest species;some nutritional competition in symbioses with trees should not be disregarded.
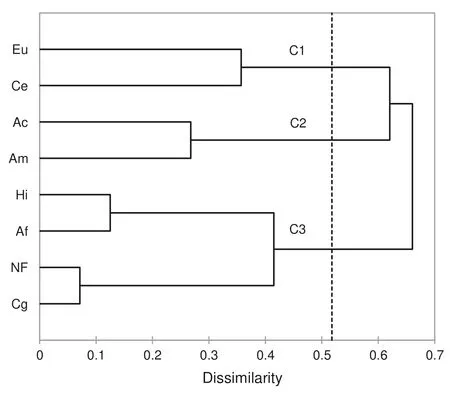
Fig. 1 Dendrogram constructed by UPGMA using mean values of arbuscular mycorrhizal and ectomycorrhizal attributes assessed in forested and non-forested areas; plantations separated by the cluster C1 [Eu ( Eucalyptus urophylla), and Ce ( Casuarina equisetifolia)];cluster C2 [Ac ( Anadenanthera colubrina), and Am ( Acacia mangium)]; and cluster C3 [Hi ( Handroanthus impetiginosus), Af ( Astronium fraxinifolium), Cg ( Colubrina glandulosa), and a non-forested(NF) area]

Fig. 2 Principal component analysis using the mean values of arbuscular mycorrhizal and ectomycorrhizal attributes in forested and nonforested areas. Plantations with native species: Ac ( Anadenanthera colubrina), Af ( Astronium fraxinifolium), Hi ( Handroanthus impetiginosus), Cg ( Colubrina glandulosa); and exotic species: Am ( Acacia mangium), Ce ( Casuarina equisetifolia) and Eu ( Eucalyptus urophylla); N (fungal spores 50 −1 g soil), Ri (Richness), J′ (Evenness), Si(Simpson index), and H′ (Shannon-Wiener index) for AM fungi; root colonization by AM and ectomycorrhizal (EcM) fungi
The relationship between soil AM fungi and vegetation cover was conf irmed (Table 4). Values of IndVal (> 0.4) with high probability (P< 0.05) were found for fungi related toAcaulosporasp1 andAcaulosporasp2 on sites planted withC. glandulosaandA. fraxinifolium. In the cover with native trees, other morphotypes of the generaGlomus, ClaroideoglomusandAmbisporafrequently occurred, although they did not reach the desired level of signif icance (P< 0.1). A relationship betweenE. urophyllaandG. margaritawas also identif ied (Table 4), a combination which should be considered in future studies. In addition to the aff orestation areas, spores related toS. calosporaandR. intraradiceswere representative for the control area covered withPaspalumandPanicum.
Discussion
This study showed a high diversity of AM fungi in the topsoil under a plantation can be associated with root diversity of the mycotrophic plants and improvement in the physical and chemical properties of the soil. Soil attributes, diversity of roots and environmental factors aff ect the proliferation of fungal spores and subsequent diversity of AM fungi (Mirzaei and Moradi 2017; Brundrett and Tedersoo 2018).
Some of the spore morphotypes detected could not be related to the fungal species described to this point. They were related to the generaGlomusandAcaulospora. It should be noted that in tropical forest systems new species of AM fungi can occur. Thus, the identif ication of the fungal spores up to the level of the genus can be considered,especially for monitoring forest systems, similarly to that carried out in natural environments of the semiarid region(Pagano et al. 2013; Zangaro et al. 2013; Retama-Ortiz et al.2017; Souza and Freitas 2017; Sousa et al. 2018). However, to characterize fungus-plant relationships in a better way, molecular analyses of the symbionts can be used(Dawkins and Esiobu; 2017; Sheldrake et al. 2017; Toju and Sato 2018) as they are complementary to the morphological descriptors used in the monitoring of AM fungal communities.
Fungal spores such asClaroideoglomus, Acaulospora, Paraglomus,GlomusandScutellosporawere frequent within forest covers. The speciesC. etunicatumis common in semiarid regions and usually included in artif icial inoculation tests, as positive responses of these plants to symbiosis have been observed (Pedone-Bonf im et al. 2018). Other fungal species with high frequencies in the soil under forest cover (Table 1) have already been detected in the Caatinga environments, apart fromParaglomus(=Glomus)albidum(Sousa et al. 2018) andAcaulospora(=Ambispora)brasiliensis. The occurrence ofA. brasiliensishas been reported in agroforestry systems of the Atlantic Forest and Cerrado biomes, as well asP. albidumin forest succession ecosystems in the Amazon region (Winagraski et al. 2019).
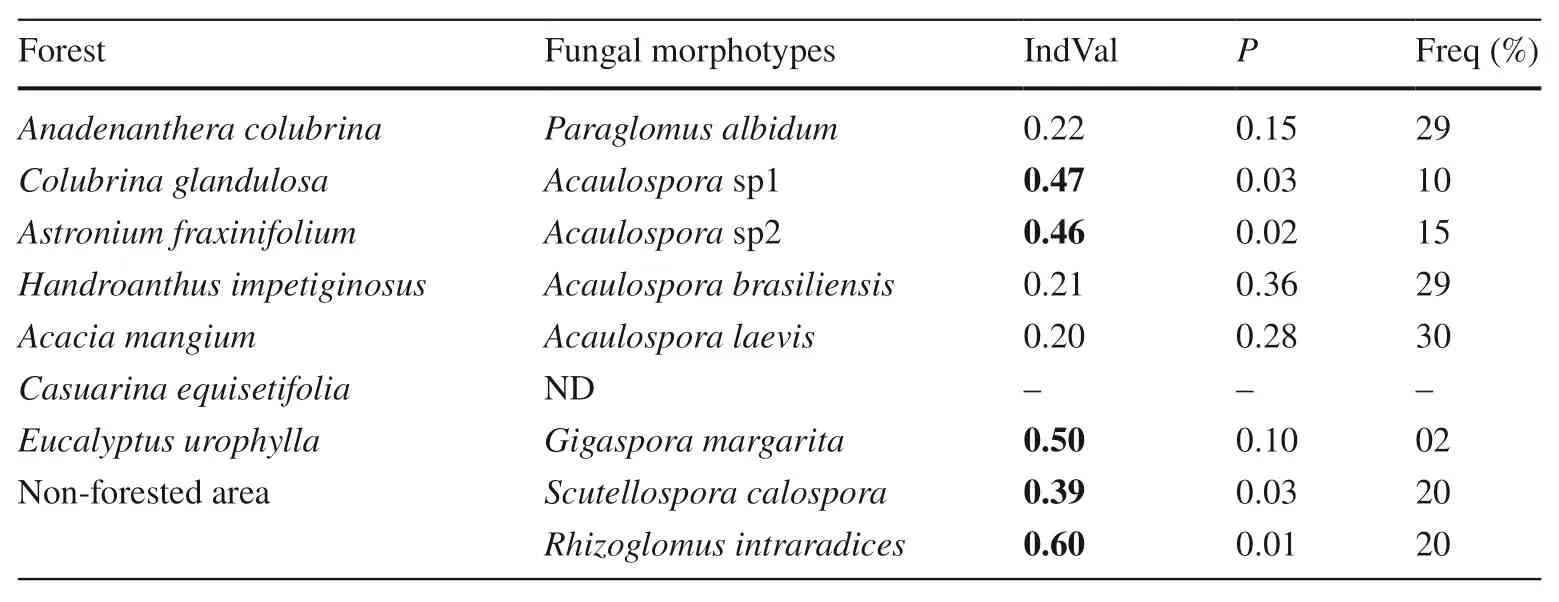
Table 4 IndVal values of arbuscular mycorrhizal (AM)fungi in soil under forested and non-forested areas
Equally important were the rare fungi associated withA.excavataandG. margarita, detected in areas planted withA. mangiumandE. urophylla, respectively, in addition toG. fasciculatumdetected within two forest covers (C. glandulosaandA. colubrina). This may be related to litter type and the consequent increase of soil carbon as detected in a forest fragment of Bahia State by Pereira et al. ( 2018).Microenvironmental factors also aff ect the proliferation of spores in the rhizosphere. It is recognized that AM fungi are not specif ic plant hosts but are responsive to the environmental conditions where the host grows (Winagraski et al.2019), so diff erent tree species could benef it from native mycorrhizal associations. Camara et al. ( 2017) observed the benef icial response ofC. glandulosaseedlings to inoculation with mixtures ofRhizoglomus clarum,G. margaritaandDentiscutata heterogamain an organo-mineral substrate.Pedone-Bonf im et al. ( 2013) also found that seedlings ofA.colubrinaresponded positively to inoculation with a mixture ofGigaspora albidaandA. longulain soil with low availabilty ofP(up to 15 mg dm −3 soil).
The AM fungal community status as observed through the ecological indeces (Table 2) could explain the adaptability of the Brazilian species to the soil conditions of this study. Souza and Freitas ( 2017) also observed higher indices of diversity and greater richness of AM fungal species in the root zones of nativeMimosa tenuif lorathan in the rhizosperic soil of exotic species such asProsopis julif lora. However, the exotic species also showed root colonization by EcM fungi in addition to native trees ofA. colubrina. EcM symbiosis is common inCasuarina(Diagne et al. 2013),Eucalyptus(Campos et al. 2011),Acacia(Aggangan et al.2010), and other families and genera of tree species (Brundrett and Tedersoo 2018), suggesting the possibility of the management of mycorrhizal associations in plantations in the region.
The ecological indices of soil AM fungi and the intensities of root colonization by AM and EcM fungi allowed the formation of three clusters:A. colubrinaandA. mangium;E. urophyllaandC. equisetifolia; and other vegetation covers including the non-forested area. Distinctions between tree covers could also be made by PCA analysis (Fig. 2).Trees ofH. impetiginosusshowed AM fungal richness and high intensity of root colonization by AM fungi, grouped withC. glandulosaandA. fraxinifolium,presenting high populations of fungal spores and high values of Simpson and Shannon-Wiener indices. Data from the clusters may be suitable for management of symbioses in any new reforestation program in the region.
Some of the mycorrhizal variants have positive correlations (Simpson dominance and Shannon and Weiner’s diversity or population of soil AM fungal spores), and others negative correlations (colonization by EcM fungi and Simpson or Shannon and Weiner’s indices). There is possibly a nutritional competition in the mycorrhization of tree species. According to Liu et al. ( 2018), plants colonized by AM and EcM fungi share sources ofPin the soil (dissolved phosphate, simple phosphate monoesters, and inositol phosphate) and both tree symbioses favor the coexistence of plant species.
The relation between two native species (C. glandulosaandA. fraxinifolium),andAcaulosporacould be, along withGlomus, environmental indicators in coastal ecosystem(restinga) vegetation in northeast Brazil (Silva et al. 2017).Otherwise,G. margarita, which showed a low frequency of spores, has been reported in forest fragments cultivated with the introducedE. urophyllain Bahia State (Santos et al.2013), and inEucalyptusplantations in southern Brazil(Mello et al. 2006). In addition to the three AM morphotypes, indicative of forest covers, we detected relationships betweenS. calosporaandR. intraradicesin the non-forested area covered in the wet season withPaspalumandPanicum.The two fungal species were equally common in the sandy,unfertile soils in the coastal ecosystems in Cuba (González et al. 2016), and they could be considered for the management of pastureland in the region.
To guarantee high diversity of AM fungi in the topsoil under a plantation, mycotrophic native species such asA.colubrina, H. impetiginosusandA. fraxinifoliumcan be introduced. The f irst also associates with EcM and potencially with rhizobia, which can result in a more resilient plant cover.
Conclusions
Plantations of native Brazilian species favor the maintenance of the diversity of AM fungi in sandy Argisol of tropical coastal regions. Moreover, in these ecosystems, exotic species ofA. mangium,E. urophyllaandC. equisetifoliaform symbiotic relationships with AM and EcM fungi. The most frequent AM fungi in plantations ofA. colubrina, A. fraxinifolium, H. impetiginosus, C. glandulosa, A. mangium, C.equisetifolia, E. urophyllaareAcaulospora,Claroideoglomus, Paraglomus,andScutellospora. On one hand, morphotypes of fungi related toAcaulosporaserved as indicators of the covers with nativeA. fraxinifoliumandC. glandulosa.On the other hand,Gigasporawas an indicator of tree covers withEucalyptus, andRhizoglomusandScutellosporawere indicators of the non-forested area.
The fungus-plant relationships may be considered in the management of forest systems, and the evidence from mycorrhizal associations reinforce the inclusion of the Brazilian species in reforestation programs for tropical regions. In addition, the selection will depend on the desired economic and environmental benef its. Evidence of mycorrhizal associations may lead to improvements in plant growth as well as benef its for nutrient cycling.
Publisher’s NoteSpringer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affi liations.
杂志排行
Journal of Forestry Research的其它文章
- Sacred groves of India: repositories of a rich heritage and tools for biodiversity conservation
- Relationship between H 2 O 2 accumulation and NO synthesis during osmotic stress: promoted somatic embryogenesis of Fraxinus mandshurica
- Changes in leaf stomatal traits of diff erent aged temperate forest stands
- Somatic embryogenesis and plant regeneration in Betula platyphalla
- Hydrogen peroxide as a systemic messenger in the photosynthetic induction of mulberry leaves
- Production and quality of eucalyptus mini-cuttings using kaolin-based particle f ilms
