Geographical distribution of Aralia elata characteristics correlated with topography and forest structure in Heilongjiang and Jilin Provinces, Northeast China
2021-04-30HongxuWeiGuoshuangChenXinChenHengtianZhao
Hongxu Wei · Guoshuang Chen · Xin Chen ·Hengtian Zhao
Abstract The Araliaceae family consists of numerous species of medical plants of signif icant value as non-wood forest products. To conserve and culture these plants in natural forest stands is an important undertaking which should be implemented according to the relationship between forest structure and understory population. In this study, thirtyf ive plots were established in natural Aralia elata stands.Taller individual and denser populations were found in the northern and in the eastern regions, respectively. Both population densities and individual leaf weight increased along longitude. In contrast, their relationship with elevation and DBH were negative. Along with the altitude gradient, both height and root-collar diameter increased but population density declined. Root-collar diameter and population density decreased with latitude and DBH, respectively. Overall,dominant trees unlikely enforced strong disturbance to the development of understory A. elata populations unless concerning some specif ic topographic factors.
Keywords Aralia elata · Chinese angelica-tree · Stand quality · Geographical distribution · Undergrowth · Nontimber forest species
Introduction
Non-wood forest products (NWFPs) are those goods other than timber from wooded lands (FAO 1999) and provide unique commercial and recreational values (FAO 1998).Due to the demand for the sustainable utilization of forest resources, NWFPs in European, Asian, Mediterranean and Scandinavian countries have received considerable attention for their incorporation into forest management regimes (Calama et al. 2010; Abraham et al. 2015) Numerous NWFPs come from understory plants, mushrooms and animals (Abraham et al. 2015). Worldwide, the total number of understory fauna and f lora that may have some commercial value has been estimated to be 4000-6000 species(Nasi and Cunningham 2001). The majority of recorded taxa can be classif ied into at least one category with signif icant economic value and potential use (Abraham et al. 2015).To model the specif ic distribution of NWFPs species is important for the management of natural forest resources(Yang et al. 2006).
Araliaceae is a large family of 50 genera and over 1400 species. TheAraliagenus contains more than 70 species,several of which are highly valued for medicinal use. Therefore, the large-scale harvest of Araliaceae is increasing globally from natural forests (Schmidt et al. 2019). In some regions, the ban on the collection of Araliaceae plants from natural forests is a result of degraded understories and has restricted natural product supplies. This created a promotion of rehabilitation by culturing new resources which rely on natural shade and available soil (Nadeau and Olivier 2003;Wan et al. 2015; Liu et al. 2016; Wei et al. 2019). Therefore,forest structure as determined by dominant trees at various topographies is a main concern for the success of raising Araliaceae species.
Araliaceae species require specif ic habitats of cool climates and well-drained soils (Schmidt et al. 2019). Forests in these regions show parallel responses of overstory growth and understory development to regional environment (Gilliam 2007; Wan et al. 2015). The distribution of dominant trees is primarily controlled by the local climate but the distribution of understory Araliaceae species is shaped by competition from neighbours (Hara et al. 1995 ; Wang et al.2006; Wixted and McGraw 2010). In natural old-growth forests, dominant tree density was insuffi cient to suppress Araliaceae. (Hara et al. 1995). Canopy gaps determined the germination, growth, and distribution of Araliaceae species which may be explained by the need for shade (Yamamoto and Nishimura 1999; Wagner and McGraw 2013; Chandler 2017; Chandler et al. 2017) Further information about the Effects of dominant trees on Araliaceae species is scarce.
The Chinese angelica-tree,Aralia elata(Miq.) Seem,belongs to the Araliaceae family and is a thorny, dwarf shrub of temperate forests (Goto et al. 1996; Moore et al.2009; Wei et al. 2019).A. elatahas dually edible and medical usages that are widely accepted in northern China, the Russian Far East, Japan, and Korea (Gao et al. 2019; Wei et al. 2019; Yu et al. 2019). The resource is highly utilized due to abundant anti-oxidant bioactive compounds that may be extracted from its buds (Wang et al. 2018), leaves(Sun et al. 2017), and roots (Luo et al. 2015). NaturalA.elatavegetation has suff ered severe pressure of over exploitation, and there is an urgency to reserve and rehabilitate their populations. To determine the relationship between forest structure and characteristics ofA. elatapopulations,requires adequate knowledge to succeed in conservation of these natural forests.A. elatacan germinate from buried seeds to avoid wildf ires (Goto et al. 1996; Higo et al. 1995)and avian frugivores (Moore et al. 2009). The growth and physiology of this species are shade intolerant (Gao et al.2019) and therefore, presence is frequently on ridges or the upper parts of slopes above 9° (Goto et al. 1996; Wei et al.2019). However, these f indings are insuffi cient to explain the response of natural populations to forest structure, hence more relevant information is needed, for example, information on current distribution ofA. elatapopulations, and on the relationship between their characteristics, topography and dominant trees.
In this study, thirty-f ive naturalA. elatapopulations were investigated in six forest types in Northeast China. Measurements were taken presence-only plots of stem density and individual growth of populations. The distribution of these variables were mapped and their relationship with the structure of dominant trees and topographical factors were determined. It was hypothesized that: (1) cooler habitats in northern regions and at higher elevations would result in higherA. elatastem-density; and, that (2) better growth of individuals would be found in forests with taller dominant trees with less canopy shade.
Materials and methods
Study area
This study was conducted in montane regions in eastern Jilin and Heilongjiang provinces in Northeast China (41°52ʹ-48° 12ʹ N, 126° 31ʹ-133° 50ʹ E). According to the IUCN Red List, there is no natural distribution ofA. elatain Inner Mongolia (Botanic Gardens Conservation International and IUCN SSC Global Tree Specialist Group 2018),and no investigation was carried out there. Because the focus was on naturalA. elatapopulations in forests, regions with natural but disturbed stands were also excluded. The study area covered 0.29 million km 2 , characterized by mountains with elevations from 122 to 950 m and slopes up to 20°. Soils were dominated by Acnsols, Alisols, Plinthosols, Gleysols, Histosols, and Fluvisols (World Reference Base 2019). In the eastern mountains of Heilongjiang and Jilin provinces, mean annual temperatures vary between− 4.7 and 10.7 °C with average annual precipitation of 866 mm. Relative humidity is about 65% annually.
Study sites
NaturalA. elatapopulations were investigated in all local forest types within the study area from mid-July to mid-August 2018. Stands that were selected had the following characteristics: (1)A. elatadominated the understory shrub layer in a density > 200 stems per hectare; (2) there no obvious disturbances to the stands and toA. elatapopulations;(3) allA. elataindividuals were at least 30 cm in height;and, (4) stands were dominated by timber trees. Stands which have been clear-cut and dominated by secondary coppice were excluded. Seedling nurseries with beds of uniformA. elatastock were also excluded from site selection. Eventually, thirty-f ive sites were chosen for study in six forest types (Fig. 1). These forests were often dominated by a single species, such asAbies nephrolepis,Betula platyphylla,Larix olgensis,Pinus koraiensis, orPinus sylvestris(Table 1). Dominant trees were those accounting for more than 70% of the total basal area (Wang et al. 2006).In deciduous broadleaf forests, dominant species includedBetula platyphylla,Fraxinus mandshurica,Populus davidiana,Quercus mongolica, andTilia amurensis. Some sites that were dominated byPinus koraiensis, other species includedAbies nephrolepis,Acer palmatum,Betula platyphylla,Pinus syluestriformis, andLarix gmelinii.
Field investigation
On each site, a 20 m × 20 m plot was established in an area of 400 m 2 and the latitude, longitude, slope angle, and elevation of the plot center recorded. Height and root-collar diameter(RCD) of allA. elataplants in the plot were measured, and the total number recorded to determine stand density. On some sites, some individuals had regenerated by vegetative resprouting (Goto et al. 1996). Because the transformation of rachillae to stems results in the highly partial growth of new shoots (Charlton 2009), branching and sprouting are common forA. elataplants. When branching occurred underground, the two sprouts aboveground were recorded as two individuals. When branching occurred 3-cm above the ground, the entire sprout was recorded as one plant. To measure leaf weight per-piece, four leaves were randomly collected in the middle of every shoot of an individual and all leaves were oven dried at 68 °C for 3 days and measured for dry weight.
All timber species in a plot ≥ 4 cm DBH at 1.3 m (DBH)were included as part of the canopy (Hara et al. 1995).Height was recorded as a vertical canopy layer for each treeaccording to the distance from the ground to the top of the crown. Canopy area (CA) was calculated by the oval model(Rodriguez-Trejo et al. 2003) and canopy density (CD)was averaged by three estimates along the longest length of canopy.

Fig. 1 Distribution of study sites in Northeast China
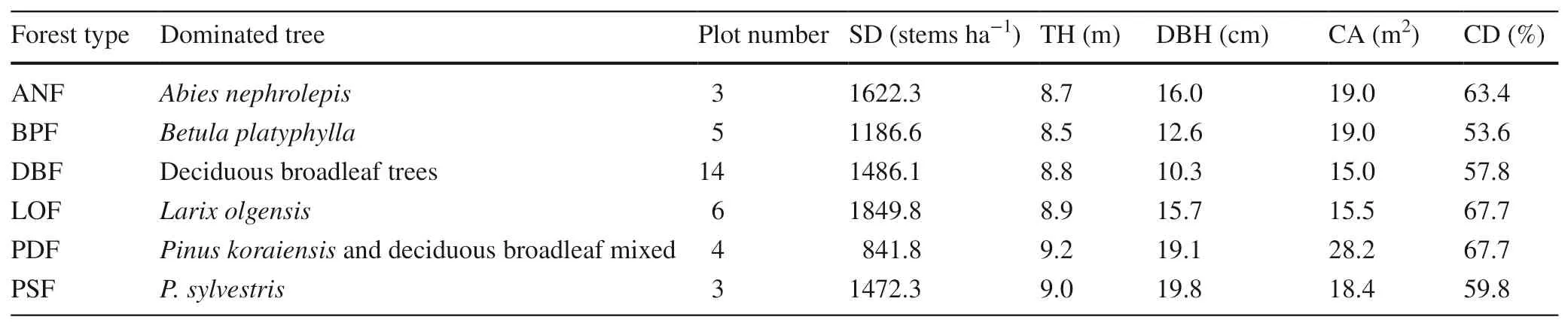
Table 1 Stand characteristics of forest types harboring natural Aralia elata populations
Statistical analysis
All plot data on dominant trees andA. elatapopulations were mapped using ArcGIS software V. 9.3 (Esri China Information Technology Inc., Beijing, China). Statistical analyses were carried out using SAS software (ver. 9.4 64-bit, SAS Institute, Gary, NC, USA). Data were tested for normal distribution and log-transformation made when necessary. All normally distributed data were tested by one-way analysis of variance (ANOVA) to detect diff erences among the six forest types. When a signif icant Effect was identif ied by ANOVA atP= 0.05, the means were compared and arranged according to the Duncan test (α= 0.05) regarding the non-uniform sizes of replicates. Pearson correlation was conducted to detect the linear relationship between paired variables. Finally, a stepwise regression was employed to detect the multiple contributions of the independent variables of topography and dominant trees to traits ofA. elatapopulations.
Results
Distribution of variables of dominant tree population structure
Dominant tree heights were greater in the southern part of the study area than in the north (Fig. 2 a). Plots in the northern and eastern parts had tree heights < 7 m. Along the latitude gradient, DBH was highest in the southern part, declined in the middle, and increased again to about 14-cm in the northern part (Fig. 2 b). Stand density was< 900 stems ha −1 in the southern part, increased to over 2000 stems ha −1 in the middle regions, and declined again in the north (Fig. 2 c). Canopy area was higher around the center of the study area and lower around the edges(Fig. 2 d). Canopy density had a similar range in most parts of the study area, but the edge plots in the north part had levels less than 40% (Fig. 2 e).
Distribution of variables of Aralia elata populations
Height ofA. elatawas higher along the latitude gradient across the middle of the study area but lower on the extreme western and eastern edges (Fig. 3 a). Root-collar diameter was about 0.8 cm in the south but much lower in the northern areas(Fig. 3 b). Stand density was less than 1500 stems ha −1 in the plots near the edges of the area in the south and northern parts(Fig. 3 c). Leaf weight was lowest at 50 mg per piece in the middle part of the study area (Fig. 3 d).
Diff erences among forest types
No dominant tree population trait was statistically diff erent among the six forest types (Table 1). Stand density of dominant trees was between 840 and 1890 stems per hectare.Height and RCD were approximately 8.85 m and 15.6 cm,respectively. Dominant trees also had average canopy areas of 19.14 m 2 and 61.7% density. The average height ofA. elatapopulations was higher in stands dominated byAbies nephrolepisthan in others except for deciduous broadleaf species(Fig. 4).A. elataheights in forests dominated byBetula platyphyllawas higher than in stands dominated byPinus koraiensisand deciduous broadleaf mixed trees andPinus sylvestrisby 22.5% and 23.1%, respectively.
Linear correaltionships
Positive correlation between latitude and longitude suggests the orientation of plots from the northeast to the southwest(Table 2). Negative correlation between elevation and both latitude and longitude indicates that areas in the southwest had higher elevations and areas in the northeast had lower elevations.
Height and DBH of dominant species were negatively correlated with latitude and longitude, respectively, but both were positively correlated with elevation (Table 2). In addition, tree height also had a positive correlation with slope but its relationship with SD was negative.
Both height and RCD ofA. elatapopulations had positive correlations with elevation while RCD had a negative regression with latitude (Table 2).A. elataheight and RCD had positive regression with each other. Leaf weight was positively correlated with longitude. Plant density ofA. elataindividuals was positively correlated with latitude and longitude but negatively with elevation and DBH.
Stepwise regression
Both longitude and log-transformed crown area contributed positively toA. elatapopulation heights, while elevation was negative(Table 3). Tree height and elevation had contrasting contributions toA. elataheight. Latitude and longitude had negative and positive contributions to RCD and leaf weight, respectively.

Fig. 2 Spatial distribution of height ( a), diameter at breast height ( b), stem density ( c), canopy area ( d), and canopy density ( e) of dominant trees of forests with natural Aralia elata populations
Discussion
Dominant tree height and A. elata height growth

Fig. 3 Spatial distribution of height ( a), root-collar diameter ( b), stand density ( c), and leaf weight per piece ( d) of A. elata

Fig. 4 Height of A. elata in natural populations in forest types dominated by diff erent tree species. Letters indicate signif icant diff erences according to Duncan’s test at 0.05 level. ANF, Abies nephrolepis forest; BPF, Betula platyphylla forest; DBF, Deciduous broadleaf forest; LOF, Larix olgensis forest; PDF, Pinus koraiensis and deciduous broadleaf mixed forest; PSF, Pinus sylvestris forest
A. elataheight was higher inAbiesforests than inPinusforests.Abiesforests were found at elevations of 350-650 m;Pinusforests were generally located at lower elevations below 350 m. There was also a positive relationship betweenA. elataheight and elevation. Therefore, high altitudeAbiesforests have tallerA. elataplants than lower altitudePinusforests. Previous studies also indicated thatAbiesforest understory was richer in diversity and greater in density compared to thePinusforest (Abella et al. 2012;Canales et al. 2018). The denser understory population under theAbiescanopy with its more diverse species can stimulate height growth ofA. elata(Cornett et al. 1998).Dominant tree height increased with elevation as well, but tree height had a negative Effect on height growth ofA.elata.Therefore, dominant trees had increasingly competing pressure on the height growth ofA. elataalong the elevation gradient. However, the estimated coeffi cient from stepwise regression indicated that this negative impact was too weak to aff ectA. elataheights on ridge tops.
Slope had no Effect onA. elataheight. However, this is in contrast to a recent study whereA. elataheights declined on a 14° slope compared to heights on 5° and 9° slopes on plots in the central area of this study (Wei et al. 2019). Our results may be more reliable because our plots were more evenly distributed. We found a positive relationship between slope and dominant tree height and therefore the negative contribution of tree height along the elevation gradient was irrelevant.

Table 2 Pearson correlation between abiotic factors and traits of Aralia elata populations
A. elata population related to canopy
The density ofA. elatapopulations ranged from 200 to 7400 stems ha −1 with an average of 2000 stems ha −1 . Stand density was lower than that forA. elataseedlings in mountains in northern Japan in the 1990s which was estimated to be 100,000 stems ha −1 (Goto et al. 1996). However, stand density in this study was close to that ofA. elatasprouts in f ields in Japan (Goto et al. 1996). Based on these comparisons,A. elataseedlings in our plots possibly resulted from sprouts than seedings. Continuous protection of forests in Northeast China has resulted in increasing number of reddeer (Cervus elaphusxanthopygus Milne-Edwards) and their foraging may have contributed to the increase resproutingA. elata(Feng et al. 2018).

Table 3 Estimates of variables from stepwise regression of parameters on topography and forest structure with understory Aralia elata populations
There was a negative relationship of DBH of dominant trees and density ofA. elatapopulations. These results partly concur to those of Vleut et al. ( 2013) where balsa tree (Ochroma pyramidale[Cav. Ex Lam.] Urb.) secondary forest had higher DBH-based basal area than diverse secondary forests, but shrub density of the understory showed the opposite relationship. However, compared to these two forest types, rain forests had higher levels in both basal area and shrub density (Vleut et al. 2013). In contrast, research on European temperate forests showed no relationship between DBH and understory shrub density (Adam et al. 2013). In this study, DBH was positively related with tree height and these two parameters indicated the dominance of trees to utilize site resources. Therefore, a greater DBH suggests an ability of dominant trees to utilize local resources for growth. This results in limited resources to recruit newA.elataindividuals. Future research is required to determine soil water and nutrient availability to conf irm the mechanism of this relationship.
Stepwise regression also indicates that along the longitudinal gradient, the elevation declined but the canopy area of dominant trees signif icantly contributed toA. elatapopulation density. Little information is available on the Effect of canopy shade onA. elatadensity in the literature. From previous studies,A. elatacan adapt to heavy shade during the ramet stage (Higo et al. 1995; Goto et al. 1996)Seeds can become buried deep in the soil during a forest f ire and regenerate as seedlings when exposed to the sunlight (Higo et al. 1995). Other studies on another species ofAralia,A. nudicaulis, may provide references to explainA. elata(Landhausser et al. 1997; Whitman et al. 1998; Zenner and Berger 2008). As a shade-obligate species,A. nudicaulishad higher rates of net assimilation and gas exchange under low light conditions (Landhausser et al. 1997).A. nudicauliscan also adapt to canopy removal but also to sprouts as well(Whitman et al. 1998; Zenner and Berger 2008). Because natural populations were selected,A. elatahad fully become acustomized to the canopy hade through natural selection.Therefore, a larger canopy tended to shade a larger area which was suitable for seedings, generating high density.
Age-impact on the relationship between height and altitude
For understory seedlings and saplings, annual height growth results from the extension of current year shoots based on the previous year’s buds. As height is a response to gradient changes of altitude-related abiotic factors (Aiba and Kitayama 1999), height may be impacted in years of extreme climates. However, shoot growth ofA. elatahas a diff erent pattern along an altitude gradient as reported in this study because of the protection from climate extremes by the overstory canopy. Annual growth ofA. elatais mainly by the elongation of lateral branches in the f irst 5 to 7 years,without apparent growth in height (Fig. S1). Thereafter,stems start to elongate in older individuals, and height growth results from not only the annual transformation of apical buds to new shoots but also from the growth of lateral branches as well (Fig. S1). Although some extreme climatic events occurred at some point during growth, they likely did not impactA. elataheight as height of seedlings depend on the length of stem. In addition,A. elatais not a perennial species like other understory NWFP species (An et al. 2018).Perennial plants will depend heavily on the age of roots to produce sprouts every year, which may be impacted by annual climate. In contrast, height growth ofA. elatawould not be aff ected by age of the plant along the altitude gradient as are other Araliaceae family members (Guo et al. 2019).
Diameter growth of A. elata along latitude
Root-collar diameter declined along the latitude gradient from south to north. Our results conf irm those reported for other woody species (Repo et al. 2000). Lower temperatures and shorter growing seasons contributed to the decline in RCD growth in northern forests. Although the linear correlation indicated a positive relationship between RCD and elevation, the Effect of elevation was eliminated by stepwise regression. Therefore, RCD growth in naturalA. elatawas not responsive to topographic changes (Wei et al. 2019).Gao et al. ( 2019) found that stem diameter ofA. elataseedlings signif icantly responded to shade but no relationship was found with canopy-related variables to RCD in this study. Variations in shade within natural forests of this study unlikely had any Effect on diameter growth inA. elata.
With regards to increasing height ofA. elataalong the elevation gradient, RCD increased as well with decreasing stand density. These results reveal the feature ofA. elatapopulations on ridges as strong but sparse individuals.Because dominant trees also had stronger features at higher elevations, it might be speculated thatA. elataindividuals competed to become overstory trees through strengthening individual growth at the cost of population density.
Leaf weight in A. elata along longitude
Leaves are important plant organs and leaf dry weight has ecological signif icance. Leaf weight is the accumulation of photosynthetic products, and the increasing investment in dry weight would ref lect enlarging area to fulf ill photosynthetic ability (Huang et al. 2019). Increases in leaf weight would promote a series of issues of transpiration, respiration,and large systems of venation (Huang et al. 2019).
In this study, the leaf weight ofA. elatawas greater in the eastern than in the western regions, unrelated to forest structure or topography. Wei et al. ( 2019) also reported that leaf weight ofA. elatadid not respond to slopes. Therefore,we believe that environmental factors may have inf luenced the geographical distribution of leaf weights. Compared to the western part of our study area, the east had less annual rainfall (Guo et al. 2017). It is unlikely that leaf weight can increase with lower available water (Rezaei et al. 2019;Yin et al. 2019). In addition, longitude had a negative relationship with elevation which ranged from 120 to 950 m.Therefore, it is reasonable to suggest that higher leaf weights in eastern plots may be due to less exposure to the stress of ultraviolet radiation. There is evidence that leaf weight in woody plants can be decreased by ultraviolet radiation by impairing photosynthesis (Verdaguer et al. 2012). Future research is required to detect the possible Effect of ultraviolet radiation on weight and other leaf parameters ofA. elata.
Conclusions
In this study, 35 plots of natural stands withA.elatapopulations were investigated for structure of dominant forest trees, topography, and characteristics of theA. elatapopulations. Diameter at breast height of dominant trees is the factor limitingA. elatapopulation density, which can be improved by canopy area in eastern plots at lower elevations.Dominant tree heights had a negative impact on the height ofA. elataalong the elevation gradient. Therefore, dominant trees unlikely had any inf luence on the development of understoryA. elatapopulations. Several of the results of this study cannot be substantiated in the literature and therefore much of our discussion has been by analyzing and explaining. Controlled experiments are essentially needed in future to detect the possible mechanism that drove the formation of results about relationships in this study.
Acknowledgements The authors acknowledge the assistances of Dan Wang, Wenbo Zhang, Feng Zhu, Peiyuan Chen, and Zhibin Ren in f ield investigation. Numerous individuals are also appreciated for their assistance in f ield investigation and data collection.
Publisher’s NoteSpringer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affi liations.
杂志排行
Journal of Forestry Research的其它文章
- Sacred groves of India: repositories of a rich heritage and tools for biodiversity conservation
- Relationship between H 2 O 2 accumulation and NO synthesis during osmotic stress: promoted somatic embryogenesis of Fraxinus mandshurica
- Changes in leaf stomatal traits of diff erent aged temperate forest stands
- Somatic embryogenesis and plant regeneration in Betula platyphalla
- Hydrogen peroxide as a systemic messenger in the photosynthetic induction of mulberry leaves
- Production and quality of eucalyptus mini-cuttings using kaolin-based particle f ilms
