固相烧结法制备锂离子电池正极材料Li2FeP2O7及其电化学性能研究
2021-04-17王任衡孙一翎范姝婷郑俊超钱正芳贺振江
王任衡,肖 哲,李 艳,孙一翎,范姝婷,郑俊超,钱正芳,贺振江
(1.深圳大学物理与光电工程学院,深圳518060;2.中南大学冶金与环境学院,长沙410083)
1 Introduction
With the demand for high energy storage batteries for portable electronic devices and electric vehicles,lithium-ion battery as a high energy density,long cycle life,environmentally friendly,and high open circuit voltage energy storage device,has received numerous attentions[1—3]. Since the energy density and rate capacity of lithium ion battery are limited,finding a suitable cathode material is the most immediate task[4—6]. In 1997,it was first reported that olivine structure lithium iron phosphate(LiFePO4)can be used as a cathode material for lithium ion batteries[7],which had been considered as high-energy power batteries in many studies[8—14]. However,many problems are still exposed,such as the bad electronic conductivity and poor cycling performance. In 2010,a new pyrophosphate structural material,Li2FeP2O7,was reported[15]. The specific capacity of Li2FeP2O7reached 110 mA·h·g-1and the discharge platform was 3.5 V,which had the highest potential among all the reported phosphate series materials[16—19]. Compared with LiFePO4,Li2FeP2O7can basically achieve the theoretical capacity without carbon coating and nano-modification,and showed high specific capacity and good chemical stability as a cathode material[20—22].
In general,the sintered temperature of Li2FeP2O7is required to be least 550 ℃to reduce impurities produced during the synthetic process. Researchers mainly improved the electrochemical performance of Li2FeP2O7from the following aspects. Firstly,the specific surface area of the Li2FeP2O7can be increased by carbon-coating,which can improve the migration rate of lithium ions[23—27]. Various methods for the synthesis of carbon-coated Li2FeP2O7,such as solid states reaction,sol-gel method,spatter combustion and spray pyrolysis,have been reported. Secondly,the Li2FeP2O7material was doped with ions to improve the intrinsic conductivity[28,29]. Common doping methods included cationic(V,Mo,Al,Ni,Sn),anionic(Cl,F),anion and cation co-doping,and the like. Finally,Li2FeP2O7can be synthesized from different kinds of lithium sources and organic carbon sources[30].
The freeze-drying method uses the principle of the sublimation of ice crystals. In a highly vacuum environment,the frozen water in the material is directly lifted from ice into water vapor. The freeze-drying method has obvious advantages because the water is directly sublimated at low temperature and low pressure[31]. The obtained material has light weight and small size after freeze-drying. Compared with other drying methods,the crystal size of the material is smaller,and its appearance and shape are preserved much better[32,33]. In the process of vacuum freeze-drying,the problem of surface hardening cannot occur and a porous sponge shape will be formed,which is conducive for the material to sinter. In addition,the oxidation reaction about the material is effectively suppressed due to the low temperature and vacuum environment.
In this paper,we chose CH3COOLi as the lithium source,and citric acid was added into the solution of CH3COOLi,Fe(NO3)3·9H2O,and NH4H2PO4as an organic carbon source. The Li2FeP2O7powder was obtainedviafreeze-drying,and then sintering at different temperatures. The results showed that the appropriate sintering temperature was 590 ℃at which Li2FeP2O7was obtained completely clean and evenly distributed. It was found that the Li2FeP2O7material exhibited outstanding specific capacity and large exchange current density.
2 Experimental
2.1 Reagents and Instruments
CH3COOLi(≥97%),Fe(NO3)3·9H2O(98%),NH4H2PO4(98%)and citric acid(C6H8O7·H2O,98%)were purchased from Sigma-Aldrich Co. ltd.(Shanghai,China).
Thermogravimetric analysis instrument(TGA,Q600 SDT,TA Instruments,New Castle,DE);X-ray diffractometer(XRD,Rint-2000,Rigaku,Japan);Fourier transform infrared spectrometer(FTIR,Nicolet Avatar 360,USA);transmission electron microscope(TEM,JEM-2100)and scanning electron microscope(SEM,JSM-7600F)(JEOL,Japan).
2.2 Synthesis of Li2FeP2O7 Material
The preparation process of Li2FeP2O7material is shown in Scheme 1. First,CH3COOLi,Fe(NO3)3·9H2O,NH4H2PO4and citric acid(C6H8O7·H2O)were weighed in a molar ratio of 2∶1∶2∶1. The above four reagents were separately dissolved in a certain amount of deionized water,and the concentration of citric acid was 0.05 mol/L. Then,the citric acid solution was stirred at a constant speed in a water bath(50 ℃),and Fe(NO3)3·9H2O solution and CH3COOLi solution were added slowly dropwise in turn. When the color of solution became yellowish brown. NH3·H2O was slowly instilled until the color of the solution turned light green,and 5% ethylene glycol was added to enhance the complexation. Finally,the NH4H2PO4solution was added into the mixed solution. The resultant solution was sonicated in an ultrasonic water bath at 50 ℃for 1 h,and then freeze-dried in a transfer freeze dryer for 8 h. The dry yellow-green powdery precursor was obtained after vacuuming for 40 h. The powder was sintered under an argon atmosphere for 8—16 h to generate black powdery Li2FeP2O7material.
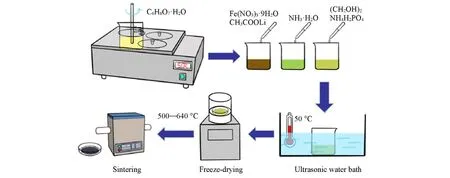
Scheme 1 Preparation process of Li2FeP2O7 material
2.3 Electrochemical and Physical Characterization
Thermogravimetric-differential scanning calorimetry(TG-DSC)analysis was used to explore the optimum synthesis temperature of Li2FeP2O7and test the change of phase. XRD and FTIR were used to characterize the structure and composition of Li2FeP2O7materials at different temperatures. The surface morphology of Li2FeP2O7electrode was detected by means of TEM and SEM. Electrochemical impedance spectra(EIS)were recorded on an electrochemical workstation(CHI660E,Chenhua,Shanghai),and the open-circuit voltages of the cells were set as the initial potentials. Cyclic voltammetry(CV)was detected at a sweep rate of 0.1 mV/s.
3 Results and Discussion
The TG-DSC curves of the freeze-dried precursor of Li2FeP2O7are shown in Fig.1(A). It can be seen that two obvious endothermic peaks appear at 100 and 200 ℃along with the continuous mass loss of material,which is caused by the loss of the water molecules contained in the precursor and some excess organic solvent. When the temperature reaches 250 ℃,a strong exothermic peak appears from Fe(NO3)3·9H2O decomposition. When the temperature exceeds 500 ℃,the mass of the sample is basically unchanged,which is mainly due to the stable phase formation,and the Li2FeP2O7formation process has been completed.

Fig.1 TG⁃DSC curves of the freeze⁃dried precursor of Li2FeP2O7(A) and XRD patterns(B) and FTIR spectra(C)of Li2FeP2O7 sintered at 500,550,590 and 640 ℃
In order to verify the results of TG-DSC,Li2FeP2O7that sintered at different temperatures(500,550,590 and 640 ℃)were detected by XRD,and the results are shown in Fig.1(B). When the temperature reaches 500 and 550 ℃,the main components detected in the XRD pattern are Li4P2O7and FePO4. When the temperature rises to 590 ℃,the main phase is Li2FeP2O7,and the peaks of Li4P2O7and FePO4are not detected,indicating that the obtained material sintered at this temperature is pure. When the temperature exceeds 640 ℃,LiFePO4is generated due to the occurrence of secondary reactions,which is not conducive to the synthesis of pure Li2FeP2O7. Therefore,the most suitable synthesis temperature is 590 ℃.
The samples sintered at different temperatures(500,550,590 and 640 ℃)were subjected to FTIR characterization to confirm the chemical bonds and functional groups of the Li2FeP2O7[Fig.1(C)]. In the FTIR spectra of Li2FeP2O7,the vibration absorption peaks are mainly distributed in the region of 400—1800 cm-1.The peaks of bending vibration modes of the typical O—P—O in the PO4structure locate at the positions of 499,568 and 638 cm-1. The absorption peaks at 746 and 941 cm-1belong to the antisymmetric and symmetric vibration of P—O—P,which is typical for pyrophosphate structure. The peaks at 1004,1118 and 1195 cm-1correspond to the stretching vibration mode of the P—O bond in PO4. In particular,the peak at 1195 cm-1corresponds to the stretching vibration of,which is the most direct evidence of the existence ofComparing the infrared spectra of the samples sintered at different temperatures,it can be found that the samples sintered at 590 ℃has less impurities and the peak ofis the most obvious among all the samples.
The SEM images of the Li2FeP2O7samples sintered at different temperatures are shown in Fig.2. It can be seen that a small amount of crystals appear at the temperature of 500 ℃,and the particle diameter is the smallest. If the particle diameter is too small,a series of serious agglomeration will occur,which is detrimental to the transport of lithium ions and electrons,resulting in poor electrochemical performance of the material.Along with the increases of temperature,the crystallinity of the sample particles improves gradually,and the secondary agglomeration causes the particle size to become larger. When the temperature reaches 590 ℃,the crystallinity of the material achieves the most suitable degree. The surface of the large particle crystal is smooth and regular,and the particle size is relatively uniform. Therefore,the characteristics of the Li2FeP2O7material particles synthesized at 590 ℃are the most suitable.
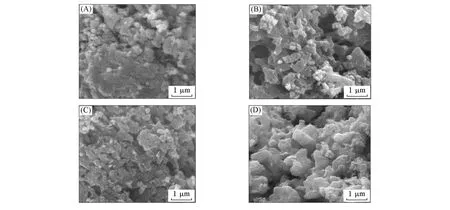
Fig.2 SEM images of Li2FeP2O7 sintered at temperatures of 500 ℃(A),550 ℃(B),590 ℃(C)and 640 ℃(D)
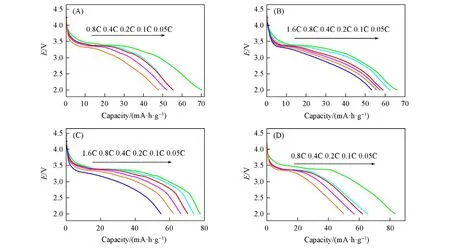
Fig.3 Discharge curves of rate performance of Li2FeP2O7 sintered at temperatures of 500 ℃(A),550 ℃(B),590 ℃(C)and 640 ℃(D)
To further investigate the electrochemical performance of Li2FeP2O7sintered at different temperatures(500,550,590 and 640 ℃),the first discharge curves of the cell at different current densities are shown in Fig.3. The discharge specific capacities of the cells with Li2FeP2O7material sintered at 500 ℃and the rate of 0.05C,0.1C,0.2C,0.4C and 0.8C between 2.0 V and 4.5 V are 70.2,55.7,56.3,52.4 and 47.8 mA·h·g−1,respectively[Fig.3(A)]. The corresponding capacities of cells with Li2FeP2O7materials synthesized at the temperature of 550 ℃are 66.7,62.4,59.2,57.6 and 55.8 mA·h·g−1[Fig.3(B)],respectively. The capacity measured at a high rate of 1.6C is 53.9 mA·h·g−1. The corresponding capacities of cells with Li2FeP2O7materials sintered at 590 ℃are 77.6,74.3,70.8,66.1 and 62.0 mA·h·g−1[Fig.3(C)],respectively. The capacity measured at a high rate of 1.6C is still 55.0 mA·h·g−1. The charging platform and the discharging platform represent the delithiation and intercalation lithium reaction,respectively. In point of the Li2FeP2O7material synthesized at 590 ℃,its discharge curves are stable,indicating that the Li2FeP2O7material can exhibit less polarization and improve the rate performance. But,the corresponding capacities of cells with Li2FeP2O7materials sintered at 640 ℃are 83.9,66.1,62.0,56.9 and 50.0 mA·h·g−1[Fig.3(D)],respectively. The above results show that the Li2FeP2O7material sintered at 590 ℃has the best electrochemical performance among all the samples.
Fig.4 is the EIS results of Li2FeP2O7sintered at different temperatures after charging and discharging. The alternate current(AC)impedance spectrum of each sample contains a semicircle and a diagonal line. The semicircle in the high frequency region corresponds to the charge transfer impedance inside the material[34,35].And the diagonal line in the low frequency region exhibits the diffusion property of the lithium ion,which is embedded in the active material of the electrode. It is obvious that the Li2FeP2O7material synthesized at 590 ℃ shows the steepest slope. The equivalent circuit diagram is shown as the inset in Fig.4,and theRlandRrvalues are listed in Table 1. The exchange current density of the Li2FeP2O7sample sintered at 590 ℃is as large as 3.00×10-4mA/cm2,indicating a low external current density required for an electrode to react. The small impedance helps to slow down the resistance and improve the cycle performance of the Li2FeP2O7material.
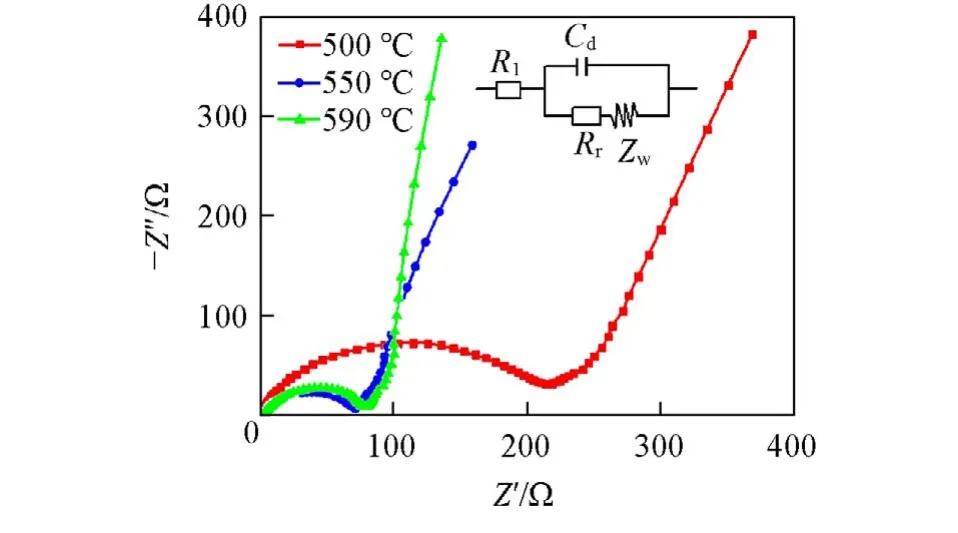
Fig.4 EIS of Li2FeP2O7 cathodes sintered at different temperatures
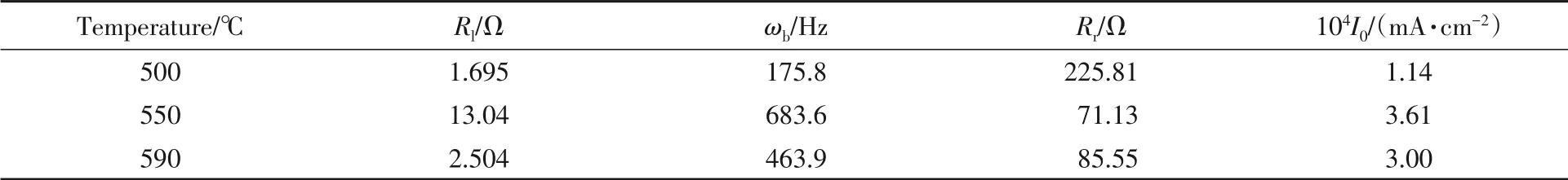
Table 1 Results of electrochemical impedance and exchange current density
In order to further study the electrochemical performance of Li2FeP2O7material,the Li2FeP2O7material sintered at 590 ℃was subjected to the CV test. The first CV curve’s range was set to 2.0—4.5 V at a scanning speed of 0.1 mV/s. It can be clearly seen from Fig.5 that the Li2FeP2O7sample contains two oxidation peaks and one reduction peak. Among them,the reduction peak at 3.32 V is produced by the intercalation of lithium ions. The two oxidation peaks at 3.64 and 3.81 V are related to the lithium ion extraction,which is connected with the oxidation process between Fe2+and Fe3+in the Li2FeP2O7material. In addition,the voltage difference between the oxidation peak and the reduction peak of the Li2FeP2O7sample is 0.49 V.
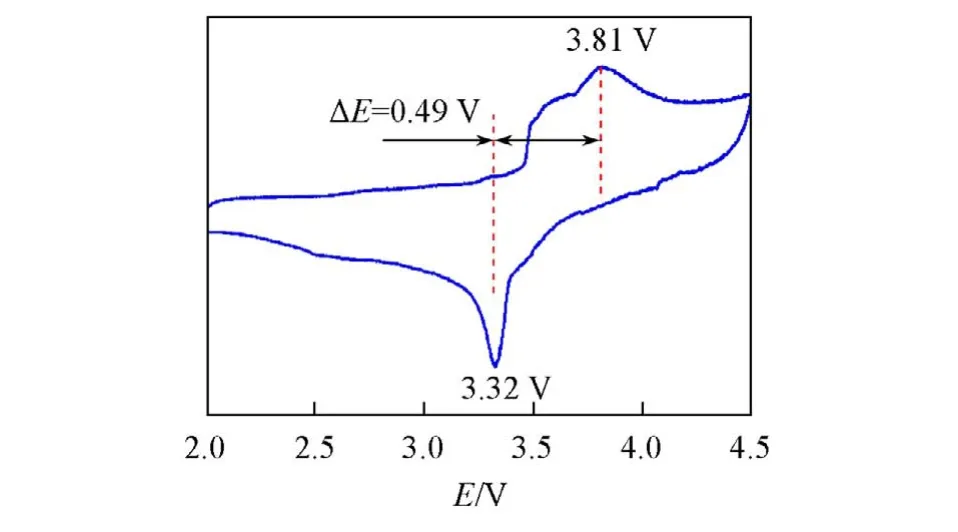
Fig.5 CV curves of Li2FeP2O7 synthesized at 590 ℃at a scan rate of 0.1 mV/s
The microstructure of Li2FeP2O7cathode synthesized at 590 ℃can be better understood by EDS mapping tests. The results show that the Li2FeP2O7material contains a large amount of C element in addition to the necessary elements Fe,P,and O(Fig.6). This points out a carbon coating on the surface of Li2FeP2O7material during the preparation process,which improves the electrochemical performance of the Li2FeP2O7material.
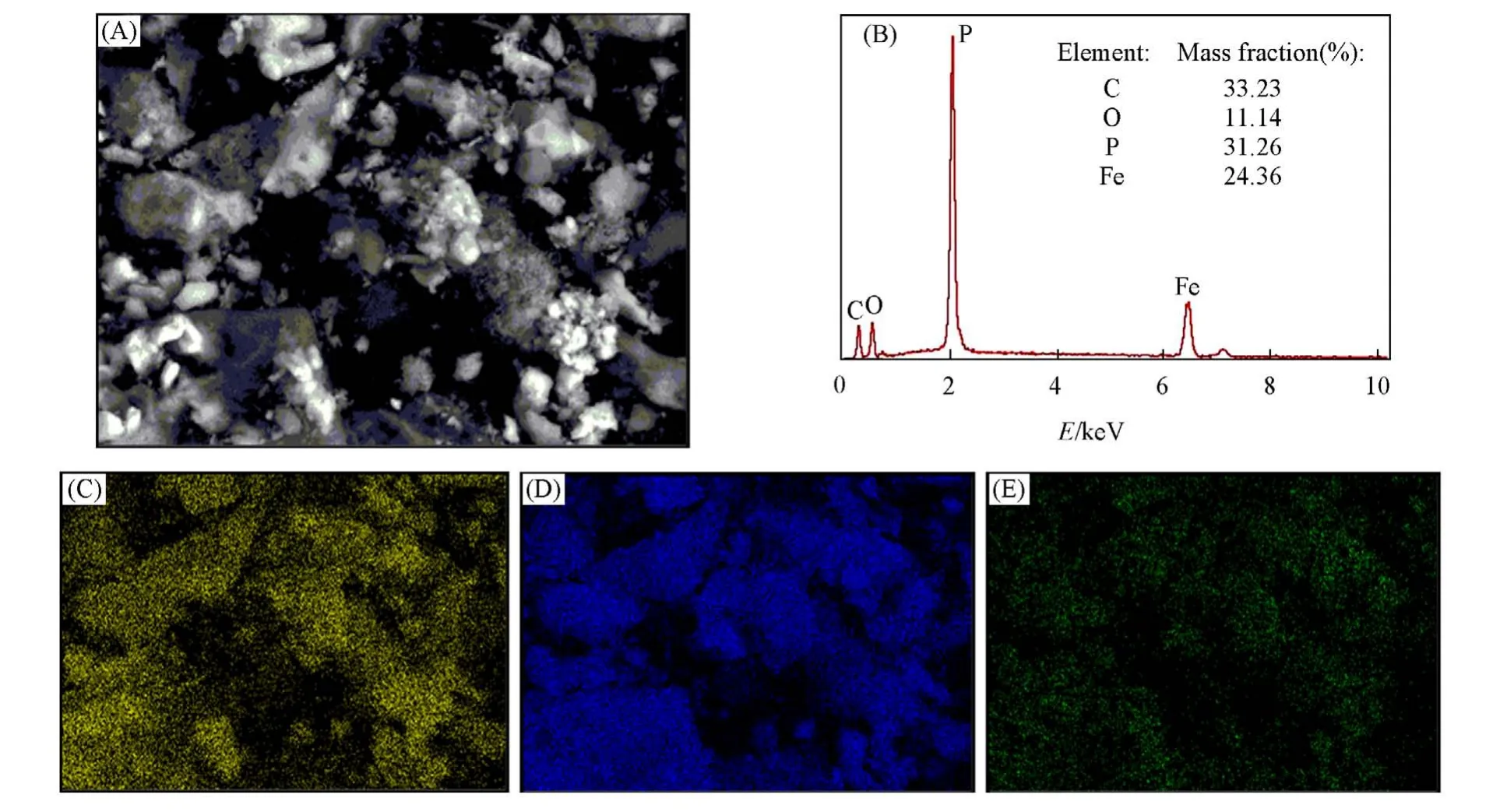
Fig.6 SEM image(A), EDS spectrum(B), and EDS elemental mapping of Fe(C), P(D) and O(E)of Li2FeP2O7 sintered at 590 ℃
In addition,it can be clearly seen from TEM image[Fig.7(A)]that there is a carbon-coated protective layer on the surface of the Li2FeP2O7material,which is helpful not only to maintain the stability of the Li2FeP2O7material during charge and discharge,but also to provide a highly conductive network,thus facilitating the deintercalation of lithium ions. As a result,the Li2FeP2O7material synthesized at 590 ℃contained minimal impurities and presented the highest discharge capacity.
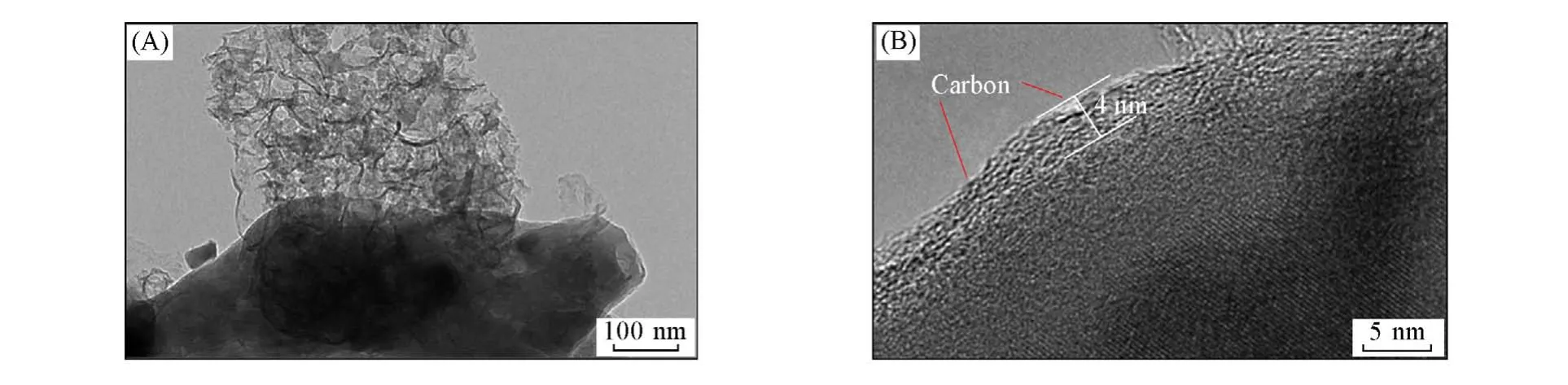
Fig.7 TEM images of the Li2FeP2O7 sintered at 590 ℃with low(A)and high(B)magnifications
4 Conclusions
In this paper,Li2FeP2O7was synthesized using CH3COOLi as lithium source,citric acid as complexing agent and organic carbon sourceviafreeze-drying and solid-state sintering method. The characterization results show that the Li2FeP2O7material synthesized at 590 ℃has the most uniform particle distribution and the smallest particle size. There is a carbon-coated protective layer on the surface of the Li2FeP2O7material,which is helpful to protect the crystal and provide a channel for lithium ion transport. The Li2FeP2O7material sintered at 590 ℃contains minimal impurities and presented the highest discharge capacity. The exchange current density of the Li2FeP2O7sintered at 590 ℃is large,indicating that the resistance of lithium ion transport is small.
This work is supported by the Science and Technology Innovation Commission of Shenzhen City,China(No.20180123 and JCYJ20190808173815205),the Guangdong Basic and Applied Basic Research Foundation of Guangdong Province,China(No. 2019A1515012111),the National Natural Science Foundation of China(No. 51804199),the Shenzhen Science and Technology Program,China(No.KQTD20180412181422399)and the National Key R&D Program of China(No.2019YFB2204500).
