Sinapic acid ameliorates D-galactosamine/lipopolysaccharideinduced fulminant hepatitis in rats: Role of nuclear factor erythroidrelated factor 2/heme oxygenase-1 pathways
2021-04-13MushtaqAhmadAnsariMohammadRaishYousefBinJardanAjazAhmadMudassarShahidSheikhFayazAhmadNazrulHaqMohammadRashidKhanSalehBakheet
Mushtaq Ahmad Ansari, Mohammad Raish, Yousef A Bin Jardan, Ajaz Ahmad, Mudassar Shahid, Sheikh Fayaz Ahmad, Nazrul Haq, Mohammad Rashid Khan, Saleh A Bakheet
Abstract
Key Words: Sinapic acid; D-galactosamine/lipopolysaccharide; Oxidative stress; Fulminant hepatitis; Antioxidant; Nuclear factor erythroid-related factor 2/heme oxygenase-1 pathways
INTRODUCTION
Naturally occurring phenolic compounds play a significant role in disease prevention and treatment[1]. Natural phenols from plants include phenolic acids, tannins,flavonoids, stilbenes, coumarins, curcuminoids, quinones, lignans, and others. Sinapic acid (SA) Hydroxycinnamic acids (HCAs) are a group of naturally occurring phenolic acid and highly abundant in the human diet, such as fruits, vegetables, and grains[2].Previous studies demonstrate that SA is a bioactive phenolic acid and has the potential to attenuate various chemically induced toxicities[3]. Fulminant hepatitis is a lifethreatening clinical syndrome that is associated with overwhelming inflammation,hepatic encephalopathy, liver injury, and eventually liver failure[4]. Several studies have identified various etiologies for acute liver failure such as viral infections,bacterial infections, alcohol, and drugs[5-7]. Lipopolysaccharide (LPS) contains endotoxin prompted inflammation, and D-galactosamine (D-GalN) consequences in lipid peroxidation via promoting the release of reactive oxygen species (ROS) in liver cells[8]. There is an urgent need for the development of novel hepatoprotective therapies. The pathogenesis of fulminant hepatitis is currently under extensive investigation using LPS/D-GalN-prompted acute liver failure (ALF) model. This is a well-known model to probe the mechanism, pathogenesis, and agents for human liver injury[9,10]. D-GalN, a well-established hepatotoxic agent, produces hepatic necrosis by inhibiting mRNA translation[11]. LPS is known to stimulate Kupffer cells and promote the release of inflammatory cytokines such as tumor necrosis factor-α and interleukin 6 (TNF-α and IL-6), that successively produce hepatonecrosis and ALF. Furthermore,ROS-induced mitochondrial dysfunction was identified as a probable mechanism in LPS/D-GalN-induced ALF[12,13].
Several reports indicated that decreasing inflammation and oxidative stress could mitigate liver damage[14]. The homeostasis of metabolism, redox equilibrium and production of ROS are easily apparent at the mitochondrial level in which superoxide is generated as a result of the electron transport chain and Kerb’s cycle reaction. The recent data demonstrate that nutritional intake, energy metabolism is also associated at nuclear level via the nuclear factor erythroid-related factor-2 (Nrf2)/antioxidant response element (ARE) pathway[15]. Several genes that contribute to oxidative stress are controlled by the key Nrf2. Before elevation in ROS, the Nrf2 transcription factor present in cytoplasm causes it to translocate to the nucleus. Activated, Nrf2 binds to the promoter region cis-acting enhancer ARE sequence (core sequence:TGAG/CNNNGC) of antioxidant genes to increase their expression of 500 genes including Gsts, Nqo1, Ugts, Gclc, Gclm, and Ho-1 etc. referred to as the “Nrf2 regulon”[16], which enhanced the capacity of the cellular radical-scavenging systems which decrease oxidative stress and activate pro-inflammatory pathways[17,18]. NRF2/ARE pathway predominantly involves in phase-II detoxification organs including liver, kidney, heart, intestine etc. A link between liver diseases and oxidative stress is indispensable. Nrf2 is a key regulator of cytodefence via mediation of antioxidant response, anti-inflammatory and chemoprotective activity[19]. The capacities of Nrf2 to enhance the expressions of antioxidant proteins and suppress oxidative stress-related injury have been broadly studied[14,20]. Therefore, implication of Nrf2/ARE activating regimens may be used for liver diseases. The generation of ROS devastates the antioxidant capacity, which contributes to the pathogenesis of several diseases,including fulminant hepatitis[10,21]. SA, also known as 3,5-dimethoxy-4-hydroxycinnamic acid, is a key phytoconstituent of citrus fruits, spices, berries, cereals, vegetables and oilseed crops commonly used in food and beverages[22]. SA is known to possess activities, such as antimicrobial, antioxidant, anti-inflammatory, anticancer,antidiabetic, antihypertensive, anti-anxiety, neuroprotective and hepatoprotective activities[3,22-26]. SA is a prominent member of the Brassicaceae family[24]. Literature reveals that SA is a bioactive phenolic acid and has the potential to attenuate various chemically induced toxicities[3]. SA is a potent scavenger of ROS; this property allows it to protect against tissue injuries[3,25,26]. SA induced NRF2/HO-1 has been reported in various disease models[25-29]. The amolearation of LPS/D-GalN-induced fulminant hepatitis through NRF2/HO-1 activation have been previously documented[30-32].However, the hepatoprotective effects of SA in LPS/D-GalN-induced fulminant hepatitis has not been previously investigated. To identify the detailed mechanisms of action for SA, we tested its antioxidant and anti-inflammatory activities in a rat model of LPS/D-GalN-induced fulminant hepatitis. Thus, the purpose of the current study was to explore the underlying hepatoprotective mechanism of SA against LPS/DGalN-induced ALF in rats. We also aimed to identify its effects on ROS production,inflammation and apoptosis and the roles of Nrf2/heme oxygenase 1 (HO-1) and NFκB pathways.
MATERIALS AND METHODS
Drugs and chemicals
SA, LPS, and D-GalN were acquired from Sigma-Aldrich (Switzerland). The following primary antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX,United States): transforming growth factor (TGF-β), Nrf2, HO-1, B-cell lymphoma 2(Bcl-2), Caspase 3, Bcl2-Associated X, Apoptosis Regulator (Bax), nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor alpha (IκBα), NF-κB, and beta-actin (β-actin). HRP-conjugated secondary antibodies were also purchased from Santa Cruz Biotechnology (Dallas, TX, United States). A nuclear and cytoplasmic protein NE-PER kit was purchased from Pierce Biotechnology (Rockford, IL, United States). Enzyme-linked immunosorbent assay (ELISA) kits for rat TNF-α, IL-6, and myeloperoxidase (MPO) were purchased from R&D Systems, Inc. (MN, United States).
Animals
Wistar adult male rats (weight, 212-226 g) were taken from the animal facility of King Saud University, Riyadh, Saudi Arabia. The experiment proposal was authorized by the Ethics Committee of the Experimental Animal Care Society, King Saud University,Saudi Arabia (KSU-SE-20-13). They were kept under standard conditions 24 ± 2 °C, 12 h light/dark cycle, and received water and food ad libitum. The studies were performed according to the protocols that were approved by the Animal Ethics Committee of King Saud University, College of Pharmacy, Riyadh, Saudi Arabia.
Experimental design
The total forty-two rats were arbitrarily categorized into four groups: The control group (n = 6), the D-GalN/LPS treated (ALF) group (n = 12), the ALF + SA (20 mg/kg)group (n = 12), and the ALF + SA (40 mg/kg) group (n = 12), To induce ALF, rats were injected intraperitoneally (i.p.) with 8 µg/kg LPS and 800 mg/kg D-GalN in 800 μL sterile normal saline. SA (20 and 40 mg/kg) solution in normal saline were administered via gavage once per day starting seven days before LPS/D-GalN treatment. The normal control group was injected i.p. with an equal volume of normal saline. Rats were anesthetized with ketamine (55 mg/kg) and xylazine (7 mg/kg) and euthanized 72 h after the D-GalN/LPS injection. Both serum and liver samples were collected and stored at -80 °C for further analysis. The dose of SA was selected based on preliminary and published data[3,23,33].
Survival rate and liver function activity
The rate of survival was observed for 48 h after the injection with D-GalN/LPS. Rats were witnessed for morbidity every hour after the injection of D-GalN/LPS. Assay kits to measure levels of aspartate aminotransferase (AST), alanine transaminase (ALT)and the ratio of bilirubin to alkaline phosphatase were purchased from Human Diagnostic Worldwide (Wiesbaden, Germany), using a spectrophotometer (Shimadzu– Model UV 2401, Kyoto, Japan).”
Histological evaluation
Hepatic tissue from each rat was fixed in 12% formalin for histopathological evaluation. The hepatic tissues were gradually dehydrated, embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin and eosin for histological inspection.Briefly, the severity of liver damage was evaluated by a blinded pathologist using four-point scale from 0 to 3 as follows: 0, l, 2, and 3 represent no damage, mild damage, moderate damage, and very severe damage, scoring system in 20 random fields at 400 × magnification per animal (n = 6) for each group. A light microscope(Olympus, Japan) examined the histological anomalies.
Preparation of nuclear and total protein extracts
Total hepatic protein was extracted by homogenizing (T 25 Digital ULTRA-TURRAX®)tissue in cold RIPA buffer (Pierce Biotechnology, United States) and the supernatant was collected after centrifuging at 2500 × g for 20 min at 4 °C. Similarly, cytosolic and nuclear proteins were prepared using the NE-PER Kit (Pierce Biotechnology, United States) following the kit instructions. The protein concentration was examined using(Pierce Biotechnology, United States) the method bicinchoninic acid (PierceTMBCA)assay[34].
Oxidative stress indices
The levels of malondialdehyde (MDA) and NO were examined in hepatic tissues using commercially available colorimetric assay kit (Sigma-Aldrich, St. Louis, MO, United States).
Antioxidant enzyme indices
Hepatic superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase(GPX) activities in homogenized hepatic tissues were examined using commercial kits(Cayman Chemical, Ann Arbor, MI, United States).
Cytokine and inflammatory marker
TNF-α, IL-6, and MPO levels in the hepatic tissue were measured using ELISA kits according to the kit instructions. Absorbance was read at 450 nm.
Western blot analysis
Protein expression was performed using western blot analysis using a previously described protocol[35]. Precisely, 30-40 µg of protein was separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), electrophoretically transferred to nitrocellulose membranes, blocked with 4% casein and BSA in TBS containing 1% Tween-20 (TBST), and incubated at 4 °C overnight with the primary antibodies. The next day, membranes were washed five times with TBST and incubated with secondary antibodies for 2 h at room temperature. Bands were observed using Luminata™ Western Chemiluminescent HRP Substrates (Millipore,Billerica, MA, United States). Densitometric analysis of the immunoblots was also performed using a LI-COR C-Di-Git Blot Scanner (Lincoln, NE, United States).
Statistical analysis
All results are presented as arithmetic means ± SE. Data were analyzed using a oneway analysis of variance followed by Dennett’s test by using Graph Pad V6.
RESULTS
Effects of SA on the survival rate of animals with D-GalN/LPS-induced ALF
The effect of SA pretreatment (20 and 40 mg/kg) on the 96 h survival rate is shown in Figure 1. After the administration of D-GalN/LPS, five rats died before 12 h with a survival rate of 58.33% after 96 h. Pretreatment with 20 and 40 mg/kg SA increased the survival rate by 66.66% and 75%, respectively, compared to the ALF group.
Effect of SA pretreatment on liver failure serum markers in D-GalN/LPS-induced ALF
The administration of D-GalN/LPS to rats caused ALF, as demonstrated by the significant increase in ALT, AST, and bilirubin levels (1485.73%, 774.10%, and 374.28%,respectively) compared with the normal controls (P < 0.05, P < 0.05, P < 0.05).Nevertheless, pretreatment with 20 and 40 mg/kg SA prevented ALF, as demonstrated by the 51.93% and 69.65% decreases in ALT levels (P < 0.05, P < 0.05), 44.29% and 73.66% decreases in AST levels (P < 0.05, P < 0.05) and 48.67% and 56.22% decreases in bilirubin levels (P < 0.01, P < 0.01) compared to the ALF rats (Table 1). Moreover, the histological examination of liver tissues showed that D-GalN/LPS caused focal central vein congestion, massive changes in lipid accumulation, ballooning formation, loss of cellular boundaries, and necrosis with inflammation. SA pretreatment, especially the 40 mg/kg dose, improved liver architecture in comparison to the ALF rats.
Effect of SA pretreatment on oxidative stress markers in the hepatic tissue of ALF rats
Liver tissue from the D-GalN/LPS-induced ALF animals showed significantly higher levels of MDA (122.90%) (P < 0.05) (Figure 2 A) and NO2-content (230.01%) (P < 0.05)(Figure 2 B) compared with the liver tissue from the control animals. However, the oral administration of 20 and 40 mg/kg SA significantly and dose-dependently reduced the MDA levels by 35.24% and 41.22%, respectively (P < 0.05; Figure 2 A) and NO2-levels by 39.05% and 51.82%, respectively (P < 0.05; Figure 2 B).
Effect of SA pretreatment on the activity of antioxidant enzyme markers in hepatic tissue of D-GalN/LPS-induced ALF rats
Hepatic tissue from D-GalN/LPS-induced ALF rats show 55.14% (P < 0.05), 70.01% (P< 0.05), and 73.37% (P < 0.05) reduction in the activities of GPx, SOD, and CAT,respectively (Figure 3). The oral administration of 20 and 40 mg/kg SA significantly restored the activity of GPx by 29.23% and 78.81%, respectively (P < 0.05); the activity of SOD by 117.64% and 185.87%, respectively (P < 0.05); and CAT activity by 123.41%and 198.41%, respectively (P < 0.05).
Effect of SA pretreatment on cytokines and inflammatory markers in hepatic tissue of D-GalN/LPS-induced ALF
The data suggested that TNF-α, IL-6 and MPO levels were significantly augmented in the D-GalN/LPS-induced ALF that is 524.61 %, 154.57 % and 118.56 % respectively (P< 0.05) as compared to control rats. SA at 20 and 40 mg/kg pretreatment significantly and dose-dependently downregulate these effects of TNF-α, IL-6 and MPO (P < 0.05;Figure 4) that is 29.69% and 50.39% for TNF-α, 37.03%, 46.53% for IL-6 and 23.52% and 32.72% for MPO as compared to D-GalN/LPS –induced ALF rats.
SA downregulates NF-κB (p65) in ALF
To examine the probable mechanism by which SA blocks TGFβ and IL-6 generation,we analyzed the activity of 20 and 40 mg/kg SA on NF-κB activation. As illustrated in Figure 5, the protein expression of IκB-α in the ALF group declined compared to the control group; however, this decrease was reversed by 20 and 40 mg/kg SA in a dosedependent fashion. Moreover, In the ALF group, we detected increased nuclear NF-κB(p65) relative to the control group. However, 20 and 40 mg/kg treatment with SA reduced NF-κB (p65) during ALF in dose-dependent manner. The data proposed that SA may inhibit D-GalN/LPS -induced TGFβ generation by blocking NF-κB activation.Induction with D-GalN/LPS encouraged the translocation of p65 into the nucleus, andinduced the inflammatory response through the NF-κB signaling pathway. As illustrated in Figure 5, D-GalN/LPS apparently upregulated the expression of Bax,caspase 3, and downregulated the expression of Bcl-2, which indicated apoptosis. On the contrary, dose-dependent downregulation of Bax, caspase 3 and upregulation of Bcl-2 was observed after pretreatment with 20 and 40 mg/kg SA in the D-GalN/LPS group. These results indicate that SA inhibited LPS/D-GalN-induced hepatocyte apoptosis by affecting the expression of apoptosis-related factors. D-GalN/LPS administration induced iNOS expression compared to the normal control.Pretreatment with 20 and 40 mg/kg SA significantly and dose-dependently inhibits iNOS expression, thus suggestive its potent anti-inflammatory effects. The antioxidant activity of SA is controlled via the modulation of the Nrf2/HO-1 pathway. Hepatic tissue from D-GalN/LPS-induced ALF show lower levels of Nrf2 and HO-1 expression as compared to the normal control rats. However, treatment with 20 mg/kg and 40 mg/kg SA significantly and dose-dependently upregulated the expression of Nrf2 and HO-1 compared with tissues from D-GalN/LP -induced animals (P < 0.05).

Table 1 Effect of sinapic acid pretreatment on liver function serum markers in D-galactosamine/lipopolysaccharide-induced acute liver failure
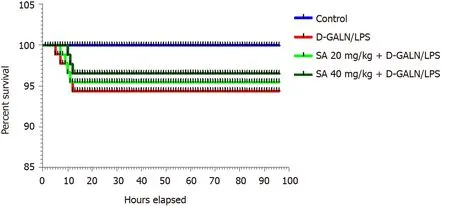
Figure 1 Effect of sinapic acid (20 and 40 mg/kg bodyweight) pretreatment on the survival rate of D-galactosamine/lipopolysaccharideinduced acute liver failure. SA: Sinapic acid; D-GalN/LPS: D-galactosamine/lipopolysaccharide.

Figure 2 Effect of sinapic acid pretreatment on oxidative stress markers in hepatic tissue of D-galactosamine/lipopolysaccharide-induced acute liver failure. The results are presented as mean ± SE with six animals per group. aDenotes significant differences compared to the control group (P < 0.05);bDenotes significant differences compared to the D-galactosamine/lipopolysaccharide group (P < 0.05). MDA: Malondialdehyde; SA: Sinapic acid; D-GalN/LPS: Dgalactosamine/lipopolysaccharide.
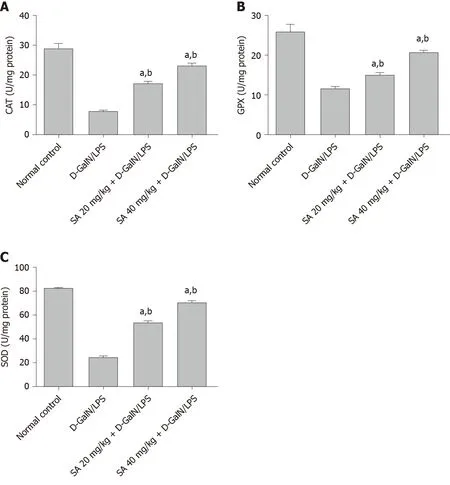
Figure 3 Effect of sinapic acid pretreatment on antioxidant enzyme activity in hepatic tissue from D-galactosamine/lipopolysaccharideinduced acute liver failure rats. The results are presented as mean ± SE with six animals per group. aDenotes significant differences compared to the control group (P < 0.05); bdenotes significant differences compared to the D-galactosamine/lipopolysaccharide group (P < 0.05). CAT: Catalase; SA: Sinapic acid; DGalN/LPS: D-galactosamine/lipopolysaccharide.
Effects of SA on histoarchitecture of liver tissues in ALF
As illustrated in Figure 6 A, the hepatic tissues from the normal control group exhibited normal cellular and lobular architecture. Hepatic tissue from the ALF group exhibited prominent pathological alterations comprising widespread portal inflammation, hepatic cell necrosis, and infiltration of inflammatory cells (Figure 6 B).However, pretreatment with 20 mg/kg and 40 mg/kg SA significantly ameliorated the D-GalN/LPS-induced pathological alterations in a dose-dependent manner as demonstrated by the reduced cell infiltration and restored lobular architecture. The severity of liver damage score has been illustrated in Figure 7.

Figure 4 Effect of sinapic acid pretreatment on cytokines and inflammatory markers in hepatic tissue of D-galactosamine/lipopolysaccharide-induced acute liver failure. The results are presented as mean ± SE with six animals per group. aDenotes significant differences compared to the control group (P < 0.05); bdenotes significant differences compared to the D-galactosamine/lipopolysaccharide group (P < 0.05). TNF-α: Tumor necrosis factor-α; IL-6: Interleukin 6; MPO: Myeloperoxidase; SA: Sinapic acid; D-GalN/LPS: D-galactosamine/lipopolysaccharide.
DISCUSSION
There is an overwhelming amount of evidence showing that SA protects against liver damage in animal models via various mechanisms, such as its antioxidant activity,anti-inflammatory activity, and ability to downregulate NF-κB p65. Therefore, SA has potential as a hepatoprotective agent for decreasing inflammation in CCL4and dimethyl nitrosamine-induced acute liver fibrosis[23]. The fulminant hepatitis rodent model was not investigated, and more detailed mechanisms remain unclear. Since SA has potent anti-inflammatory and antioxidant functions, we hypothesized that SA could also have hepatoprotective effects against D-GalN/LPS-induced fulminant hepatitis. The D-GalN/LPS-induced animal model of ALF is widely used to check the efficacy of hepatoprotective agents[36,37]. To examined the hepatoprotective effect of SA,serum levels of AST and ALT were analyzed to study the extent of liver damage.Several reports have shown that SA decreased AST and ALT[23]. AST and ALT are two known serum biomarkers of liver dysfunction[38,39]. Elevated levels of AST are indicative of tissue necrosis[40]. D-GalN/LPS-induced ALF rats exhibit enhanced serum levels of AST and ALT that were accompanied by enhanced inflammatory infiltration,hemorrhage, hepatocyte necrosis, and the loss of hepatic architecture. Pretreatment with SA (20 and 40 mg/kg) dose-dependently downregulated the AST and ALT levels and helped restore liver functions and structures. These results are consistent with previous reports[3,23,24,41,42]. Mitochondrial dysfunction and oxidative stress are two linked cellular events[43]. The dysfunction of mitochondrial dynamics leads to accumulation ROS and encourages induction of DNA damage, up or down regulation of apoptotic/anti-apoptotic factors, phosphatases and apoptotic/antiapototic factors leading to redox imbalance leading to wide range of disease including fulminant hepatic disease[42,44,45]. Previous evidence indicate that mitochondrial defects contribute to liver damage via a number of pathways[46,47], involving inhibition of mitochondrial βoxidation and respiratory chain activity, failure of mitochondrial membrane potential and damage to the antioxidant protection mechanism.


Figure 5 Sinapic acid downregulates nuclear factor kappa B in acute liver failure. A-H: Effect of sinapic acid on the protein expression of transforming growth factor-β1 (A), heme oxygenase-1 (B), nuclear factor erythroid 2-related factor 2 (C), nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor alpha protein (D), nuclear factor kappa B (E), anti-apoptotic protein BCl2 (F), caspase 3 (G), and Bax in D-galactosamine/lipopolysaccharide (D-GalN/LPS)-induced acute liver failure (ALF) (H). The results are presented as the mean ± SE of six animals per group. aDenotes significant differences to the D-GalN/LPS-induced ALF group (P < 0.05); bdenotes significant differences compared to the normal control. TGF-β1: Transforming growth factor-β1; HO-1: Heme oxygenase-1; Nrf2: Nuclear factor erythroid 2-related factor 2; IkBα: Nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor alpha; NF-κb: Nuclear factor kappa B; SA: Sinapic acid; D-GalN/LPS: D-galactosamine/lipopolysaccharide.
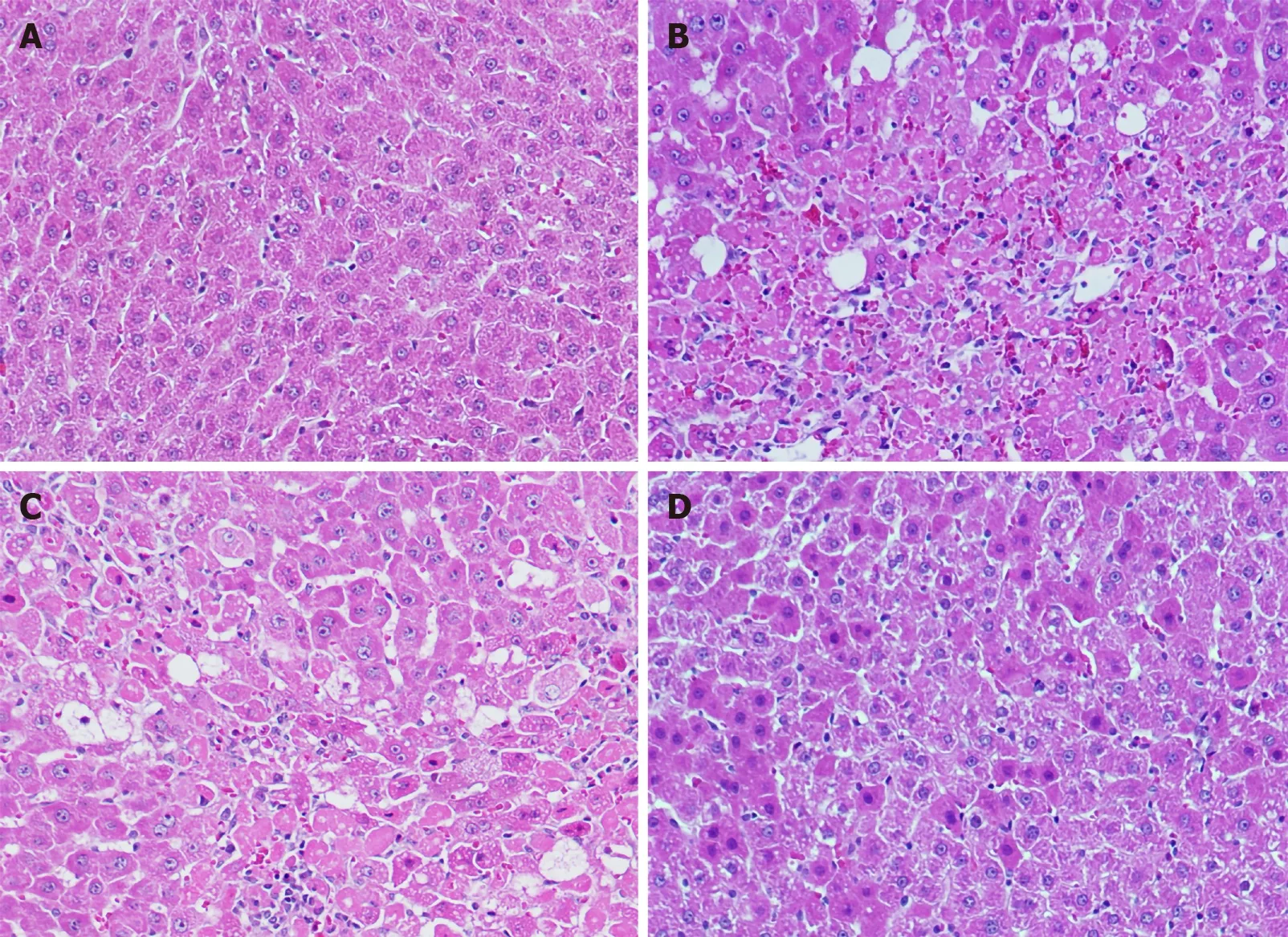
Figure 6 Light photomicrographs of hepatic tissues. Hematoxylin and eosin stains, magnification 100 ×. A: Hepatic section of normal control rat exhibits normal architecture of hepatic cord of cells; B: Hepatic section of D-galactosamine/lipopolysaccharide (D-GalN/LPS) treated rats exhibiting massive fatty changes,focal central vein congestion, ballooning formation, necrosis with inflammation, and loss of cellular boundaries, massive cellular infiltration; C: Hepatic section of rats treated D-GalN/LPS and 20 mg/kg of sinapic acid (SA) showing mild central vein congestion, mild fatty changes, ballooning, necrosis with sinusoidal dilatation, mild cellular infiltration; D: Hepatic section of rats treated D-GalN/LPS and 40 mg/kg of SA exhibiting the absence of ballooning, inflammatory cells, and regeneration of hepatocytes around central vein toward near-normal liver architecture but slight congestion in the central vein.

Figure 7 The liver damage score was examned using four-point scale from 0 to 3. 0, l, 2, and 3 represent no damage, mild damage, moderate damage, and very severe damage, scoring system in 20 random fields at 400 × magnification per animal (n = 6 per group). aDenotes significant differences compared to the control group (P < 0.05); cDenotes significant differences compared to the D-galactosamine/lipopolysaccharide group (P < 0.05). SA: Sinapic acid; D-GalN/LPS:D-galactosamine/lipopolysaccharide.
ROS play a significant role in the pathogenesis of ALF[21,48]. Reactive nitrogen species(RNS) and ROS can react with polyunsaturated fatty acids (PUFAs) to cause lipid peroxidation; this process may further damage the cellular membrane and trigger apoptosis[49,50]. Kupffer cells and neutrophils undergo apoptosis during vascular oxidative stress, which leads to ALF[51]. Several reports have implicated oxidative stress in D-GalN/LPS-induced ALF[52]. MDA is the end product of lipid peroxidation and the accumulation of ROS/RNS. It is also an excellent marker for oxidative stress[53]. The level of MDA and NO increased significantly in D-GalN/LPS-induced ALF rats as compared to normal control rats. The pretreatment with 20 and 40 mg/kg SA pretreatment substantially and dose-dependently curbed the oxidative stress as indicated by reduced MDA and NO levels. Therefore, SA might have potent antioxidant activity through its ability to inhibit ROS/RNS production induced by DGalN/LPS. There are several reports that SA has potent antioxidant activity[3]. DGalN/LPS-induced oxidative stress leads to the accumulation of hepatic lipid peroxides and the depletion of antioxidant enzymes such as GPx, SOD, and CAT.
D-GalN/LPS-induced ALF rats exhibit lower levels of GPx, SOD, and CAT as compared to normal control animals. SA (20 and 40 mg/kg) pretreatment significantly restored levels of GPx, SOD, and CAT in hepatic tissues, which is possibly an adaptive response to oxidative stress. SA has been previously shown to restore levels of GPx,SOD and CAT in a CCL4 and dimethyl nitrosamine-induced model of acute liver injury[23]. D-GalN/LPS-induced liver failure caused the production of TNF-α and IL-6,which triggered an inflammatory cascade in hepatocytes causing ALF[54,55]. Several natural polyphenols have been reported to prevent liver injuries[52,56]. Thus, levels of TNF-α and IL-6 in the liver were analyzed in the study. Pretreatment with SA reduced levels of these cytokines in a dose-dependent fashion. This finding may partially explain the hepatoprotective activity of SA. NF-κB is activated by D-GalN/LPS through the phosphorylation and degradation of IκB-α; this allows NF-κB to translocate to the nucleus and increase the gene expression of cytokines such as TNF-α and IL-6[57]. Out data indicate that SA downregulated NF-κB activation by decreasing IκB-α degradation in a dose-dependent fashion.
Nrf2, a crucial transcription factor, controls these enzymes such as SOD, CAT, GPx,and HO-1 by binding to the elements of antioxidant response, inducing adaptive cytoprotective responses, and having a strong influence on the response to oxidative stress[58]. Several antioxidant genes are controlled by Nrf2. Upon activation by oxidative stress, Nrf2 translocates to the nucleus and binds to antioxidant transcription elements of Phase II to increase the expression of antioxidant genes[13,52,59]. The restoration of antioxidant enzymes SOD, CAT, GPx, and HO-1 and down regulation of TNF-α and IL-6 due to upregulation of NRF2 and down regulation of NF-kB indicates towards cytoprotective effect due to its antioxidant and antinflamatory nature[58]. The data indicates SA mediates Nrf2 nucleus translocation as evident upregulation of NRF2 expression in nucleus that cause upregulation in antioxidant enzyme SOD, CAT,GPx, and HO-1 that performing a defending role against D-Gal/LPS induced inflammation and oxidative stress[58,60]. Down regulation of NRF2 is linked with enhanced inflammation, while its upregulation reduces transcriptionally mediated NF-kB, pro-inflammatory and immune responses[44,61]. HO-1 is a rate-limiting enzyme that translates heme into equimolar amounts of iron, carbon monoxide, and biliverdin.HO-1 has cytoprotective and antioxidant activities and is activated in D-GalN/LPSinduced hepatitis[6,52]. The pretreatment of D-GalN/LPS-induced ALF rats with SA induced the expression of HO-1 through increased Nrf2 expression. Several studies have shown that there is a reduction in the expression of HO-1 and Nrf2 in DGalN/LPS-induced ALF, which is similar to our results (Figure 2)[23,48,52]. Our data show that HO-1 and Nrf2 were downregulated in liver tissue after D-GalN/LPS administration, but SA pretreatment enhanced their expression in a dose-dependent manner. These outcomes showed that the Nrf2/HO-1 signaling pathways are implicated in the protective mechanism of SA. Apoptosis plays a key role in the pathogenesis of D-GalN/LPS-induced ALF[62]. Our data showed that D-GalN/LPS treatment may increase apoptotic and necrotic hepatocellular death through the upregulation of Bax and caspase-3 and the downregulation of Bcl-2. SA pretreatment significantly downregulates the expression of Bax and caspase-while upregulating Bcl2 in a dose-dependent manner, thus reducing apoptotic cell damage in hepatocytes.The aforesaid data is consistent with previously published studies[63,64].
CONCLUSION
This work demonstrated for the first time that the SA has hepatoprotective effects in the D-GalN/LPS-induced rat model through its ability to suppressing oxidative stress,inflammation, and apoptosis. The protective mechanism of SA depends on the downregulation of NF-κB and the restoration of antioxidant enzyme levels through the activation of the Nrf2/HO-1 pathway. Thus, SA could be applied to treat or prevent D-GalN/LPS -induced ALF in the future (Figure 8).
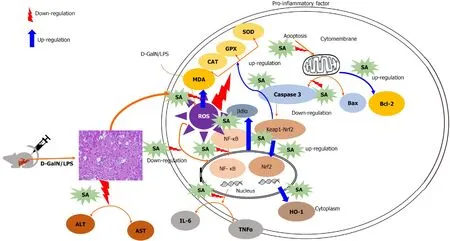
Figure 8 Graphical abstract. SA: Sinapic acid; D-GalN/LPS: D-galactosamine/lipopolysaccharide; ALT: Alanine transaminase; AST: Aspartate aminotransferase;TNF-α: Tumor necrosis factor-α; IL-6: Interleukin 6; TGF-β1: Transforming growth factor-β1; HO-1: Heme oxygenase-1; Nrf2: Nuclear factor erythroid 2-related factor 2; IkBα: Nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor alpha; NF-κb: Nuclear factor kappa B; ROS: Reactive oxygen species; CAT:Catalase; MDA: Malondialdehyde; GPX: Glutathione peroxidase; SOD: Superoxide dismutase.
ARTICLE HIGHLIGHTS
Research background
Sinapic acid (SA) has been shown to have various pharmacological properties such as antioxidant, antifibrotic, anti-inflammatory, and anticancer activities. Its mechanism of action is dependent upon its ability to curb free radical production and protect against oxidative stress-induced tissue injuries.
Research motivation
In the current study, the hepatoprotective effects of SA against lipopolysaccharide(LPS)/D-galactosamine (D-GalN)-induced acute liver failure (ALF) in rats were studied.
Research objectives
In the current study, the hepatoprotective effects of SA against LPS/D-GalN-induced acute liver failure (ALF) in rats were studied.
Research methods
Experimental ALF was induced with an intraperitoneal (i.p.) administration of 8 μg LPS and 800 mg/kg D-GalN in normal saline. SA was administered orally once daily starting 7 d before LPS/D-GalN treatment.
Research results
Data showed that SA ameliorates acute liver dysfunction, decreases serum levels of alanine transaminase (ALT), and aspartate aminotransferase (AST), as well as malondialdehyde (MDA) and NO levels in ALF model rats. However, pretreatment with SA (20 mg/kg and 40 mg/kg) reduced nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation and levels of inflammatory cytokines (tumor necrosis factor-α and interleukin 6). Also, SA increased the activity of the nuclear factor erythroid-related factor 2/heme oxygenase-1 (Nrf2/HO-1) signaling pathway.
Research conclusions
In conclusion, SA offers significant protection against LPS/D-GalN-induced ALF in rats by upregulating Nrf2/HO-1 and downregulating NF-κB.
Research perspectives
The amolearation of LPS/D-GalN-induced fulminant hepatitis through NRF2/HO-1 activation have been previously documented. However, the hepatoprotective effects of SA in LPS/D-GalN-induced fulminant hepatitis has not been previously investigated.To identify the detailed mechanisms of action for SA, we tested its antioxidant and anti-inflammatory activities in a rat model of LPS/D-GalN-induced fulminant hepatitis. Thus, the purpose of the current study was to explore the underlying hepatoprotective mechanism of SA against LPS/D-GalN-induced ALF in rats. We also aimed to identify its effects on ROS production, inflammation and apoptosis and the roles of Nrf2/HO-1 and NF-κB pathways.
ACKNOWLEDGEMENTS
The authors thank the Deanship of Scientific Research for the funding of this work through the Research Group Number, RG-1439-083 and RSSU for their technical support at King Saud University.
杂志排行
World Journal of Gastroenterology的其它文章
- Digestive system involvement of infections with SARS-CoV-2 and other coronaviruses: Clinical manifestations and potential mechanisms
- Xiangbinfang granules enhance gastric antrum motility via intramuscular interstitial cells of Cajal in mice
- Quantitative multiparametric magnetic resonance imaging can aid non-alcoholic steatohepatitis diagnosis in a Japanese cohort
- Clinicopathological features and prognostic factors associated with gastroenteropancreatic mixed neuroendocrine non-neuroendocrine neoplasms in Chinese patients
- Effect of liver inflammation on accuracy of FibroScan device in assessing liver fibrosis stage in patients with chronic hepatitis B virus infection
- Simultaneous partial splenectomy during liver transplantation for advanced cirrhosis patients combined with severe splenomegaly and hypersplenism
