中药活性成分口服结肠靶向纳米系统治疗溃疡性结肠炎的研究进展
2021-03-17钟雪梅傅超美章津铭
鲜 静,张 晨,钟雪梅,傅超美,高 飞,章津铭
中药活性成分口服结肠靶向纳米系统治疗溃疡性结肠炎的研究进展
鲜 静,张 晨,钟雪梅,傅超美,高 飞*,章津铭*
成都中医药大学药学院,四川 成都 611137
溃疡性结肠炎(ulcerative colitis,UC)为现代常见病、难治病。因其发病反复,临床常需反复长期使用柳氮磺胺吡啶、免疫抑制剂等药物,但其疗效有限且易产生不良反应。现代研究发现中药多酚类、生物碱类、醌类、萜类等活性成分通过多靶点机制,表现出较好缓解UC的作用,且不良反应相对较低,但存在水溶性差、胃肠道不稳定和结肠靶向性差等制剂学问题。针对以上制剂学问题,研究者构建了多种中药活性成分的口服结肠靶向纳米系统,通过避免胃肠道破坏、延长肠滞留、实现药物在病灶部位控释等方式显著提升了对UC的治疗效果。对具有UC防治作用的中药活性成分及其作用机制,以及口服结肠靶向纳米系统用于UC治疗的研究进展进行综述,以期为中药活性成分口服靶向治疗UC提供思路。
中药活性成分;溃疡性结肠炎;纳米制剂;结肠靶向;递药体系
溃疡性结肠炎(ulcerative colitis,UC)是一种病程长且难以治愈的非特异性炎症性肠病,病区可至直肠黏膜下层,累及直肠、乙状结肠乃至整个结肠区域[1]。其临床表现为腹痛、腹泻、体质量减轻及便血,可反复发作,已经被世界卫生组织列为现代难治病之一,其发病机制复杂,与黏膜通透性增加、微生物水平失调和肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)、白介素-6(interleukin-6,IL-6)、白介素-10(interleukin-10,IL-10)等炎症因子水平失调等因素相关[2]。目前治疗UC的常用药物有5-氨基水杨酸类、皮质醇类和免疫抑制类等,但是长期服用这些药物会引起严重的不良反应。根据疾病症状,UC在中医上属于“休息痢”“久痢”“肠癖”等范畴[3]。基于辨证论治理念,中医药防治UC历史悠久,临床疗效显著且不良反应较小。中药治疗UC具有多成分、多靶点、改善机体内环境等优势,然而,中药活性成分经口服治疗时,存在水溶性差、胃肠稳定性低、口服生物利用度差等不足。如何实现中药活性成分在结肠病变部位的高效递送,是亟待解决的一个问题。本文通过查阅近年来的中英文文献,总结并综述了具有UC防治作用的中药代表活性成分及其作用机制,以及口服结肠靶向纳米系统用于UC治疗的研究进展,以期为中药活性成分高效治疗UC提供参考思路。
1 具有UC防治作用的中药活性成分及其作用
中医药治疗UC历史悠久且疗效明显,特别是中药多酚类、生物碱类、醌类、萜类等活性成分显示出对UC的治疗潜力。
1.1 多酚类
多酚类化合物是一类具有多个酚羟基的化合物,存在于多种中药中,其中白藜芦醇、芦丁、水飞蓟素、姜黄素等化合物均被报道有治疗UC的作用。白藜芦醇常存在于决明、藜芦、虎杖等常用中药中,在治疗和预防各种疾病中具有重要作用。Rauf等[4]、Marques等[5]研究发现白藜芦醇可通过下调TNF-α、环氧合酶2、C反应蛋白、干扰素等炎症生物标志物而治疗炎症。芦丁,又名芸香苷,存在于槐米、荞麦叶、蒲公英等中药中,主要用于抗炎、抗病毒、抗氧化[6]。Nones等[7]、Habtemariam等[8]研究发现芦丁可通过抑制TNF-α和核因子κB(nuclear factor kappa-B,NF-κB)。从水飞蓟中提取得到的黄酮木脂素类化合物水飞蓟素具有抗癌、抗炎症的作用,水飞蓟素可清除自由基和活性氧物质,减少组胺释放和TNF-α、IL-6、IL-8的表达,进而有效改善UC的炎症症状[9-10]。姜黄素来源于姜科植物姜黄,常作为一种食品添加剂,具有抗炎、抗氧化、抗肿瘤、保肝、抗血管生成等多种功效。因而,姜黄素在治疗UC上具有良好的作用[11]。
1.2 生物碱类
生物碱类成分是一类含氮的碱性有机化合物。近年来,一些中药中的生物碱类成分显示出良好的UC治疗作用。从毛茛科植物黄连中提取出的一种异喹啉生物碱小檗碱,常用于治疗细菌相关的腹泻。临床可用于抗炎、抗肿瘤、免疫调节。研究表明,小檗碱可通过抑制某些炎症因子的表达来改善UC[12]。青藤碱是从防己科植物青藤的根中提取的一种生物碱类成分,常被用于治疗风湿性关节炎。近年来,研究者发现青藤碱在治疗慢性炎症方面也具有一定作用[13]。Tang等[14]、Zhou等[15]发现青藤碱能通过调节Nrf2/NQO-1信号通路减轻由硫酸葡聚糖钠(dextran sulfate sodium,DSS)诱导的UC小鼠的炎症情况。氧化苦参碱是从豆科植物苦参中分离得到的活性成分,已有研究证实,氧化苦参碱具有抗肝炎病毒、抗肿瘤等作用。熊永爱等[16]开展实验证明氧化苦参碱对三硝基苯磺酸(trinitrobenzene sulfonic acid,TNBS)诱导的SD大鼠UC的疗效明确。从黑胡椒中提取出的胡椒碱和防己中提取的粉防己碱也被证实具有一定的抗UC作用[17-18]。
1.3 醌类
具有UC治疗作用的醌类成分主要是大黄素和大黄酸。大黄素是大多数中药中含有的一种蒽醌类衍生物,具有抗肿瘤、抗菌、利尿、舒张血管的作用。研究发现大黄素能通过降低血液中抗鞭毛蛋白抗体的水平和下调Toll样受体5(toll like receptor 5,TLR5)、NF-κB p65通路的表达来治疗UC[19-20]。大黄酸是一种常被用于治疗胃肠道疾病的蒽醌类化合物,可以减轻巨噬细胞RAW264.7因脂多糖诱导后产生的促炎症因子的水平[21]。也有研究发现从百花酸藤果中提取出的信筒子醌亦可减少诱导型一氧化氮合酶(inducible nitric oxide synthase,iNOS)、TNF-α、IL-1β、IL-6的表达来缓解炎症[22]。
1.4 萜类
萜类是异戊二烯的聚合物及其衍生物的总称,研究发现某些倍半萜类、二萜类、三萜类成分具有治疗UC的作用。从雷公藤中提取出的一种五环三萜类成分雷公藤红素,对炎症、癌症和关节炎等均有潜在的治疗作用[23]。Jia等[24]研究发现雷公藤红素可抑制坏死性凋亡和缓解DSS诱导的UC小鼠炎症。小白菊内酯是一种倍半萜烯内酯化合物,是菊科植物的重要有效成分,具有抗氧化应激、抗炎、抑制细胞凋亡等生物学活性。研究发现,小白菊内酯可作为一种NF-κB通路的抑制剂,缓解由DSS诱导的小鼠UC[25]。二萜类成分酯穿心莲内酯是穿心莲中的一种重要成分,可影响UC患者体内Th1/Th2/Th17反应[26]。此外,Zhu等[27]发现穿心莲内酯对TNBS诱导的UC小鼠模型有较好的治疗效果。二萜类成分二氢丹参酮Ⅰ也被报道具有治疗UC的作用[28]。
1.5 其他
中药中含有的其他成分,如西红花酸、莽草酸、牛蒡子苷元、鸦胆子苦素以及多糖类成分果胶、纤维素、壳聚糖、车前籽胶等均被报道具有治疗UC的作用[29-34]。一些中药提取物,如生姜提取物亦被证明有抗炎作用。研究发现生姜提取物中的成分6-姜烯酚、6-姜辣素能够抑制炎症相关基因的表达,从而改善炎症病情[35-37]。
具有UC防治作用的代表性中药活性成分见图1,其作用及其机制见表1。

图1 具有UC防治作用的代表性中药活性成分

表1 具有UC防治作用的代表性中药活性成分及其作用机制

续表1
MPO-髓过氧化物酶 PPARγ-过氧化物酶体增殖物激活受体γ STAT-信号传导及转录激活蛋白 COX-2-环氧化酶2 IFN-γ-γ干扰素 TGF-β-转化生长因子β Nrf2-核因子E-2-相关因子2
MPO-myeloperoxidase PPARγ-peroxisome proliferator-activated receptor γ STAT-signal transducer and activator of transcription COX-2- cyclooxygenase 2 IFN-γ-interferon γ TGF-β-transforming growth factor β Nrf2-nuelear factor E2-related factor 2
2 口服结肠靶向纳米系统用于UC的治疗效果
虽然中药活性成分治疗UC具有多途径、多靶点、不良反应小、改善机体内环境等优势,但由于其本身存在的疏水性强、渗透性差、口服稳定性差等不足,限制了其在治疗UC方面的应用。为了使中药活性成分更有效地治疗UC,一些基于粒子尺寸依赖、pH依赖、酶响应、主动靶向等原理的中药活性成分口服结肠靶向纳米系统被开发出来。与传统的口服制剂相比,口服结肠靶向纳米制剂具有更多的优势。首先,其可以提高药物水溶性和稳定性,避免肠道环境和某些酶对药物的破坏,减少物理或化学降解,延缓药物释放时间,达到缓释的目的;其次,当把药物制备成口服结肠靶向纳米制剂时,纳米级别的粒子在结肠部位有更好的靶向性,能有效地减轻系统不良反应[53-54],当使用pH敏感材料、结肠微生物易降解材料构建纳米体系时,还能进一步增强药物的结肠靶向性。最后,由于纳米制剂所具有的微小粒径,使得药物更易在结肠炎症部位聚集,且以较低的药物浓度便可以达到很好的治疗效果[55]。
2.1 粒子尺寸依赖型
研究表明,UC患病区域肠道黏膜层被破坏,巨噬细胞等炎症相关细胞增多,原有的肠道环境被破坏。纳米级别的粒子可被巨噬细胞优先吸收,同时可以通过结肠上皮高通透性和滞留效应(enhanced permeability and retention effect,EPR)效应将活性药物递送到结肠炎症组织内[56]。Lamprecht等[57]分别制备带有荧光色素的3种纳米粒子(粒径分别为0.1、1、10 μm),给TNBS诱导的UC大鼠ig 3 d后观察各种粒子的分布,3种不同粒径的纳米粒子在结肠部位的分布指数分别为(5.2±3.8)%、(9.1±4.2)%、(14.5±6.3)%。因此,在UC患病区域,当粒径足够小时,药物粒子能够渗透进入细胞,从而促进其快速地通过黏膜屏障,与免疫系统作用,提高药物的摄取、吸收、分布以及代谢[58-59]。Ma等[60]利用乳化溶剂蒸发法分别制备装载姜黄素的微粒(粒径1.7 μm)和纳米粒(粒径270 nm),与微粒相比,纳米粒具有更高的释放率,动物实验中,姜黄素纳米粒更易缓解DSS诱导的小鼠UC炎症情况。
2.2 pH依赖型
胃肠道的pH值从胃到结肠逐渐升高。根据结肠pH 7~8的特点可以设计出pH依赖的纳米递送体系,该纳米系统可在结肠部位靶向释放[54,61-62]。通过在药物表面包裹pH敏感的生物降解高分子材料可以设计出pH依赖的药物递送系统。甲基丙烯酸聚合物Eudragit®是常见的pH敏感材料。通过改变其侧链可使其在不同的pH中溶解。Raj等[63]制备出pH敏感的姜黄素纳米制剂,先用粒子凝胶法制备出壳聚糖包载的纳米核结构,再使用乳化溶剂蒸发法制备Eudragit FS 30D壳,得到核-壳结构的纳米,该纳米制剂具有控释药物、结肠靶向的能力,释放实验中,包裹pH敏感材料的壳聚糖纳米粒在模拟结肠液(pH 7.4)中的累积释放率达84.7%,且在24 h内持续有药物释放。Beloqui等[11]也将姜黄素、pH敏感剂Eudragit®S100、聚乳酸-羟基乙酸共聚物[poly(lactic-co-glycolic acid),PLGA]溶解于有机相中后滴入含有聚乙烯醇的水溶液中搅拌制得pH敏感纳米制剂,该纳米制剂系统在pH 1.5胃模拟液中和pH 4.5小肠模拟液中几乎不释放姜黄素,在pH 7.2结肠模拟液中1 h后的姜黄素释放率达90%。细胞摄取实验中,姜黄素纳米粒子比姜黄素游离溶液更易穿过上皮屏障,增加结肠癌Caco-2细胞对姜黄素的吸收。体内实验中,pH敏感的姜黄素纳米粒制剂明显减少DSS诱导小鼠中性粒细胞的浸润和TNF-α的分泌,降低MPO的水平,缓解DSS诱导的小鼠体质量下降,抑制结肠缩短;HE染色中,pH敏感纳米制剂组明显缓解结肠部位炎症情况。芦丁[64]、鞣花酸[65]、青藤碱[66]等也被报道有pH敏感型纳米制剂用于UC的治疗。
2.3 酶响应型
研究显示结肠含有大量有益微生物,这些微生物可以产生高活性的蛋白酶和多肽酶,可利用该特点制成结肠酶敏感的纳米制剂。制剂到达结肠后,酶敏感材料降解使药物释放,提升药物的生物利用度[67-69]。Castangia等[70]利用超声波反应法制备酶敏感的槲皮素脂质体治疗UC。Nutriose是一种可溶性玉米糊精,利用Nutriose的酶降解性制得酶敏感的复合脂质体。体内分布实验显示未包裹Nutriose的脂质体ig 4 h后在回肠和盲肠有较强的荧光,而在结肠只有微弱荧光;包裹Nutriose的脂质体ig 4 h后在结肠有明显的荧光。此外,该纳米递送体系能延迟药物释放,释放实验中,包裹Nutriose的脂质体在pH 7.0的结肠模拟液中8 h的累积释放率比未包裹的低15%。体内药效实验中,纳米制剂组明显减轻结肠区域的出血和溃疡情况,降低结肠水肿组织(colonic edema tissue,CAS)指数,降低MPO活性。壳聚糖包被海藻酸钙的微球装载淫羊藿苷也可通过酶响应将药物靶向递送至结肠[71]。
2.4 主动靶向型
研究发现,结肠的炎症部位具有大量的炎症相关蛋白,当在纳米粒上连接能特异性与其结合的物质后,可通过配体-受体作用达到结肠靶向的目的。硫酸软骨素(chondroitin sulfate,CS)是一种糖胺聚糖物质,可特异性地与CD44受体结合。CD44是活性巨噬细胞表面的一种跨膜转运糖蛋白。Gou等[72]利用丝素蛋白制备姜黄素纳米粒后在其表面接上CS,达到靶向结肠巨噬细胞的目的。巨噬细胞摄取实验中,给药处理4 h后硫酸软骨素纳米粒(chondroitin sulfate nanoparticles,CS-NPS)荧光明显强于姜黄素纳米粒。CS-NPS ig后,明显抑制小鼠体质量下降,下调TNF-α、IL-6的水平,上调IL-10的水平;HE染色实验中,免疫细胞的积累减少。CS-NPS ig后,15 d DSS处理后的小鼠存活率达50%。研究发现,结肠炎症老鼠体内结肠上皮细胞中的CD98上调,可通过干扰其合成来达到治疗UC的目的[73]。Xiao等[74]制备含CD98合成干扰RNA(CD98 siRNA,siCD98)的姜黄素水凝胶用于靶向治疗UC。利用PLGA包裹姜黄素和siCD98形成纳米粒,然后外包壳聚糖后与透明质酸连接,最后在壳聚糖和海藻酸钠存在下交联成水凝胶。细胞摄取实验中,透明质酸纳米粒组荧光强于姜黄素纳米组,证明表面修饰透明质酸(hyaluronic acid,HA)后能提升细胞对纳米粒子的摄取。进一步的细胞实验显示,该纳米制剂系统降低结肠癌Colon-26细胞中CD98的表达,下调MPO和粪便中载脂蛋白2(lipocalin-2,Lcn-2)的水平,减轻结肠溃疡情况,促进结肠组织恢复正常。也有研究者将具有靶向性的siRNA载入生姜来源的天然纳米粒中用于靶向治疗UC[75]。
2.5 其他
除了上述提到的靶向纳米系统外,还有一些如多孔纳米制剂、自组装纳米递送系统用于中药活性成分的结肠靶向递送。Chen等[76]利用双重乳化溶剂蒸发技术使用PLGA/PF127包载姜黄素,以碳酸氢铵(ammonium bicarbonate,ABC)作为致孔剂,产生多孔纳米粒,巨噬细胞摄取实验中,多孔纳米粒制剂荧光强于无孔纳米粒制剂。体内实验显示多孔纳米粒在结肠组织有更多的积累。Dou等[77]用自组装纳米乳化法构建鸦胆子苦素纳米粒,将鸦胆子苦素(bruceine D,BD)溶解在(聚乙二醇-15-羟基硬脂酸酯)-丙二醇-中链三酰甘油(4∶2∶1)溶液中,搅拌即得自组装纳米粒。与BD游离溶液相比,BD自组装纳米系统具有更好地抑制促炎症因子的作用,且能明显缓解UC组织的损伤。
口服结肠靶向纳米系统的递送原理见图2,中药活性成分治疗UC的口服结肠靶向纳米系统见表2。
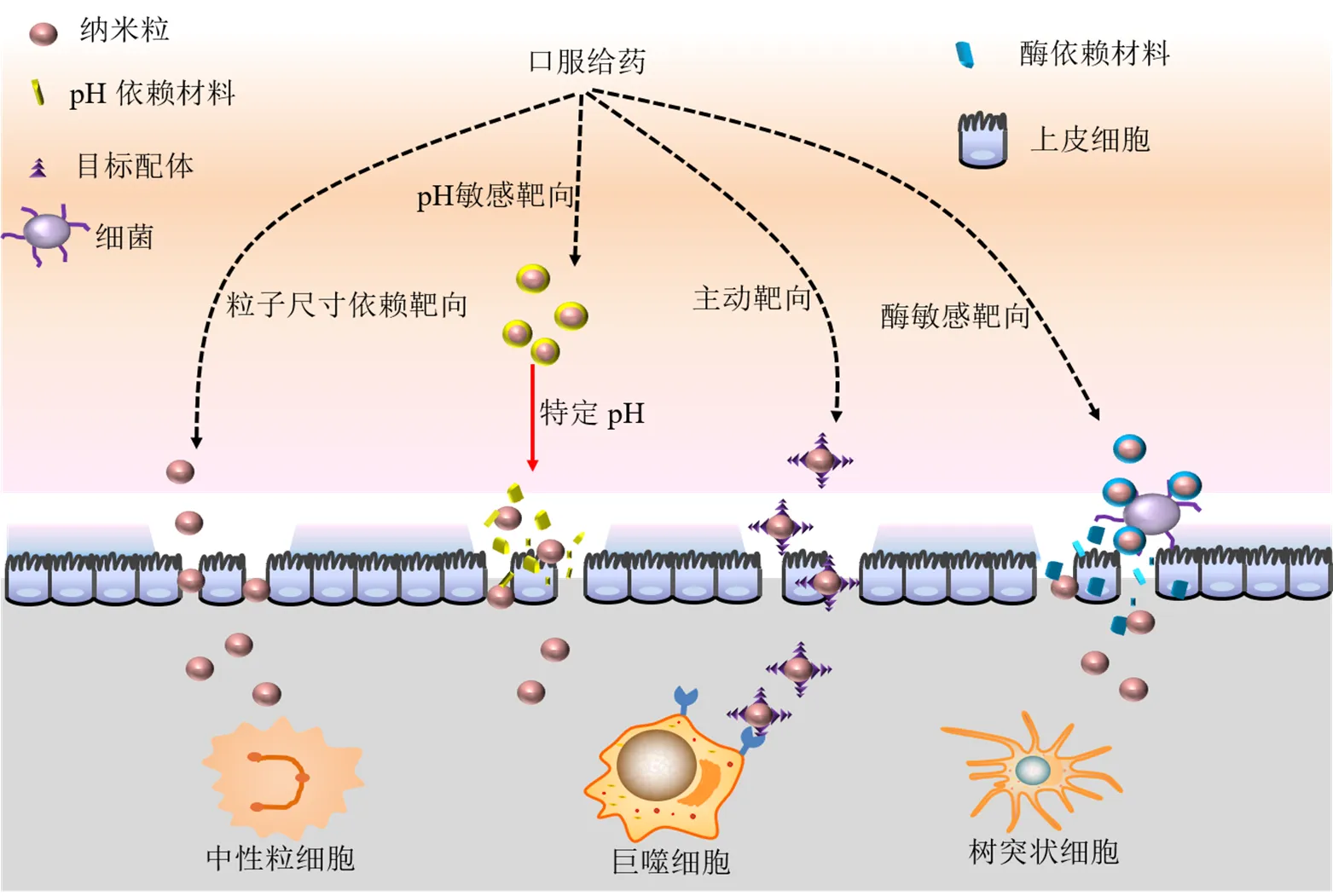
图2 口服结肠靶向纳米系统的递送原理

表2 中药活性成分治疗UC的口服结肠靶向纳米系统
↑-上升 ↓-下降 PF127-泊洛沙姆127 SF-丝素蛋白 PG-丙二醇 DAI-疾病活动指数 SBO-大豆油 VCO-天然椰油 MyD88-髓样分化因子;TRAF6-肿瘤坏死因子受体相关蛋白6 LPO-脂过氧化物 GSH-谷胱甘肽 LDH-乳酸脱氢酶
↑-increase ↓-decrease PF127-poloxamer F127 SF-silk fibroin PG-propylene glycol DAI-disease active index SBO-soya bean oil VCO-virgin coconut oil MyD88-myeloid differentiation factor 88 TRAF6-TNF receptor associated factor 6 LPO-lipid peroxidation GSH-glutathione LDH-lactate dehydrogenase
3 结语
传统中药具有多成分、多靶点的特点,在治疗以UC为代表性的慢性、复杂性疾病方面优势显著。随着研究的深入,中药干预UC的有效成分被发现且机制逐渐清楚,如姜黄素、雷公藤红素、白藜芦醇等。但是,在制剂开发过程中,其水不溶性、低生物利用度和结肠靶向性差等问题,局限了其使用。因此,如何通过口服有效递送药物到达结肠患病部位释放药物发挥治疗效果是亟待解决的问题。口服结肠靶向递药系统的构建可以解决这一问题,可根据粒子尺寸依赖、pH依赖、酶响应和配体-受体特异性配对等原理设计治疗UC的纳米制剂,一方面使药物溶解度增大,另一方面通过纳米功能化设计,使药物靶向结肠,富集于结肠部位从而提升对UC的疗效。然而,结肠靶向纳米制剂仍然存在一些问题:结肠靶向纳米制剂虽然能增加药物溶解度,但在某些体系中,药物的累积释放率不高且抗胃酸环境破坏力较弱;许多纳米的胃肠稳定性局限于体外的研究,其体内的变化有待进一步观察,且其长期稳定性有待深入;某些纳米制剂抵达结肠部位后其释放规律的准确性需要进一步的探讨;某些纳米制剂通过结肠黏膜细胞时渗透性不高,存在炎症或免疫相关细胞摄取率不高等问题,这些问题都会进一步限制结肠靶向纳米制剂运用于临床。但是,近年来,随着新型材料的运用、结肠特异性受体的发现和UC发病机制的研究,将会进一步提升口服制剂的结肠靶向性和专属性,为口服中药结肠靶向纳米制剂的设计提供思路。
利益冲突 所有作者均声明不存在利益冲突
[1] Gao C F, Liu L J, Zhou Y Y,. Novel drug delivery systems of Chinese medicine for the treatment of inflammatory bowel disease [J]., 2019, 14: 23.
[2] 申睿, 刘苗, 朱向东, 等. 中医药治疗溃疡性结肠炎实验研究进展 [J]. 中草药, 2018, 49(7): 1721-1725.
[3] 赵振营, 李亚卓, 于飞, 等. 溃疡性结肠炎中药组方规律及整合药理学分子机制探索 [J]. 中草药, 2018, 49(13): 3042-3050.
[4] Rauf A, Imran M, Suleria H A R,. A comprehensive review of the health perspectives of resveratrol [J]., 2017, 8(12): 4284-4305.
[5] Marques F Z, Markus M A, Morris B J. Resveratrol: Cellular actions of a potent natural chemical that confers a diversity of health benefits [J]., 2009, 41(11): 2125-2128.
[6] Chua L S. A review on plant-based rutin extraction methods and its pharmacological activities [J]., 2013, 150(3): 805-817.
[7] Nones K, Dommels Y E, Martell S,. The effects of dietary curcumin and rutin on colonic inflammation and gene expression in multidrug resistance gene-deficient (mdr1a-/-) mice, a model of inflammatory bowel diseases [J]., 2009, 101(2): 169-181.
[8] Habtemariam S, Belai A. Natural therapies of the inflammatory bowel disease: The case of rutin and its aglycone, quercetin [J]., 2018, 18(3): 234-243.
[9] Park J W, Shin N R, Shin I S,. Silibinin inhibits neutrophilic inflammation and mucus secretion induced by cigarette smoke via suppression of ERK-SP1pathway [J]., 2016, 30(12): 1926-1936.
[10] Kim B R, Seo H S, Ku J M,. Silibinin inhibits the production of pro-inflammatory cytokines through inhibition of NF-κB signaling pathway in HMC-1 human mast cells [J]., 2013, 62(11): 941-950.
[11] Beloqui A, Coco R, Memvanga P B,. pH-sensitive nanoparticles for colonic delivery of curcumin in inflammatory bowel disease [J]., 2014, 473(1/2): 203-212.
[12] Zhu L, Gu P, Shen H. Protective effects of berberine hydrochloride on DSS-induced ulcerative colitis in rats [J]., 2019, 68: 242-251.
[13] Li S, Han J, Wang D S,. Sinomenine attenuates chronic inflammatory pain in mice [J]., 2017, 32(1): 211-219.
[14] Tang J, Raza A, Chen J,. A systematic review on the sinomenine derivatives [J]., 2018, 18(11): 906-917.
[15] Zhou Y, Liu H Y, Song J,. Sinomenine alleviates dextran sulfate sodium‑induced colitis via the Nrf2/ NQO‑1 signaling pathway [J]., 2018, 18(4): 3691-3698.
[16] 熊永爱, 韩丽, 王淼, 等. 氧化苦参碱干预IκB-α蛋白对溃疡性结肠炎的治疗作用机制研究 [J]. 中国实验方剂学杂志, 2012, 18(8): 152-155.
[17] Li Q P, Zhai W W, Jiang Q L,. Curcumin-piperine mixtures in self-microemulsifying drug delivery system for ulcerative colitis therapy [J]., 2015, 490(1/2): 22-31.
[18] Zhang D K, Cheng L N, Huang X L,. Tetrandrine ameliorates dextran-sulfate-sodium-induced colitis in mice through inhibition of nuclear factor-kappaB activation [J]., 2009, 24(1): 5-12.
[19] Monisha B A, Kumar N, Tiku A B. Emodin and its role in chronic diseases [J]., 2016, 928: 47-73.
[20] Luo S, Deng X L, Liu Q,. Emodin ameliorates ulcerative colitis by the flagellin-TLR5 dependent pathway in mice [J]., 2018, 59: 269-275.
[21] Ge H, Tang H, Liang Y B,. Rhein attenuates inflammation through inhibition of NF-κB and NALP3 inflammasomeand[J]., 2017, 11: 1663-1671.
[22] Thippeswamy B S, Mahendran S, Biradar M I,. Protective effect of embelin against acetic acid induced ulcerative colitis in rats [J]., 2011, 654(1): 100-105.
[23] Zhao J, Luo D, Zhang Z,. Celastrol-loaded PEG-PCL nanomicelles ameliorate inflammation, lipid accumulation, insulin resistance and gastrointestinal injury in diet- induced obese mice [J]., 2019, 310: 188-197.
[24] Jia Z Y, Xu C F, Shen J Q,. The natural compound celastrol inhibits necroptosis and alleviates ulcerative colitis in mice [J]., 2015, 29(2): 552-559.
[25] Zhao Z J, Xiang J Y, Liu L,. Parthenolide, an inhibitor of the nuclear factor-κB pathway, ameliorates dextran sulfate sodium-induced colitis in mice [J]., 2012, 12(1): 169-174.
[26] Zhu Q, Zheng P F, Zhou J Y,. Andrographolide affects Th1/Th2/Th17 responses of peripheral blood mononuclear cells from ulcerative colitis patients [J]., 2018, 18(1): 622-626.
[27] Zhu Q, Zheng P F, Chen X Y,. Andrographolide presents therapeutic effect on ulcerative colitis through the inhibition of IL-23/IL-17 axis [J]., 2018, 10(2): 465-473.
[28] Guo Y, Wu X, Wu Q,. Dihydrotanshinone I, a natural product, ameliorates DSS-induced experimental ulcerative colitis in mice [J]., 2018, 344: 35-45.
[29] 陶赟, 龚国清, 钱之玉, 等. 西红花酸对溃疡性结肠炎大鼠模型的作用及其机制研究 [J]. 中国临床药理学与治疗学, 2013, 18(3): 263-270.
[30] Singh B. Psyllium as therapeutic and drug delivery agent [J]., 2007, 334(1/2): 1-14.
[31] Laroui H, Dalmasso G, Nguyen H T,. Drug-loaded nanoparticles targeted to the colon with polysaccharide hydrogel reduce colitis in a mouse model [J]., 2010, 138(3): 843-853.
[32] Wu X, Dou Y N, Yang Y,. Arctigenin exerts anti-colitis efficacy through inhibiting the differentiation of Th1 and Th17 cells via an mTORC1-dependent pathway [J]., 2015, 96(4): 323-336.
[33] Dong K, Zeng A G, Wang M L,.andstudy of a colon-targeting resin microcapsule loading a novel prodrug, 3,4,5-tributyryl shikimic acid [J]., 2016, 6(20): 16882-16890.
[34] Huang Y F, Zhou J T, Qu C,. Anti-inflammatory effects ofoil emulsion by suppressing NF-κB activation on dextran sulfate sodium-induced ulcerative colitis in mice [J]., 2017, 198: 389-398.
[35] Grzanna R, Lindmark L, Frondoza C G. Ginger: An herbal medicinal product with broad anti-inflammatory actions [J]., 2005, 8(2): 125-132.
[36] Zhang M, Viennois E, Prasad M,. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer [J]., 2016, 101: 321-340.
[37] Brown A C, Shah C, Liu J,. Ginger's (Roscoe) inhibition of rat colonic adenocarcinoma cells proliferation and angiogenesis[J]., 2009, 23(5): 640-645.
[38] Kwon K H, Murakami A, Tanaka T,. Dietary rutin, but not its aglycone quercetin, ameliorates dextran sulfate sodium-induced experimental colitis in mice: Attenuation of pro-inflammatory gene expression [J]., 2005, 69(3): 395-406.
[39] Guazelli C F, Fattori V, Colombo B B,. Quercetin-loaded microcapsules ameliorate experimental colitis in mice by anti-inflammatory and antioxidant mechanisms [J]., 2013, 76(2): 200-208.
[40] Luo X P, Yu Z L, Deng C,. Baicalein ameliorates TNBS-induced colitis by suppressing TLR4/MyD88 signaling cascade and NLRP3 inflammasome activation in mice [J]., 2017, 7: 16374.
[41] Zhang C L, Zhang S, He W X,. Baicalin may alleviate inflammatory infiltration in dextran sodium sulfate-induced chronic ulcerative colitis via inhibiting IL-33 expression [J]., 2017, 186: 125-132.
[42] Tao F F, Qian C, Guo W J,. Inhibition of Th1/Th17 responses via suppression of STAT1 and STAT3 activation contributes to the amelioration of murine experimental colitis by a natural flavonoid glucoside icariin [J]., 2013, 85(6): 798-807.
[43] Chao L, Zheng P Y, Xia L,. Calycosin attenuates dextran sulfate sodium (DSS)-induced experimental colitis [J]., 2017, 20(9): 1056-1062.
[44] Al-Rejaie S S, Abuohashish H M, Al-Enazi M M,. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats [J]., 2013, 19(34): 5633-5644.
[45] He X X, Wei Z K, Wang J J,. Alpinetin attenuates inflammatory responses by suppressing TLR4 and NLRP3 signaling pathways in DSS-induced acute colitis [J]., 2016, 6: 28370.
[46] Arafa H M, Hemeida R A, El-Bahrawy A I,. Prophylactic role of curcumin in dextran sulfate sodium (DSS)-induced ulcerative colitis murine model [J]., 2009, 47(6): 1311-1317.
[47] Yadav V R, Suresh S, Devi K,. Novel formulation of solid lipid microparticles of curcumin for anti-angiogenic and anti-inflammatory activity for optimization of therapy of inflammatory bowel disease [J]., 2009, 61(3): 311-321.
[48] Abdin A A. Targeting sphingosine kinase 1 (SphK1) and apoptosis by colon-specific delivery formula of resveratrol in treatment of experimental ulcerative colitis in rats [J]., 2013, 718(1/2/3): 145-153.
[49] Yum S, Jeong S, Lee S,. Colon-targeted delivery of piceatannol enhances anti-colitic effects of the natural product: Potential molecular mechanisms for therapeutic enhancement [J]., 2015, 9: 4247- 4258.
[50] Marín M, María Giner R, Ríos J L,. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis [J]., 2013, 150(3): 925-934.
[51] Shen P, Zhang Z C, He Y,. Magnolol treatment attenuates dextran sulphate sodium-induced murine experimental colitis by regulating inflammation and mucosal damage [J]., 2018, 196: 69-76.
[52] Shaker M E, Ashamallah S A, Houssen M E. Celastrol ameliorates murine colitis via modulating oxidative stress, inflammatory cytokines and intestinal homeostasis [J]., 2014, 210: 26-33.
[53] Xiao B, Merlin D. Oral colon-specific therapeutic approaches toward treatment of inflammatory bowel disease [J]., 2012, 9(11): 1393- 1407.
[54] Collnot E M, Ali H, Lehr C M. Nano- and microparticulate drug carriers for targeting of the inflamed intestinal mucosa [J]., 2012, 161(2): 235-246.
[55] Hua S S, Marks E, Schneider J J,. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue [J]., 2015, 11(5): 1117-1132.
[56] Lamprecht A, Yamamoto H, Takeuchi H,. Nanoparticles enhance therapeutic efficiency by selectively increased local drug dose in experimental colitis in rats [J]., 2005, 315(1): 196-202.
[57] Lamprecht A, Schäfer U, Lehr C M. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa [J]., 2001, 18(6): 788-793.
[58] de Jong W H, Borm P J. Drug delivery and nanoparticles: Applications and hazards [J]., 2008, 3(2): 133-149.
[59] Powell J J, Faria N, Thomas-McKay E,. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract [J]., 2010, 34(3): J226- J233.
[60] Ma P P, Si X Y, Chen Q B,. Oral drug delivery systems for ulcerative colitis therapy: A comparative study with microparticles and nanoparticles [J]., 2019, 19(4): 304-311.
[61] Zhang M Z, Merlin D. Nanoparticle-based oral drug delivery systems targeting the colon for treatment of ulcerative colitis [J]., 2018, 24(7): 1401-1415.
[62] Nugent S G, Kumar D, Rampton D S,. Intestinal luminal pH in inflammatory bowel disease: Possible determinants and implications for therapy with aminosalicylates and other drugs [J]., 2001, 48(4): 571-577.
[63] Raj P M, Raj R, Kaul A,. Biodistribution and targeting potential assessment of mucoadhesive chitosan nanoparticles designed for ulcerative colitis via scintigraphy [J]., 2018, 8(37): 20809-20821.
[64] Abdel Ghaffar A M, Radwan R R, Ali H E. Radiation synthesis of poly(starch/acrylic acid) pH sensitive hydrogel for rutin controlled release [J]., 2016, 92: 957-964.
[65] Jeong Y I, Yv̄ R P, Ohno T,. Application of Eudragit P-4135F for the delivery of ellagic acid to the rat lower small intestine [J]., 2001, 53(8): 1079-1085.
[66] Xiong H, Tian L, Zhao Z,. The sinomenine enteric-coated microspheres suppressed the TLR/NF-κB signaling in DSS-induced experimental colitis [J]., 2017, 50: 251-262.
[67] Chourasia M K, Jain S K. Polysaccharides for colon targeted drug delivery [J]., 2004, 11(2): 129-148.
[68] Basit A W, Short M D, McConnell E L. Microbiota- triggered colonic delivery: Robustness of the polysaccharide approach in the fed state in man [J]., 2009, 17(1): 64-71.
[69] Pinto J F. Site-specific drug delivery systems within the gastro-intestinal tract: From the mouth to the colon [J]., 2010, 395(1/2): 44-52.
[70] Castangia I, Nácher A, Caddeo C,. Therapeutic efficacy of quercetin enzyme-responsive nanovesicles for the treatment of experimental colitis in rats [J]., 2015, 13: 216-227.
[71] Wang Q S, Wang G F, Zhou J,. Colon targeted oral drug delivery system based on alginate-chitosan microspheres loaded with icariin in the treatment of ulcerative colitis [J]., 2016, 515(1/2): 176-185.
[72] Gou S Q, Huang Y M, Wan Y,. Multi-bioresponsive silk fibroin-based nanoparticles with on-demand cytoplasmic drug release capacity for CD44-targeted alleviation of ulcerative colitis [J]., 2019, 212: 39-54.
[73] Kucharzik T, Lugering A, Yan Y T,. Activation of epithelial CD98 glycoprotein perpetuates colonic inflammation [J]., 2005, 85(7): 932-941.
[74] Xiao B, Zhang Z, Viennois E,. Combination therapy for ulcerative colitis: Orally targeted nanoparticles prevent mucosal damage and relieve inflammation [J]., 2016, 6(12): 2250-2266.
[75] Zhang M Z, Wang X Y, Han M K,. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis [J].(Lond), 2017, 12(16): 1927-1943.
[76] Chen Q B, Gou S Q, Ma P P,. Oral administration of colitis tissue-accumulating porous nanoparticles for ulcerative colitis therapy [J]., 2019, 557: 135-144.
[77] Dou Y X, Zhou J T, Wang T T,. Self- nanoemulsifying drug delivery system of bruceine D: A new approach for anti-ulcerative colitis [J]., 2018, 13: 5887-5907.
[78] Chen Q B, Si X Y, Ma L J,. Oral delivery of curcuminporous polymeric nanoparticles for effective ulcerative colitis therapy [J]., 2017, 5(29): 5881-5891.
[79] Xiao B, Si X Y, Zhang M Z,. Oral administration of pH-sensitive curcumin-loaded microparticles for ulcerative colitis therapy [J]., 2015, 135: 379-385.
[80] Varshosaz J, Minaiyan M, Khaleghi N. Eudragit nanoparticles loaded with silybin: A detailed study of preparation, freeze-drying condition and/evaluation [J]., 2015, 32(3): 211-223.
[81] Yen C C, Chen Y C, Wu M T,. Nanoemulsion as a strategy for improving the oral bioavailability and anti-inflammatory activity of andrographolide [J]., 2018, 13: 669-680.
[82] Badamaranahalli S S, Kopparam M, Bhagawati S T,. Embelin lipid nanospheres for enhanced treatment of ulcerative colitis-Preparation, characterization andevaluation [J]., 2015, 76: 73-82.
Research progress on oral colon targeting nano system of Chinese medicine active ingredients in treatment of ulcerative colitis
XIAN Jing, ZHANG Chen, ZHONG Xue-mei, FU Chao-mei, GAO Fei, ZHANG Jin-ming
School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China
Ulcerative colitis (UC) is a common and refractory disease. Because of its repeated onset, it is often necessary to use sulfasalazine, immunosuppressor agents and other drugs for a long time, but its efficacy is limited and it is easy to produce side effects. Modern studies have found that polyphenols, alkaloids, quinones, terpenoids and other active components of traditional Chinese medicine can alleviate UC better through multi-target mechanism, and the side effects are relatively low, but there are pharmaceutical problems such as poor water solubility, gastrointestinal instability and poor colon targeting. In view of the above problems, the researchers have constructed a variety of oral colon targeted nano system of active ingredients of traditional Chinese medicine, which can significantly improve the therapeutic effect of UC by avoiding gastrointestinal tract damage, prolonging intestinal retention, and achieving controlled release of drugs in the focus. In this paper, active components of traditional Chinese medicine with prevention and treatment effect of UC and their mechanisms, and the research progress on oral colon targeting nano system of Chinese herbal active ingredients in treatment of ulcerative colitisare reviewed, in order to provide ideas for active components of traditional Chinese medicine in oral targeted treatment of UC.
active components of traditional Chinese medicine; ulcerative colitis; nano-preparations; colon targeting; drug delivery system
R283.6
A
0253 - 2670(2021)06 - 1816 - 11
10.7501/j.issn.0253-2670.2021.06.031
2020-07-06
国家自然科学基金青年基金资助项目(81903811);2018—2020年度中华中医药学会青年人才托举工程资助项目(QNRC1-01)
鲜 静(1996—),女,硕士研究生,研究方向为中药药剂学。Tel: 18728456014 E-mail: xianjing4320@126.com
章津铭,男,博士,教授,主要从事中药新制剂与新剂型研究。Tel: (028)61800101 E-mail: zhangjinming1987@126.com
高 飞,男,博士,副研究员。Tel: (028)61800101 E-mail: feigao207@yeah.net
[责任编辑 崔艳丽]
