Carbazole ring:A delicate rack for constructing thermally activated delayed fluorescent compounds with through-space charge transfer
2021-03-14KuofeiLiToWngBingYoYunnnChenHoDengHongmeiZhnZhiyunXieYnxingCheng
Kuofei Li,To Wng,Bing Yo,Yunnn Chen,Ho Deng,Hongmei Zhn,∗,Zhiyun Xie,Ynxing Cheng,∗
a State Key Laboratory of Polymer Physics and Chemistry,Changchun Institute of Applied Chemistry,Chinese Academy of Sciences,Changchun 130022,China
b School of Applied Chemistry and Engineering,University of Science and Technology of China,Hefei 230026,China
Keywords:Thermally activated delayed fluorescence Through-space charge transfer Carbazole Solution-processed devices Sky-blue emission
ABSTRACT Three carbazole derivatives,AcPTC,PxPTC and PtPTC,consisting of two 9,9-dimethyl-9,10-dihydroacridine,phenoxazine or phenothiazine donor groups and one diphenyltriazine acceptor group fixed at 1,8,9-positions of a single carbazole ring via phenylene,are designed and synthesized.X-ray diffraction analysis of AcPTC reveals that there exist multiple π-π interactions between the donor and acceptor groups to form a sandwich-like structural unit with edge-to-face interaction model.The compounds thus show obvious thermally activated delayed fluorescence with through-space charge transfer character and possess considerable photoluminescence quantum yields of up to 73% in doped films with sky-blue to yellow emissions.The solution-processed electroluminescent devices achieve the highest maximum external quantum efficiencies of 10.0%,11% and 5.6% for AcPTC,PxPTC and PtPTC,respectively,with small effi-ciency roll-offs.
Thermally activated delayed fluorescent (TADF) materials have attracted more attention as the third-generation emitters in recent years since they can achieve 100% internal quantum efficiency by harvesting both singlet and triplet excitons simultaneously in the electroluminescence (EL) process without using noble metals[1–3].In most purely and simply organic TADF compounds,a general strategy is to construct a twisted donor and acceptor structural unit,in order to spatially separate the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital(LUMO),and reduce the energy gap (ΔEST) between the lowest singlet (S1) and triplet (T1) excited states.In this case,the TADF radiative transition is from through-bond charge transfer (TBCT) [4–8].Alternatively,employing through-space charge transfer (TSCT) can also realize efficient TADF [9–12].In the emitters,donor and acceptor are fixed onto the specific positions of a framework and enable strong through-spaceπ-πconjugation,which can improve electronic coupling and thus enhance the photoluminescence quantum yield (PLQY).For example,an external quantum efficiency (EQE) of up to 30.8% was achieved based on such an emitter,proving the success of the way [13].
Carbazole (Cz) is an excellent electron-donating group with high T1energy level,hole transport ability and good thermal and photochemical stability,and thus often used to build optoelectronic materials including TADF emitters [14–18].In previous work,two donors and one acceptor are linked at 1,8,9-positios of a single Cz ring and form a firm sandwich structural unit with a closely parallel arrangement.The unit hence ensures multipleπ-πinteractions,achieving efficient TADF and high EQEs of up to 24.3% [19].Inspired by the result,here a successive work is carried out by introducing three six-membered central ring donors of 9,9-dimethyl-9,10-dihydroacridine (Ac),phenoxazine (Px) or phenothiazine (Pt)at 1,8-positions of the Cz ring,respectively,to provide three compounds AcPTC,PxPTC and PtPTC,as shown in Fig.1a.Unlike the face-to-face stacking in the compounds reported previously [11,19-23],these compounds adopt an edge-to-face model with strong conjugation,and thus form an efficient TSCT channel.Moreover,owing to the restricted rotation of the cyclic donor,the double twisted conformation along Cz,1,8-position bridged phenylene and donor prevents the HOMO distribution from diffusing to Cz ring.The complete separation of HOMO and LUMO produces a suffi-ciently smallΔEST.The compounds all show legible TADF features with over 82% proportions of delayed components.The solutionprocessed OLEDs using AcPTC exhibits sky-blue emission with a peak at 484 nm and a maximum EQE of 10.0%,while the devices using PxPTC and PtPTC show green and yellow emissions with the maximum EQEs of 11.0% and 5.6%,respectively.
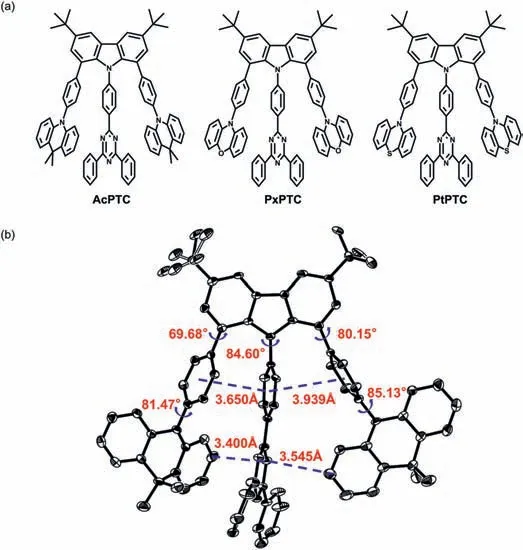
Fig.1.(a) Chemical structures of the compounds AcPTC,PxPTC and PtPTC.(b) Crystal structure of AcPTC.
Three compounds were synthesized by the Suzuki crosscoupling reaction with the good yields and detailed synthetic procedures and analytical data are provided in Supporting information.As shown in Fig.1b,the crystal structure of AcPTC clearly shows the strong edge-to-faceπ-πinteraction with the shortest distances of 3.400 and 3.545 ˚A between the Ac and triazine groups.The torsion angles between Cz and bridged benzene rings are 69.68,84.60 and 80.15°,respectively,while the similar torsion angles of 81.47 and 85.13° are found between Ac groups and bridged benzene rings.Owing to the steric effect of three substituents at adjacent 1,8,9-positions and bonding directionality of Cz ring,two Ac groups have to adopt the closely parallel arrangement with Cz ring,whereas three bridged benzene rings have the nearly perpendicular linkage with Cz ring and meanwhile become cofacially aligned.The structural factors therefore compel the donor Ac groups to only conjugate to the acceptor triazine group with the edge-to-face stacking.
The through-space conjugation is also confirmed by reduced density gradient analysis (Fig.S3 in Supporting information)[24,25].Furthermore,the large torsion angles among Cz,bridged benzene rings and donor groups,giving rise to a supposed double twisting along their connection,restrict the distributions of HOMOs.As shown in Fig.2,the HOMOs almost entirely distribute on donors and no HOMOs spread onto Cz ring,while the LUMOs mainly locate on triphenyltriazine with a little amount extending to Cz ring.The former is visibly different from the HOMO distributions in the compounds with diphenylamine as a donor group[19–21].Meanwhile no overlap of HOMO and LUMO on Cz is observed.The facts imply that there solely exists a TSCT transition pathway in the three compounds,rather than involving potential TSCT and TBCT two ones.In addition,the sandwich-like structures derived from the multipleπ-πinteractions bring about the excellent thermal stability of three compounds with the high decomposition temperatures of above 410 °C (Fig.S2 in Supporting information).
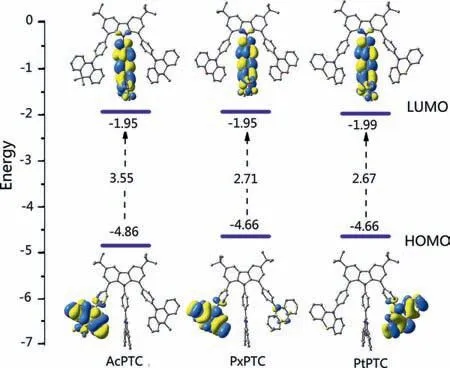
Fig.2.The distributions of the HOMOs and LUMOs of AcPTC,PxPTC and PtPTC obtained from TD-DFT at the B3LYP/6–31G(d) level.
The UV–vis absorption and PL spectra of three compounds both in toluene and film were measured to investigate their photophysical properties.The absorption of TSCT is very weak due to the completely separated HOMO and LUMO [26,27],as shown in Fig.3,whereas the intense absorption before 360 nm is attributed toππ∗transition.Nevertheless,the gradually intensified absorptions are still observed from the low energy edge of absorption in the order of AcPTC,PxPTC and PtPTC,which is consistent with the gradually increased electron donating ability of three donor groups[5,28],verified by their HOMO energy levels of-5.31,-5.19 and-5.05 eV with the close LUMO ones of-2.93,-2.97 and-2.99 eV determined by the cyclic voltammetry experiments.Correspondingly,three compounds in toluene exhibit the orderly red-shifted emissions with the peaks from 498,544 to 561 nm,which are close to those both in neat films and analogue exciplexes composed of the corresponding donor and acceptor with molar ratios of 2:1 (Fig.S7 in Supporting information).The PL data testify again that the emissions of the compounds only originate from the TSCT transition,which is in agreement with theoretical calculation and crystal structural analysis.In the 20 wt% doped films with SimCP2 (bis[3,5-di(carbazol-9-yl)phenyl]diphenylsilane)as host,however,their emissions obviously blue-shift relative to those both in solution and neat film,implying that the intermolecularπ-πinteractions are effectively eliminated in the doped systems (Fig.S3d in Supporting information).The effect is also reflected in the PLQYs of the compounds,which are all less than 22%in toluene,but sharply improved to 73%,61% and 51% for AcPTC,PxPTC and PtPTC,respectively,together with the increased rigidity from the restricted intramolecular rotation and vibration in the doped films [29].The T1energy levels measured from 77 K phosphorescence spectra are 2.82,2.64 and 2.55 eV (Fig.S5 in Supporting information),respectively,and thus the smallΔESTs of less than 0.05 eV are generated,indicating highly efficient TADF features in the three compounds (Table 1).
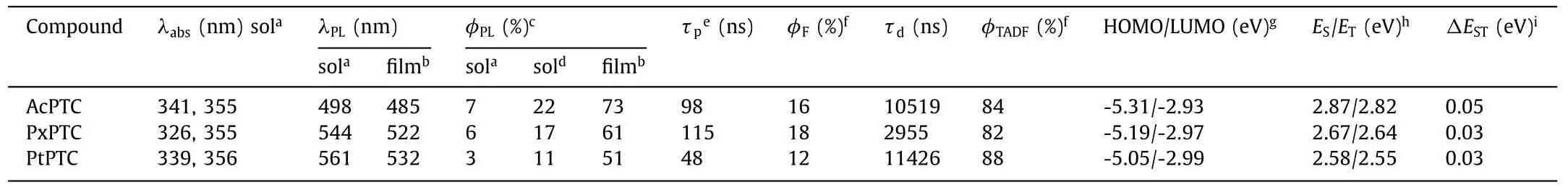
Table 1 Photophysical properties of AcPTC,PxPTC and PtPTC.
The TADF characteristics are further confirmed by the transient PL decay spectra shown in Figs.S8 and S9 (Supporting information).The compounds all exhibit prompt and delayed fluorescent components at room temperature both in solution and doped film.It should be noted that the proportions of the delayed components are more than 82% for all compounds in doped films,while the lifetimes of 10.5,2.96 and 11.4 μs are longer than those in solution.Furthermore,the proportion of the delayed component of AcPTC is positively related to the rising temperature,which directly proves that the delayed fluorescence is from the RISC process.
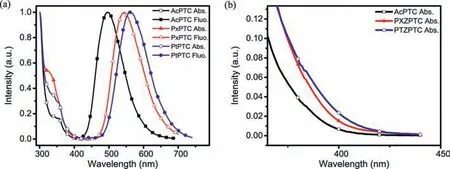
Fig.3.(a) The UV–vis absorption and PL spectra of AcPTC,PxPTC and PtPTC measured in toluene with the concentration of 10-5 mol/L.(b) The details of the edge of the absorption spectra.
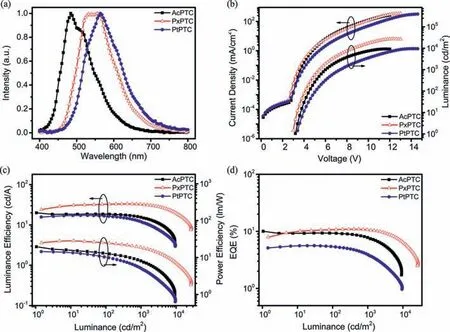
Fig.4.Device performances for AcPTC-,PxPTC-and PtPTC-doped films:(a) EL spectra;(b) current density–voltage–luminance curves;(c) luminous efficiency–luminance–power efficiency curves;(d) EQE-luminance curves.
In view of the large molecular weight and good solubility of three compounds,the solution-processed OLEDs were fabricated to explore their EL behaviors.The device structure is ITO/PEDOT:PSS(40 nm)/SimCP2:20wt% emitter (40 nm)/TmPyPB (60 nm)/LiF(1 nm)/Al (100 nm),in which PEDOT:PSS and LiF act as holeand electron-injecting layers,and TmPyPB (1,3,5-tri(m-pyrid-3-ylphenyl)benzene) is electron-transporting layer.The EL characteristic curves are presented in Fig.4 and the device performances are summarized in Table S5.The device using the compound AcPTC shows sky-blue emission peaked at 484 nm with the CIE coordinates of (0.18,0.26),while the devices with PxPTC and PtPTC as dopants display green and yellow emissions with the maximum emissive wavelengths at 533 and 564 nm,which are in agreement with their PL spectra.The maximum EQEs of the OLEDs all exceed the upper-limit (5%) of the conventional fluorescent device,indicating that the electro-generated triplet excitons can be harvested for EL through TADF mechanism.Among them,the device with AcPTC achieves the maximum EQE of 10.0%,while the device with PxPTC shows more slowly efficiency roll-off from the maximum EQE 11.0% to 10.7% at a luminance of 1000 cd m2with a roll-off rate of 3%.In contrast,probably due to the relatively redshifted emission and/or not well matched host,PtPTC just realizes 5.6% of EQE,which should be improved by using an optimized device structure [11].
In conclusion,three TADF compounds containing a single Cz ring have been successfully synthesized and fully characterized.The Cz ring almost completely acts a rack role to anchor two donor and one acceptor groups at its 1,8,9-positions,instead of donor character.Benefiting from the bonding directional nature of the aromatic Cz ring and the rigid linkage between the Cz ring and donor/acceptor groups,a sandwich-like structural unit is formed by the two donor and one inserted acceptor groups through the edge-to-face conjugation.Therefore,the compounds possess TADF features with the full TSCT transition,completely separated HOMO and LUMO,smallΔESTs and over 82% proportion of delayed component.The emissive wavelength can be shifted from sky-blue,green to yellow emissions by means of replacing the donor groups at 1,8-positions of the Cz ring.The solutionprocessed OLEDs using three compounds achieve the considerable EQEs with the small efficiency roll-off.Especially for AcPTC,its device shows sky-blue emission of 484 nm with a maximum EQE of 10.0%.The results mean that this strategy is feasible to develop new TADF emitters including full spectrum emissive materials by altering donor/acceptor groups.Further work is in progress in our group.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.51973210,21805271 and 21674110) and the Science and Technology Development Project of Jilin Province,China (No.20190201071JC).The authors are grateful to Network and Computing Center,Changchun Institute of Applied Chemistry,Chinese Academy of Sciences for the essential support.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.05.054.
杂志排行
Chinese Chemical Letters的其它文章
- Long-wavelength (red to near-infrared) emissive carbon dots:Key factors for synthesis,fluorescence mechanism,and applications in biosensing and cancer theranostics
- Nanotechnology combining photoacoustic kinetics and chemical kinetics for thrombosis diagnosis and treatment
- The point-of-care-testing of nucleic acids by chip,cartridge and paper sensors
- Sodium bicarbonate,an inorganic salt and a potential active agent for cancer therapy
- New advances in gated materials of mesoporous silica for drug controlled release
- Current development in wearable glucose meters
