Current development in wearable glucose meters
2021-03-14QiuyuanChenYanZhaoYunqiLiu
Qiuyuan Chen,Yan Zhao,Yunqi Liu
Department of Materials Science,Fudan University,Shanghai 200433,China
Keywords:Diabetes Glucose sensors Noninvasive Electrochemical Healthcare systems Wearable devices
ABSTRACT Diabetes is one of the most disturbing chronic diseases in the world.The improvement of treatment efficiency brought by self-monitoring of blood glucose can relieve symptoms and reduce complications,which is considered as the gold standard of diabetes diagnosis and nursing.Compared to the traditional finger pricking measurement with painful and discontinuous processes,continuous blood glucose monitoring (CGM) presents superior advantages in wearable and continuous assessment of blood glucose levels.However,widely used implantable CGM systems at present require implantation operation and are highly invasive,so it is hard to be accepted by users.Except for the blood,available fluids in humans,such as interstitial fluid (ISF),sweat,tears and saliva,also contain glucose associated with blood sugar and can be extracted more easily.Therefore,these more accessible fluids are expected to realize minimized traumatic blood glucose monitoring.This review introduces the latest development of wearable minimally-/non-invasive CGM device,focusing on the types of blood substitute biological fluid and suitable monitoring approaches.We also analysis the merits and drawbacks of each method,and discuss the properties such as sensitivity,stability and convenience of each meter.Beyond highlighting recent key work in this field,we discuss the future development trend of wearable minimally-/non-invasive glucose meters.
1.Introduction
Diabetes is an incurable global disease and has become one of the greatest threats to humans at this stage.According to the International Diabetes Federation,in 2019,463 million adults aged 20–79 worldwide had diabetes,accounting for 1/11 of the world’s adults;by 2030,the number of diabetics would reach 578.4 million;by 2045,it would reach 700.2 million.In addition,according to calculations by the American Diabetes Association (ADA),the total cost of diabetes in 2017 is estimated to be 327 billion USD,which is about 26% higher than in 2012 after deducting inflation.What is more,for individuals,the medical expenses of diabetic patients are on average about 2.3 times higher than that of people without diabetes [1].
Diabetes is caused by impaired glucose metabolism.Most diabetes mellitus can be divided into type Ⅰdiabetes and type Ⅱdiabetes according to the etiology [2].Type Ⅰdiabetes is caused by autoimmune attacks that destroy insulin-producingβcells in the pancreas,accounting for about 10% of all diabetes cases.This type of diabetes usually begins in childhood,also known as adolescent diabetes or insulin-dependent diabetes.Type Ⅱdiabetes is a metabolic disease characterized by hyperglycemia,including insulin resistance and relative insulin deficiency,accounting for about 90%.This type of diabetes usually occurs in people over 40 years of age,also known as adult-onset or non-insulin-dependent diabetes [2-4].Diabetes can bring serious and dangerous complications,including retinopathy,renal failure,diabetic foot,and cardiovascular diseases (such as atherosclerosis,coronary artery disease,heart disease).As a supplement,people with diabetes are at risk of hypertension,blindness,stroke,epilepsy,and amputation [2].Selfmonitoring of blood glucose allows patients to implement strict blood glucose control outside the hospital [5],combined with precise drug treatments can effectively improve treatment efficiency and reduce the risk of complications [6].Thus,millions of diabetics test their blood sugar levels every day,making glucose the most tested analyte [7].
The overwhelming blood sugar test method is the electrochemical monitoring method using enzymes.There are already kinds of enzymes that can be used to detect glucose concentrations such as glucose oxidase (GOx) or glucose dehydrogenase (GDH) [7].GOXcatalyze the oxidation of glucose to gluconic acid with the help of oxygen,while producing the by-product hydrogen peroxide (H2O2) (Reaction 1).

Initially,the degree of the above reaction was characterized by measuring the amount of oxygen reduction.At the cathode of the detector,oxygen reduction occurs (Reaction 2).

Advances in technology occurred in 1973 when Guilbault and Lubrano designed an enzyme electrode (anode) to detect hydrogen peroxide products to measure blood sugar (Reaction 3).The improved detection method brings accuracy and precision [8].

To date,electrochemical detectors have spanned three technical generations.The history began in 1962,when Clark and Lyons of Cincinnati Children’s Hospital jointly developed the first device using a glucose enzyme electrode [9].The first generation relied on oxygen in the air to oxidize (Reaction 1) and measure the production of hydrogen peroxide (Reaction 3).The improvement of the second generation lies in the use of a non-physiological electron acceptor to shuttle electrons,which increases oxygen efficiency and accounts for most of the market share.However,due to the charge transfer between the enzyme and the electrode is hindered,an additional electronic medium is required to enhance the charge transfer [10].Therefore,the third-generation sensors are aiming to get rid of artificial mediators and even eliminate glucose enzymes[6,7,11].
At the state of art,most diabetic patients use painful and invasive pricking method such as finger-pricking to detect daily blood glucose concentrations.Finger-pricking is a simple and reliable method,but on the contrary,it will also cause discomfort,inconvenience and pain to patients,and even damage the tissue health of fingers [12].In addition,patients were unable to collect blood sample by themselves at night,so it is unattainable for patients to continuously understand the changes in their blood glucose levels in 24 h [13].
Therefore,continuous blood glucose monitoring (CGM) has attracted much attention because the real-time information about the magnitude,direction and duration of glucose concentration changes provided by CGM devices improve blood glucose control [14].However,the implantable CGM system requires clinical surgery,and it also has potential pain,biological contamination,and immune response problems [15,16].
People never give up optimizing blood sugar detection methods.This emerging area involves the non-invasive or at least minimally invasive measurement of blood glucose by human integrated electronic sensors,which are interested in the collection,measurement and analysis of previously less involved biological fluids.Because these biological fluids,such as sweat,tears,saliva and interstitial fluid (ISF),also contain glucose,and their glucose concentrations are related to blood glucose concentrations [11,17,18].Specifically,the glucose concentration in blood ranges from 2~40 mmol/L,in ISF is 1.99~22.2 mmol/L,in sweat is 0.01~1.11 mmol/L,in tears is 0.05~5 mmol/L,and in saliva is 0.008~1.77 mmol/L [3,19,20].In recent years,wearable glucose detectors based on these body fluids are becoming a research hotspot (Fig.1).The wearable glucose detector provides detailed information that cannot be obtained by intermittent blood sampling,and can display parameters such as blood glucose concentration,rate of change in real time (24/7).It is worth noting that the constructive development of flexible and stretchable materials and electronic devices will explode with great opportunities in wearable healthcare meters.
In this review,we provide a timely and comprehensive overview of wearable minimally-/non-invasive blood glucose detectors for sampling biological fluid other than blood.In this article,we highlight four biological fluids,including:ISF,sweat,lacrimal fluid,and oral cavity’s saliva.This review provides an exhaustive analysis of the advantages and disadvantages of various methods for detecting each kind of body fluid,and consequently offer novel insights into the future development prospects toward new-generation electronics.
2.Glucose monitoring in epidermal fluid
2.1.Glucose monitoring in interstitial fluid (ISF)
Cell-to-cell spaces in multicellular animals are filled with thin layers of fluid generally,known as interstitial fluid (ISF).It is a fluid environment in which ordinary cells live directly and can easily exchange substances with surrounding cells,capillaries or lymphatic capillaries.Therefore,the composition (such as glucose,fatty acids,salts and minerals [21]) of ISF is very similar to that of blood.In the subcutaneous tissue of human beings,glucose molecules diffuse from the blood to the ISF [16],which is a channel for cells to obtain material/energy supplies,so the concentration of glucose in the epidermal ISF is in accurate equilibrium with the concentration of blood glucose [17].However,other interfering substances,such as proteins,lipids and blood cells,cannot be transferred to the ISF because of their high molecular weight [16],which reduces the interference in measurement and the scale,and peculiar smell in epidermal detectors formed by accumulated proteins.Therefore,the detection of glucose concentration in ISF has the merits of high correlation with blood glucose [17,22],relatively high glucose concentration (at least one order of magnitude higher than those in the human serums mentioned later),good stability (compared with the discontinuous and astable secretion of sweat,and tears,the amount of ISF is very steady [23]),and mature technologies(several commercial devices have been developed [24-30]).Gleaning ISF requires pathognomonic installations,where micron needles [31]/needles arrays,iontophoresis/reverse iontophoresis [30],sonophoresis [32],etc.are potential candidates.
In 1976,microneedles (MNs) were first introduced by Gestel and Place for the purpose of administration [33].The MNs arrays usually grow neatly on a substrate,with lengths of hundreds of micrometers,and with an internal diameter less than 100 μm[34,35](exceptionally,hydrogel MNs are solid [36,37]).Owing to the MNs are short in length,they can only penetrate into the depth at epidermis [34].Human skin includes three layers of epidermis,dermis and subcutaneous tissue,in which the epidermis is mainly composed of living cells and dead tissues with few nerves and without blood vessels [38].As shown in Figs.2a and b,the MNs penetrate the superficial skin without contacting the nerves endings and blood vessels in the dermis,so it can be implanted painlessly,meeting the minimum invasive requirements from the patients [14,36],especially from the juvenile and elderly patients.
Caliò and his collaborators’ poly(ethylene glycol)diacrylatebased MNs could simultaneously detect the concentration of glucose and lactic acid in ISF [36,39].Similarly,the MNs sensor created by Gao and his colleagues could measure the ISF glucose,uric acid and cholesterol consistence synchronously [40].The virtues of the above two MNs-based devices are that they could integrate the analysis of multiple biomarker molecules in ISF on one patch,while the two designs to be mentioned next focused on solving the common defect of the slowly ISF collecting speed.To improve the speed of sampling of fluid through MNs,Strambiniet al.designed densely-packed MNs with a density of up to 1×106needles/cm2as shown in Fig.2c.Because of the capillary action of dense MNs,the glucose sensor can extract ISF at a rate up to 1 mL/s [41].MNs shown in Fig.2b made of high swelling material,namely methacrylated hyaluronic acid (MeHA),are another feasible solution improved the extraction speed of ISF.The MeHA’s high-speed water absorption features,coupled with the addition of maltose as an osmotic pressure enhancer,made this platform can extract ISF three times faster compared with most existing platforms [37].However,none of the aforementioned MNs glucose detectors had been tested in live animals,let alone on humans.It has been confirmed that animal experiments and clinical experiment will prevent MNs from causing adverse reactions in organism,and will verify their wearability and biocompatibility,so as to avoid the embarrassing situation of the well-designed CGM is not suitable for patients [14,42,43].
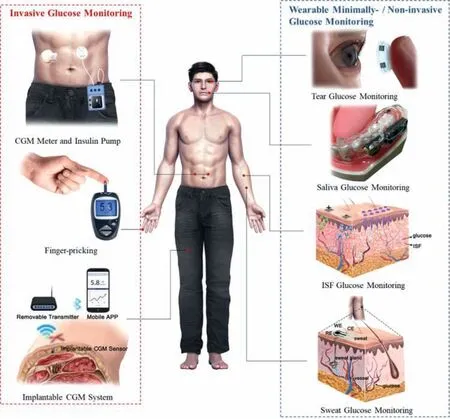
Fig.1.Common painful,invasive glycemic monitoring methods at this stage (left),and the minimally-/non-invasive modes described in this review to indirectly monitor glucose by measuring biomarker fluids (right).Graph “Saliva Glucose Monitoring” Adapted with permission [131].Copyright 2015,IEEE.
Fig.2d presents two types of MNs (tapered silicon microneedle array (TSMA) and straight silicon microneedle array (SSMA))[44].Both MNs arrays were processed by silicon micromachining method,which did not have the disadvantages of electrode corrosion and galvanic interference as metal electroplating method [45],nor the high requirements for the molds in polymer patterning or molding method [46,47].TSMA and SSMA were mounted on CGM systems respectively.From comparison results in human body experiments,SSMA designed by Jinaet al.could be a price moderate substitute of TSMA that are already commercialized (ArKal Medical Inc.2012) [48].Jinaet al.further improved the structure of the above SSMA blood glucose monitor and conducted longer-term clinical trials [15].The optimized MNs geometry design and buffer solution containing citrate ions could effectively slow down the self-healing reaction of skin,thus enormously increased the service life of the sensor.Comparing glucose measurement results and fingertip blood glucose readings during the 72-h device wearing period of 10 diabetic subjects,there were no obvious adverse reactions,reported in Fig.2e.The results proved the accuracy of the devise,and the overall mean absolute relative difference (MARD)was only 15% [15].However,the device could not realize real-time data analysis in the strict sense,requiring external data downloading and analyzing procedure when it is free.
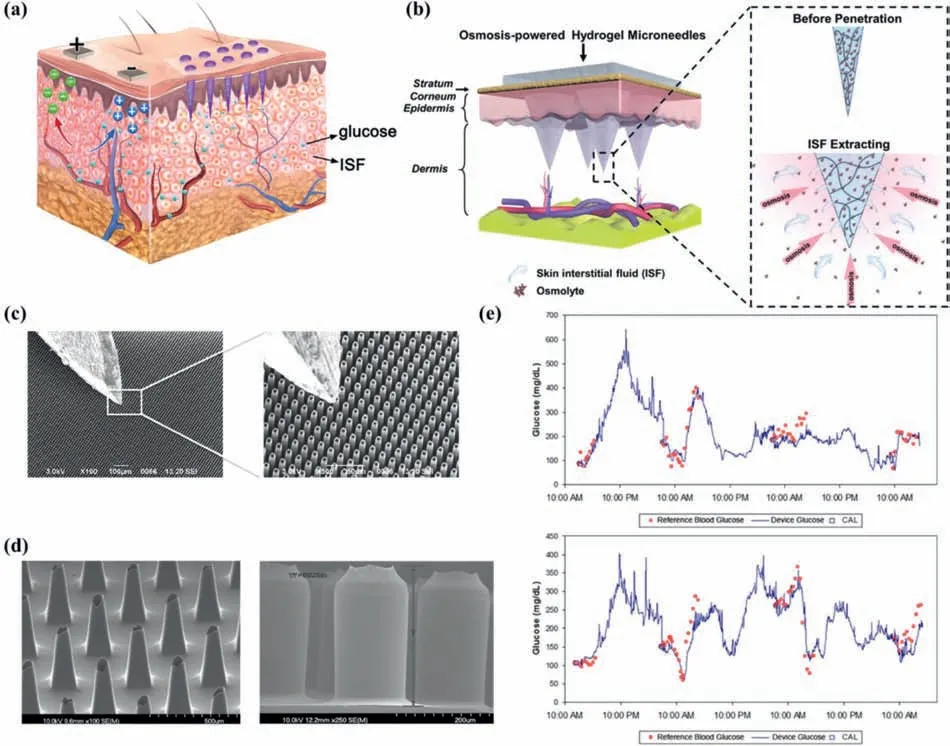
Fig.2.Principle and representative instruments for ISF glucose monitoring with MNs mode.(a) Scheme of the diffusion effect of glucose from capillaries to ISF,and the method of extracting glucose from ISF (left:RI,right:MNs).(b) Schematic of the osmolyte-containing MeHA hydrogel MNs extracting ISF.Reprinted with permission [37].Copyright 2020,John Wiley and Sons Ltd.(c) Scanning electron microscope (SEM) images at two different magnifications of MNs designed by Strambini et al.with a typical insulin needle sitting on their top for comparison.Reprinted with permission [41].Copyright 2015,Elsevier.(d) SEM image of TSMA (left) and SSMA (right).Reprinted with permission [44].Copyright 2013,Elsevier.(e) Sample plots of the device performance over 72 h,whose solid line shows device signal and the discrete points represents comparative blood glucose measurements,while square symbols means comparative points used as calibrations.Reprinted with permission [15].Copyright 2014,Sage Publications.
Despite the noteworthy progress in MNs,several crucial issues will seriously obstruct the developed enzyme-based devices mentioned above from truly practical use.For example,environmental factors such as temperature,humidity and pH can significantly affect the properties of enzymes [16,49],and at the same time,enzymes are expensive and difficult to preserve chronically.Granular metal/metal oxide materials (e.g.,Cu [50]/CuO [51]/Cu2O[52]/CuS [53],Ni [49]/NiO [54],ZnO [55]and Pt [16,33]) and carbon nanomaterials (such as graphene [56]and carbon nanotubes[57]) are potential candidates for enzyme substitutes.Park’s team designed a variety of wearable non enzymatic glucose sensors and tested them on experimental rabbits [16,33].For example,Leeet al.adopted platinum black as the sensitive material,medical grade stainless steel as the matrix of MNs,and coated the outer surface with Nafion film as a protective layer [33].Possibly due to the biological pollution caused by the imperfect coating of protective film,the service life of the device was shorter than expected in the rabbit experiment.Moreover,MNs made of stainless steel were expensive and might cause permanent skin damage [21].
ISF glucose monitoringviaMNs is a minimally invasive strategy,although its impact and trauma on human body are negligible.Potential troubles with MNs include needle tip fractures,requiring frequent calibration [58],risk of contamination [21,59],and possible pain [60].What is worse,MNs usually fail quickly due to the healing reaction of the skin [15].Hence,some attempts in seeking noninvasive glucose sensors have been made [4].The iontophoresis (IP) [61]/reverse iontophoresis (RI) [62]method applies a gentle electric current to the superficial layer of the skin so that ISF can be deposited on the cathode non-invasively,and therefore has attractive tremendous attention.
IP promotes the flow of ions/molecules in the skin through electrical rejection and electroosmosis [61,63],which is well summarized in Fig.3a.Through IP,it is easy to sample the ISF presented in epidermis non-invasively.To take one example,Emaminejadet al.introduced sweat inducer (acetylcholine,methacholine,and pilocarpine) ions into the skin to produce sweat,and measured ISF glucose,Na+and Cl–mixed in sweat real-timely(Fig.3b) [19,64].However,failure to separate ISF from sweat would reduce the accuracy,rigorous and credibility of the measurement results,while the method shown in Fig.3a cleverly used a low-cost screen printing process to achieve separate detection of this dual biological fluid [65].This panda-like device could,for the first time,detect sweat (detecting ethanol) and ISF (detecting glucose) simultaneously,by stimulating sweating at the anode (transdermal pilocarpine administration) and extracting ISF at the cathode.Changes in sweat alcohol/ISF glucose concentration was measured before and after human subjects consume food/drink alcoholic beverages,with accurate correlation compared with commercial blood glucose meter and respiratory analyzer equipment,proving that this wearable device had better accuracy,reliability,and comfort [65].
RI refers to the application of a gentle current on the epidermis to make ions and molecules precipitate out of the skin.Because human skin has a negative charge at neutral pH,sodium ions are the main charge carrier [66].As it is vividly illustrated in Figs.2a and 3c,the migration of sodium ions from the skin to the cathode causes an electroosmotic flow of ISF to the cathode,and glucose is also transported to the cathode along with the ISF flow [67].Wrist-mounted electrochemical sensor named GlucoWatch (Cygnus Inc.) that detected glucose concentration in ISF using RI was the first commercial non-invasive glucose detector allowed by the Food and Drug Administration (FDA) in early 2000s [13,22,30].Although it could achieve continuous blood glucose measurement to a certain extent (up to three blood glucose readings can be provided per hour for 12 h) [7,13,20],the products were removed from shelves in 2008 [13,20]due to the drawbacks such as skin irritation,long warm-up process (2–3 h),requiring frequent calibration by an invasive blood collection method [19],unsatisfactory measurement results during exercise [68],high false positive rate[69]and not sophisticated or convenient enough to wear [70].A tattoo-type noninvasive RI glucose monitoring platform is depicted in Figs.3c–e [67],which contained a tattoo-based paper,RI electrodes (Ag/AgCl),references/counter electrodes(Ag/AgCl),working electrodes (Prussian blue),transparent insulating layer and a hydrogel layer.By reducing the electrophoretic current of extracted ISF ions,the platform integrated with flexible substrates and amperometric glucose detector could eliminates discomfort observed in GlucoWatch.
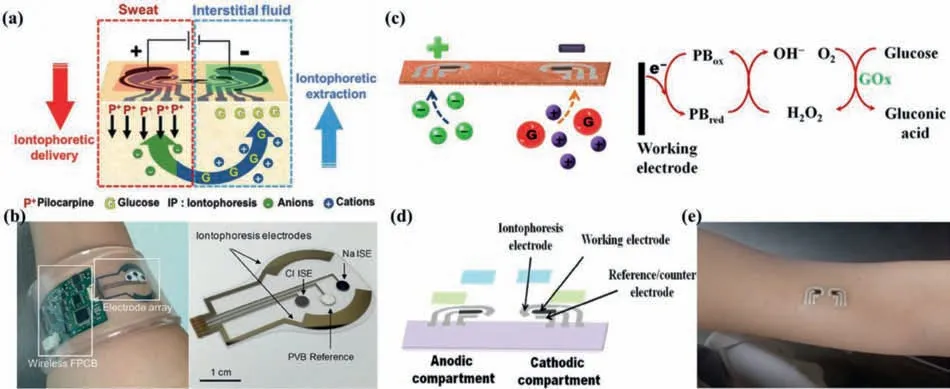
Fig.3.Principle and representative instruments for ISF glucose monitoring using IP or RI.(a) Schematic of IP operation-induced generation of alcohol-containing sweat by iontophoretic delivery of pilocarpine at the anode with simultaneous gathering of ISF glucose at the cathode.Reprinted with permission [65].Copyright 2018,Wiley-VCH Verlag GmbH &Co.(b) Image of the autonomous sweat extraction and sensing platform (left) and the detailed image of the electrode arrays part (right).Reprinted with permission [64].Copyright 2017,National Academy of Sciences.(c) Photograph of the RI and catalysing processes involved in each phase.(d) Image of the tattoo-based RI platform displaying the tattoo-based paper (purple),Ag/AgCl electrodes (silver),Prussian blue electrodes (black),transparent insulating layer (green),and hydrogel layer(blue).(e) Schematic of the RI glucose sensing tattoo device applied to a human subject.(c–e) Adapted with permission [67].Copyright 2015,American Chemical Society.For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article.
Out of the purpose of complementary advantages,Yanget al.combined MNs with RI,which can extract ISF efficiently owning to dual-extraction effect [71].Although the device was designed to analyze cell-free DNA,it could be converted into a glucose detector with a few steps.
ISF blood glucose monitoring is an effective and promising method,not only because the correlation between ISF and blood has been extensively studied,but also because the glucose concentration in ISF is higher than that in sweat,tears,and saliva.But at the same time,using ISF as the test fluid faces many challenges.The most critical restriction is the delay in glucose transmission from blood to ISF (5–20 min) [6,33].Consequently,real-time information detected from ISF sensors can be later than blood glucose,hence interstitial glucose cannot respond to blood glucose concentration in time.As a result,the demand of emergency warning for blood sugar changes in patients,especially for hypoglycemia,cannot be fulfilled [6].
2.2.Glucose monitoring in sweat
Human sweat is a kind of biological fluid that counts a great deal.Scorching,exercise,or excitement can promote sweating,and the most sweaty parts are forehead,soles of feet,palms,back,chest,and underarms [72].This acidic (pH 4–5) liquid contains ions(Na+,K+,Ca2+,NH4+,Cl-,etc.),metabolites (glucose,lactate,urea,ethanol,peptides,etc.) [73],providing various information on human physiology and metabolism.The proven correlation endows the ability to the measure of sweat glucose concentration to estimate blood glucose levels [18].
The disposable test strip was designed to measure the glucose concentration in sweat [74].However,the non-reusable feature made this type of equipment cannot meet the basic premise of CGM.Therefore,realizing continuous detection of sweat blood glucose still faces many challenges.Specifically,there are three main categories of current improvements,namely,ameliorating the way of collecting sweat,improving the reliability of enzymes,and optimizing data reading and sensing.
Electrochemical measurement of glucose content in sweat is a reliable and non-invasive method.A common electrochemical probe consists of a working electrode (WE),a reference electrode(RE) and a counter electrode (CE),as shown in Fig.4a.Because the low glucose concentration in sweat,it is more difficult to detect with non-enzymatic alternatives such as graphene than in the case of ISF.As a result,most of wearable sweat glucose detectors that claimed to be non-enzyme were still in the theoretical stage and lacking credibility [75,76].Unfortunately,the use of enzymes has many inherent disadvantages.For example,since the redox center of the enzyme is covered by peptides,it is difficult to transfer electrons at the enzyme-electrode interface.Consequently,it is usually necessary to add complex and leaky electronic media to promote electron transfer.Xiaet al.prepared a flexible and hierarchicalmeso/macro porous carbon nanotubes (CNT)-ethylene-vinyl acetate copolymer (EVA) film and utilized the formed porous structure to improve the charge transfer between the enzyme and the electrode (Fig.4b) (which can be classified as the third-generation glucose sensor mentioned earlier),increasing the sensitivity consequently [10].
Considering the issue that the activity of enzymes is easily affected by temperature,humidity and pH,construction of enzymebased sensing system is still challenging.Notably,developing comprehensive platform which contains temperature,humidity and pH sensors can vastly improve the reliability and stability of selfcalibration measurement [77].Gaoet al.proposed a flexible and integrated sensor array for multi-channelin-situsweat analysis [78].Under the calibration of the temperature sensor,the platform could simultaneously and separately measure the concentration of glucose,lactic acid,Na+and K+in sweat.Such platform is depicted in Fig.4c.Through the attached flexible integrated circuit board,signal transmission,adjustment (amplification and filtering),processing and wireless transmission could be realizedin-situon the device (that is,no external analysis is required).Compared with above-mentioned platform,Yokuset al.adopted both pH sensor and temperature sensor for self-calibration,thereby obtaining further offset effect of pH on enzyme activity [79].The most notable highlight of this work was the sensor array with redundant working electrodes (e.g.,there are 12 working electrodes for glucose sensing) (Fig.4d),which could offset the errors caused by a single electrode.
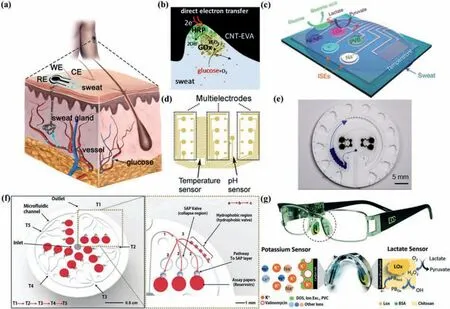
Fig.4.Principle and representative instruments for sweat glucose monitoring.(a) Schematic of a common electrochemical probe for the purpose of detecting glucose concentration in sweat,consisting of a WE,a RE and a CE.(b) Photograph of the mediator-free sweat glucose monitoring based on the bienzyme system via direct electron transfer at porous enzyme/CNT-EVA electrode interfaces.Reprinted with permission [10].Copyright 2020,Elsevier.(c) Schematic of the sensor arrays including glucose,lactate,Na+,K+ and temperature sensors for multiplexed perspiration analysis.Reprinted with permission [78].Copyright 2016,Nature Publishing Group.(d) CAD design of the sensor array with redundant electrodes.Reprinted with permission [79].Copyright 2020,Elsevier.(e) Top view illustrations of platforms with microfluidic channels and CBVs.Reprinted with permission [59].Copyright 2019,American Association for the Advancement of Science.(f) Detailed description of the microfluidic channels and SAP system (left).Illustration of the top side of the device describes the microfluidic channel design (right).The sequential hydrodynamic flow proceeds from T1 to T5.Selective hydro-dynamic flow tracks from (1) the channel linked with the inlet to (2) the SAP valves followed by (3) the hydrophobic valve region.This track enables sequential filling of the reservoirs.Reprinted with permission [86].Copyright 2018,Wiley-VCH Verlag GmbH &Co.(g) Image of the eyeglasses biosensor system integrated with a wireless circuit board along the arms (up) and a nose pad electrochemical sensor (dashed circle in left and detailed presented in right) with schematic of potassium sensor and lactate sensor,along with the corresponding recognition and transduction events (down).Reprinted with permission [90].Copyright 2017,Royal Society of Chemistry.For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article.
With regard to the aspect of collecting sweat,innovative design is highly desired instead of simply using stimulant [58]or exercise[78]to induce perspiring.It often takes 10 to 20 min to collect the lowest limit of the amount of sweat required for sensing [77].Leeet al.shortened the distance between the reference electrodes and the counter electrodes to reduce the amount of sweat required for sensing to 1 mL [80](20 times less than their previous work [77]).
However,the above-mentioned patch-type sensor seemed to be able to detect 7/24 continuously,in fact the sensors were being exposed to the initial sweat [58,81].Specifically,the initial sweat will form a film layer between skin and sensors because of the human body’s negative feedback adjustment mechanism,the subsequent sweat production in this area will gradually decrease [82].In addition,this layer of sweat film is difficult to evaporate,making the initial sweat always crosstalk to subsequent sweat which is already rare [83].The characteristics of attractive low-cost,simple design and versatility [84]endows the emerging microfluidic systems the capability of overcoming the drawbacks of the patch-type platforms.
Rogers’team improved the microfluidic system indefatigably to realize the continuous and efficient collection of sweat and the spatiotemporal detection of glucose.Starting from the work of laying the foundation [85],their attempts and efforts included:(1)Designed capillary bursting valves (CBV) [59,83,84](e.g.Fig.4e).The collected sweat flowed in the microfluidic system generates hydraulic pressure,when the hydraulic pressure exceeded the threshold,the CBV will open.Different CBVs were designed to open under different pressures to realize the orderly,precise,and designed sweat flow,avoiding crosstalk of sweat.(2) Increased the detection limit to adapt to physiologically relevant ranges of concentration such as glucose.(3) The use of super absorbent polymer(SAP) water-driven valve instead of CBV could prevent liquid backflow and allow air circulation,and it was not sensitive to deformation or contact pressure [86,87](Fig.4f).(4) Improved waterproof performance,even could be worn when swimming [88].The camera and app attached to the smartphone could easily analyze the color change after sweat with glucose flow into the cavity containing GOx and Prussian blue.The colorimetric display method eliminated the complicated,large and unsightly electrical signal processing and emission circuits in the electrochemical method.
A method that can also avoid the circuit part is fluorescence display.Cuiet al.chose luminescent porous silicon (PSi) and carbon quantum dots (CQDs) with porous structure and oxidationresponsive photoluminescence attenuation characteristics as dualnanometer fluorescent materials,while bimetallic (Au and Ag)nanoparticles (BiM) were combined together to PSi particles to enhance the initial fluorescence of Psi [89].The hydrogen peroxide produced by GOx oxidizes glucose would cause PSi to oxidize,leading to the result of attenuating the fluorescence of PSi.Therefore,when this skin pad with BiM-CQDs@Psi fluorescent materials was attached to the human body,the increase in sweat glucose concentration can be observed as a fluorescence transition[red (Psi)→blue (CQDs)].Compared with electrochemical sensors,fluorescent sensors possess the advantages of high sensitivity,convenient operation and visual readings.
The aforementioned sensors separately solved the abovementioned issues of improving sweat collection mode,improving the reliability of enzyme,optimizing data reading and sensing.At the same time,fashion and beauty is also a very important aspect.Sempionattoet al.proposed a new design concept for wearable electrochemical sensors,using spectacles [90,91].They integrated an ampere-type biosensor (which could be a lactate sensor or a glucose sensor) and a potential measuring potassium ion selective electrode into the two nose bridge pads of the glasses,and partly hid the integrated circuit in the glasses frame (Fig.4g).The metabolites and electrolytes in the sweat at the nose pads on both sides of the glasses were continuously and accurately detected,and at the same time the signal was transmitted to the host device by the Bluetooth wireless device [90].A similar design was also used forin-situmonitoring of metabolites such as glucose in tear fluid[91],which will be discussed in the next chapter.
There are still many challenges in the future dynamic,in-situand real-time sweat monitoring applications.It is difficult to solve the three issues mentioned at the beginning of this chapter simultaneously and perfectly.In addition,the discontinuity,instability and individual differences of sweat secretion are also obstacles that are difficult to overcome.In addition,there are biological communities on the surface of the skin,including bacteria,fungi,and even parasites [92].The density of bacteria on the skin is estimated to be as high as 1010cm2,and some of them consumes sweat glucose,causing a considerable deviation in glucose concentration [93].As our suggestions,in the future,it will be considered to add a disinfectant slow-release device on the existing comprehensive detection platform.In summary,there are still many efforts need to be filled into the gap between the wearable sweat glucose sensor described in papers and the marketed product.
3.Glucose monitoring in lacrimal fluid
Eyes are organs that can provide rich physiological information,and the multiple physical and chemical information contained in them can be used as an important basis for clinical diagnosis.For example,intraocular pressure is a dangerous cause of glaucoma [94],and electroretinography can be used as an objective assessment of retinal diseases [95].Abnormal eye movements are an early diagnostic sign of concussion [96],schizophrenia [97],Wallenberg syndrome [98],Joubert syndrome [99]and a series of other diseases [100,101].Matrix metalloproteinase 9 (MMP-9) is a biomarker for the diagnosis of dry eye syndrome [102].Lacrimal fluid,or called tear generally,have a relationship with blood in glucose concentration [103],due to plasma penetration.Therefore,diabetes can be diagnosed by monitoring the glucose concentration in lacrimal fluid.
Tear film is a layer of thin and transparent liquid distributed on the surface of cornea by continuous secretion of lacrimal gland and accessory gland (secretion rate is between 0.5–2.2 μL/min to continuously compensate for evaporation loss) [104],which is used to moisturize and protect eyes.The tear film flows across the surface of the eye through blinking,gravity and fluid effects,forming a film with a thickness of 3 μm and a volume of 7 μL [105],and is finally discharged through the nasolacrimal duct.Therefore,although tears flow out of the eye socket only when crying or being stimulated,the presence of the tear film makes it possible to continuously monitor the glucose concentration in the tear fluid.Contact lenses play a role in visual correction and cosmetology,becoming the most ideal carrier for tear glucose sensors for the reason that their inner surface is always in contact with the tear film (Fig.5a).
Google and Novartis have developed a smart contact lens (SCL)that provides the glucose concentration in the wearer’s tears [11].But until today,the product has not been released,and there are no scientific journal reports onin vivoglucose sensing [106].
Mitsubayashi’s team attached a PDMS-based (70 μm) test strip with sputtered platinum (200 nm,working electrode) and evaporated Ag/AgCl (300 nm,counter/reference electrode) to contact lenses (Fig.5b),and successfully carried outin-situocular biomonitoring of tear glucose on rabbits [107,108](Fig.5c).The SCL successfully realized the real-time measurement of tear glucose.However,this combination method led to unattractive appearance and required a lead wire to connect the SCL to the analysis device.Even worse,due to the lack of effective packaging,the measurement results might be interfered with such as ascorbic acid [108].Yaoet al.used Nafion® membrane to eliminate several potential interferences in the tear film (ascorbic acid,lactic acid and urea)[109].Although it had good linearity,repeatability and resistance to molecular interference,this SCL also required a hard wire to transmit the signal (Fig.5d),and it had poor time stability and air permeability.
Obviously,one of the biggest challenges faced with electrochemical SCLs is to achieve wireless signal transmission.In addition,electrochemical sensors require stable and accurate directcurrent (DC) voltage,which consumes energy.Therefore,another challenge is the supply of energy.
Wireless energy reception and information transmission can be realized by installing toroidal coils in SCLs.Park’s group proposed a series of such SCLs,whose sensor part were field effect transistors (FET) [11,110-113].FET has unique advantages in the field of biosensing,including high signal-to-noise ratio,high sensitivity and short response time.The principle of FET-based biosensors is that when the biomarker is in contact with the active area or the gate of a FET,the electrical characteristics of the transistor will change.The general composition of the FETs glucose sensor designed by Park’s team were as follows:(1) The source/drain electrodes were graphene combined with AgNF/AgNW,(2) the active area was graphene,and GOx is fixed to graphene by pp-stacking through pyrene connectors Above,(3) the connecting electrode was Cr/Au,(4) the dielectric layer was SU8.For example,Kimet al.proposed a multifunctional parylene based contact lens sensor in 2017 to monitor glucose and intraocular pressure (Fig.5e) [110].Graphene combined with AgNW electrodes were proven to have good transparency (>91%) and ductility (~25%).In vitroandin vivoexperiments using bull’s eyeball (to measure intraocular pressure)and live rabbits (to test glucose response) had proven its reliability.
Loop antennas have been commonly used by SCLs [106,110–112,114].But as a result of requiring large and expensive external induction coil/signal processing equipment which should always be held close to the eyes during measuring procedure [114],loop antennas are gradually being replaced by more efficient energy/signal transmission solutions.
Integrating micro batteries on SCLs will solve the energy supply problemin-situ.Cui’s team developed a low-temperature processed thin film battery [115](Fig.5f).The key preparation process was an olivine-based cathode by RF magnetron sputtering at 90° offaxis.This battery could be prepared at a lower temperature and was well-tolerated for moisture,making it very suitable for contact lenses.Biofuel cells,which utilize organic matter in tears,play the role of both power and sensor in SCLs,so they are ideal selfpowered power supply for contact lens.Taikiet al.used zinc-air battery to provide constant DC power for a SCL,which was generated by ring-type zinc anode and bilirubin oxidase (BOD) biocathode in artificial tears [116].The zinc-BOD battery could provide constant DC power for more than 15 h,so it was a kind of power supply suitable for soft contact lens.Hayashiet al.developed a 0.6 mm×0.6 mm CMOS-compatible solid glucose fuel cell,whose output voltage was related to the glucose concentration,which could be measured to detect the user’s blood glucose concentration [117].The power consumption of the entire device was reduced to nanowatt level (0.27 nW).More efficient energy storage is also a feasible solution to solve the power demand.For example,Park and others added a super capacitor to the SCL circuit,which could greatly extend the service life after each charge[113].
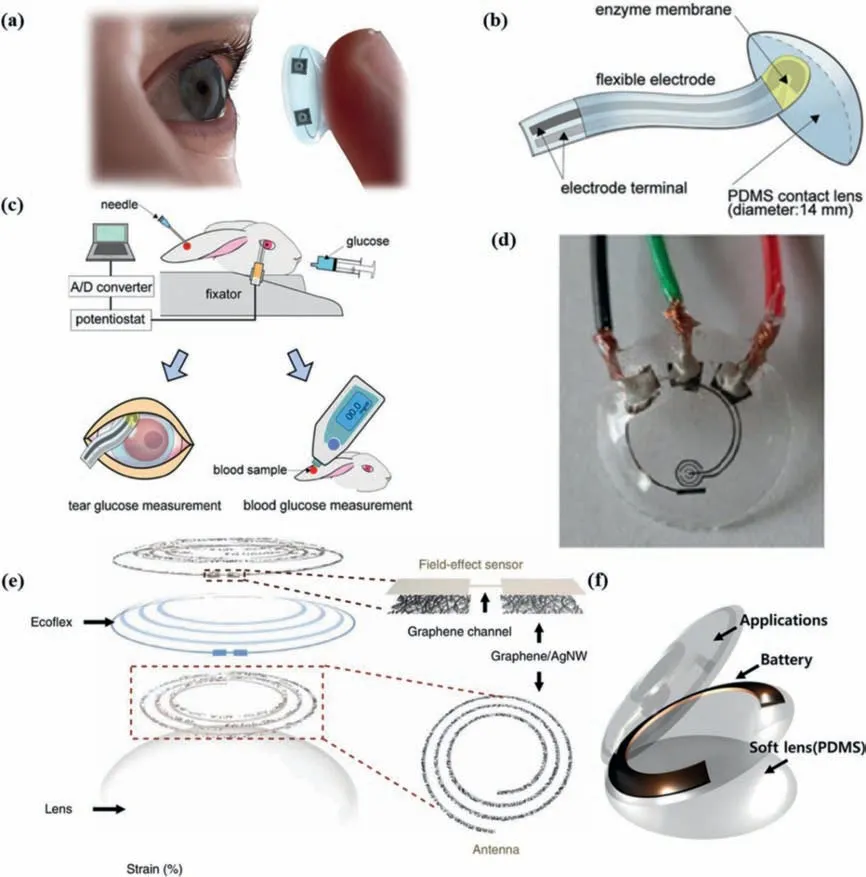
Fig.5.Representative SCLs for tear glucose monitoring through electrochemical method.(a) Schematic of a SCL.(b) The flexible electrodes with enzyme membrane were bonded onto the surface of the PDMS contact lens.(c) Measurement method of tear glucose concentration on rabbits.Blood glucose level was measured by a commercially blood glucose monitoring kit,simultaneously.(b,c) Adapted with permission [107].Copyright 2011,Elsevier.(d) The testing progress of the SCL with hard wires.Reprinted with permission [109].Copyright 2011,Elsevier.(e) Schematic of the wearable contact lens sensor,with the graphics of a FET sensor and an antenna.Reprinted with permission [110].Copyright 2017,Nature Publishing Group.(f) The schematic of the SCL embedded with thin film battery.Reprinted with permission [115].Copyright 2018,Elsevier.For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article.
In terms of signal expression,using radio frequency identification (RFID) technology can avoid the need for bulky and expensive equipment [11,118].On the other hand,adding LED to the circuit part can realizein-situdisplay of glucose sensing results [112,113].However,the switch on and off of LED light depends on whether the glucose concentration reaches the setting threshold,so quantitative analysis cannot be achieved.What is worse,the LED in SCLs designed by Parket al.would turn off when the glucose concentration was greater than the threshold (0.9 mmol/L),which was contrary to human intuition [112].
The way to realize visual signal display is not limited to accessional LED,while contact lens based on optical principle is another major type of SCLs.No matter how much effort the researchers have made,there are always circuits in electrochemical SCLs that affect vision.The circuit also affects aesthetics,and in addition,due to the Joule heating effect,the contact lens will be heated by the circuit,causing the risk of eye damage.While the optical SCLs omit the circuit part,result inin-situsignal displayed on the contact lens.Optical SCLs can be commonly divided into three types:fluorescence type,chromatographic type and holographic type.
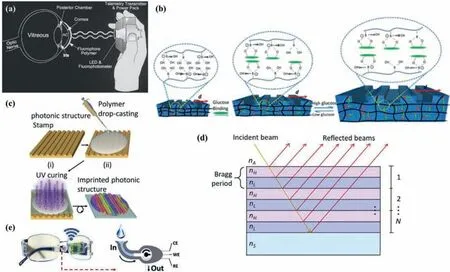
Fig.6.Principle and the structure of representative SCLs for tear glucose monitoring through optic method except for (e).(a) Schematic of the measurement progress of the contact lens glucose sensor with a hand-held photofluorometer.Reprinted with permission [120].Copyright 2004,Mary Ann Liebert,Inc.(b) Effect of the glucose-phenyl boronic acid complexation.(c) The fabrication process of the hydrogel SCL.(b,c) Adapted with permission [121].Copyright 2018,American Chemical Society.(d) Structure of a Bragg diffraction grating alternating high (nH) and low (nL) refractive index layers,which is on a substrate (nS) and in contact with the environment (nA).Reprinted with permission [125].Copyright 2020 SPIE Digital Library.(e) Schematics of the fluidic device and wireless electronics integrated into the eyeglasses platform (left) along with a representation of enzymatic detection (right).Reprinted with permission [91].Copyright 2019,Elsevier.
Baduguet al.embedded fluorophores containing boric acid in contact lenses [119].Similarly,Marchet al.added tetramethylrhodamine isothiocyanate-concanavalin A (TRITC-Con A) and 2×106molecular weight fluorescein isothiocyanate–dextran(FITC-dextran) to contact lenses [120].The fluorescence intensity produced by the glucose-sensitive fluorescent material is proportional to the glucose concentration,showing the application prospects of fluorescent SCLs in the field of non-invasive home monitors.Fig.6a shows the measurement principle of the SCL made by Marchet al.:A LED of a hand-held photofluorometer emits blue light,then SCL reflect green fluorescence,and finally the fluorescence is received by a fluorophotometer of the photofluorometer.However,fluorescent materials are expensive and possibly be toxic,and pH and O2will affect the response of the dye.Fluorescence readout equipment is also costliness,because it must include a fluorophotometer and a dedicated monochromatic light source [121].
The principle of colorimetry biosensors is that special colorchanging material exhibits a reflection peak shift when combined with tear metabolites,resulting in a color change visible to naked eyes.Moredduet al.utilized H2O2to oxidize 3,3’,5,5’-tetramethylbenzidin (TMB) under the catalysis of peroxidase (POD)[122].When the glucose concentration increased,the sensitive part of the contact lens would turn from yellow to green.However,fluorescence and colorimetry will cause color-changing in SCLs,which will also affect the aesthetics and may affect the user’s perception of hue.
Borate can reversibly combine with glucose to form a hydrophilic complex.Therefore,the glucose selective hydrogel functionalized with boric acid will absorb water and then inflate due to the binding of glucose,as shown in Fig.6b.Following the above principles,Ruanet al.[123],Elsherifet al.[121],and Linet al.[124]respectively produced similar hydrogel-based holographic SCLs,using diffracting wavelength changes (by embedding polystyrene crystalline colloidal array (PSCCA) in the matrix),the reflected power of the first-order spot (the hydrogel film is preprinted into a photon microstructure) (Fig.6c),and the change in the thickness of the hydrogel (photographed by mobile phone and analyzed by an APP) as the basis for detecting glucose concentration.This is a quick,easy and low-cost way to monitor glucose in tear fluid.The principle of the holographic SCL proposed by Llorente’s team was to some degree different from the above mentioned.As shown in Fig.6d,the basic cycle of their SCL was a pure PDMS layer plus a PDMS layer functionalized by GOx.N periodically layers formed a Bragg diffraction grating.The functional layer modified by the enzyme reacted with glucose,and the refractive index decreased,resulting in a change in the optical properties of the grating.Since the oxygen permeability index of PDMS is 30 times the average value of hydrogel,a PDMS-based holographic SCL is more comfortable and safer to wear [125].
SCLs are not the only solution for wearable tear fluid glucose monitoring.Considering that many people are not used to wearing contact lenses,and even some diabetic patients also suffer from eye diseases so that they cannot wear contact lenses,non-SCL-type devices have become alternatives.Sempionattoet al.integrated a microfluidic electrochemical detector into the nose bridge pad of glasses to monitor alcohol,glucose,vitaminetc.in tears [91].As shown in Fig.6e,the funnel-shaped collection device extending from the nose bridge pad could divert the tears generated by the stimulation to the nose bridge pad sensor.The measurement results show that the collected tear fluid could effectively monitor the blood glucose level.Unfortunately,some other research results showed that the composition of reflex tears produced by stimuli and psycho-emotional tears produced by emotions is different from that of basal tears [11,122].Therefore,it is uncomfortable and unscientific to stimulate the wearer’s eyes to collect tear fluid to detect tear fluid components.
However,using tear as a quantitative analysis medium for blood glucose detection still has many challenges.For instance,diabetes itself will not only impact the patient’s eye structure and lacrimal gland and its surrounding tissue,but also influence the patient’s tear secretion and composition [126].With increasing of the disease course and deterioration of the syndrome,the relationship between glucose concentration in tears and blood sugar will become more and more difficult to predict.On the other hand,the eyes are fragile organs,and vision is an indispensable sense.Some opaque or easily peeled or even fragile parts used in SCLs can affect vision and even damage eyes [91,112].Therefore,compared with other biological fluids,future tear glucose sensors should focus more strictly on comfort,safety and biocompatibility considerations.
4.Glucose monitoring in oral cavity’s saliva
Saliva is a complicated biological fluid,which contains many molecules that permeate from the blood,so it can be used as a detection fluid for people’s nutritional and metabolic states and various diseases theoretically [20,127,128].Saliva glucose can be measured after filtering out large biomolecules mixed in saliva[19],which is a method ofin vitromonitoring [127,128].Compared to sweat and tears,saliva secretion is more abundant and stable,so it has superiorities in wearable continuous biomarkers testing.For instance,Mannooret al.demonstrated dental tattoos that can continuously and wirelessly monitor bacteria in saliva[129].But this platform was not conducive to continuous realtime readout because it required a large active device close to the sensor [130].
Mouthguards are widely used by athletes to prevent sportsrelated dental injuries.This kind of close-to-tooth devices are attractive platforms because they have more volume than skin patches or SCLs mentioned above to install sensors,control/analysis IC chip and wireless transceiver (Fig.7a) [131].
Wang’s group claimed that they were the first group to introduce mouthguard-based wearable sensors to continuously monitor biomarker molecules in human saliva samples with high accuracy and stability.In 2014,they first proposed a smart mouthguard for detecting lactic acid in saliva [132],which was based on a printable Prussian Blue transducer and a poly(ortho-phenylenediamine)(PPD)/lactate-oxidase (LOx) reagent layer,as what is shown in Fig.7b.In 2015,they published a similar study to detect sialic uric acid [130].Added potentiostat,microcontroller and Bluetooth low energy (BLE) transceiver,the sensed information could be wirelessly transmitted to smartphones,laptops and other electronic products synchronously.They claimed that this category of sensors could be used to monitor any other substance,including glucose.This hypothesis is on solid ground,because theoretically replacing the enzyme (LOx) used in the aforementioned lactic acid mouthguard sensor with GOx will be converted into a glucose sensor.
Arakawaet al.combined glucose sensor in mouthguard,called “cavitassensors”,for noninvasive monitoring in oral cavity[133](Fig.7c).The GOx-coated Pt electrode and Ag/AgCl electrode constituted the sensing electrode part,which was adhered to the surface of the mouthguard substrate made of polyethylene terephthalate (PETG).In artificial saliva composed of salt and protein,the glucose sensor showed a highly sensitive detection result in the range of 5–1000 μmol/L,which could be monitored by remote sensing for a long duration (more than 2 h).Subsequent studies had improved the duration of remote sensing monitoring over five hours [134].In conclusion,mouthguard-type glucose detector is a promising non-invasive method for health care.
Nevertheless,wearable bio-monitoring in the buccal cavity is also confronted with many challenges.In comparison to ISF,sweat and tears,saliva has the most complicated solute composition and the smallest glucose concentration thus means the most sensitive and specific sensor is demanded.Residual food residue in the mouth along with various other impurities may hinder accurate measurement of glucose concentration [19].Daily activities such as eating,quoting alcoholic beverages or smoking will interfere with the readings,even damage the equipments and reduce the service life of the detectors.Moreover,most of the saliva glucose detectors currently reported require further human clinical trials to confirm reliability.
5.Conclusions and future prospects
With the number of diabetics patients increase rapidly,the demand for related products that support patients for routine disease screening,diagnosis,and long-term treatment is also increasing.Although direct skin pricking method to collect blood samples ensures accurate and reliable information of blood glucose concentration and changes,this method can cause discomfort and pain in patients.Moreover,it is time-consuming and expensive,and cannot achieve continuous blood glucose measurement,especially.In contrast,wearable minimally-/non-invasive glucose meters provide convenient,safe and economical assessment of glycemic levels.Current research focus is to evaluate blood glucose levels from tears,saliva,ISF and sweat through minimally invasive or noninvasive methods.In the past 60 years,the development of CGM biosensors has made extensive progress,which was reflected in simultaneous improvement of appearance and sensitivity of devices,benefiting from the exploration of novel materials and neoteric design concepts.However,wearable continuous glucose meters have not yet achieved reliable and extensive commercial application.In the United States,less than 10% of type I diabetes patients use CGM technology,statistically.To make matters worse,two-thirds of patients who used CGM finally choose to disable it [14].The main drawbacks existed in commercial CGM equipment are their unreliability and discomfort.Only when future wearable CGM devices become more precise,reliable,comfortable,affordable and fashion,can this technology be widely used.
We believe that one of the trends of wearable blood glucose detectors in the future is developing for simultaneous and multiplexed screening of multiple biomarkers/vital signs.The most representative example is the sweat in-situ analysis platforms based on wearable flexible integrated sensor array published in by Gaoet al.[78]and Yokuset al.[79](Fig.8a).In fact,many of the examples described earlier in this article are more than sense glucose.The solid foundations for this trend are as follows.Firstly,additional temperature,pH,and humidity sensors can offset the deviation of measurement results caused by changes in enzyme activity.Furthermore,human body is integral,so the mutative physiological characteristics of patients suffering from diabetes are not just elevated blood sugar.That means,biomarkers/vital signs like pyruvate,lactic acid and pH can all be used as aids in blood glucose monitoring.From the above two points,it can be concluded that a comprehensive wearable platforms are one of the best candidates for improving accuracy and reliability in the future.More impressively,although it seems that the total cost has increased,the average cost of the sensors for each analyte has decreased.Patients with diabetes often suffer from complications or other basic diseases.Therefore,personalized household comprehensive health care analyzers (based on integrated sensor platform) have a great market prospect.In the future,users can customize the sensor probe types according to their own needs.For example,Type Ⅱdiabetic patients with kidney stones have a lower sweat pH than normal [135],so such a patient should at least subscribe a sweat analysis platform containing glucose and pH sensor.
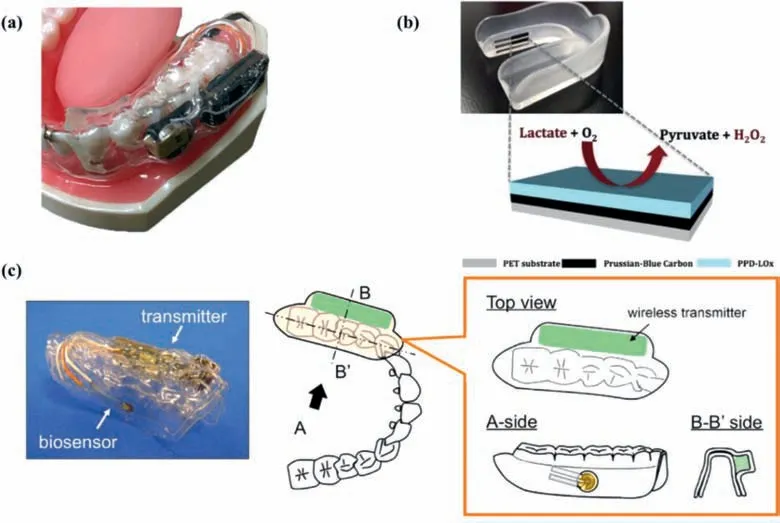
Fig.7.Representative mouthguard-type detectors for tear glucose monitoring.(a) Schematic of a typical mouthguard-type glucose monitor.Reprinted with permission [131].Copyright 2015,IEEE.(b) Photograph of the mouthguard biosensor (up),and Schematic illustration of the PB working electrode (down).Reprinted with permission [132].Copyright 2014,Royal Society of Chemistry.(c) Image of the “cavitas sensors” made by Arakawa et al.The device consists of a glucose sensor and a wireless transmitter encapsulated in the PETG,including a potentiostat for stable glucose measurement.Reprinted with permission [133].Copyright 2016,Elsevier.
Besides adopting a variety of sensor probes to offset the fluctuation of enzyme,there are many ways to improve the reliability and stability of minimally-/non-invasive glucose monitoring devices.Adopting new materials or new structures,utilizing expanding novel detection means and analysis methods,combined with emerging technology innovation,and large-scale population research are all effective ways to achieve the consistency,stability and reliability of detection among individuals.
The natural world is full of alluring inspirations,illuminating the development of advanced sensors.For example,inspired by the chitin complex of the arthropod stratum corneum,Honget al.introduced an optically transparent biomimetic composite material that hybridized silk fibroin (β-sheets) and chitin nanofibers (ChNF)[111].A SCL glucose sensor based on this hybrid bio-material showed expectant biocompatibility.Recently,Changet al.reported a highly conductive (20.4 Sm-1) and transparent (99.8%) egg white hydrogel,which had the potential to be widely used in wearable devices [136].Since there are more glucose transporters-1 on the cell membrane of cancer cells than normal cells,they show higher selective permeability to glucose.Inspired by this phenomenon,Kimet al.used breast cancer cell membranes (BCCM) to significantly improve the selectivity of glucose sensors [137].Wanget al.took afflatus from the anatomical structure of rhinophore(a sensory organ of marine mollusks) and designed a retractable bionic electrochemical sensor [138](Fig.8b).The key to realizing this biomimetic similarity lay in the successful preparation and adjustment of the folding/unfolding electrochemical surface active (ECSA) on the highly elastic Ecoflex fiber to achieve programmable sensitivity for glucose detection.Biomaterials and bioinspired/biomimetic structures will bring better glucose-specific sensitivity,biocompatibility,biodegradability and lower cost to future wearable blood glucose sensors.
Compared with sensitivity and comfort,aesthetics and fashion are often ignored by researchers.The several nose bridge cushion blood glucose sensors proposed by Sempionatto and others mentioned above are no different from ordinary glasses in appearance[90,91],which could avoid the uneasy or inferiority feeling of diabetic patients when wearing them.While Kimet al.designed the electrode of the sensor in the shape of a panda,which would make children with type I diabetes more willing to wear it (Fig.8c) [65].
Even if the process of detecting blood sugar realizes noninvasive,for diabetics,the process of injecting insulin is still painful.Kim’s team integrated sweat diabetes monitoring and a polymer microneedle drug delivery system for feedback therapy on a wearable patch [77,80].The MNs could be activated by heat to deliver drugs through the skin,which would significantly improve the quality of nursing management.Recently,Bao’s research group reported a SCL that achieved on-demand controlled drug delivery.In the diabetic rabbit model,the drug was successfully released from the reservoir for the treatment of diabetic retinopathy [106](Fig.8d).It can be seen that more and more attention is also being directed to non-invasive drug delivery.But this technology is not yet mature,because the drug intake of diabetics requires complex and accurate calculations.Hypoglycemia caused by excessive insulin injection can cause weakness,dizziness and even shock.With the advent of the information and network age,wearable electronic technology combined with wireless communication is essential for medical care through health monitoring technology[112].Through radio frequency identification (RFID),bluetooth low energy (BLE),near field communication (NFC) and other technologies,wearable blood glucose meters,personal host devices (smartphone/PAD/PC) and the insulin pumps are connected with each other to form the so-called body area network (BAN) [139].This kind of feedback-style,closed-loop,user-interactive home health care program is receiving more and more attention.
Furthermore,the use of emerging artificial intelligence (AI) and big data technology can provide patients with more accurate medication regimen,more personalized user experience and more perfect health guidance.Many competitive companies in the field of diabetes health care are developing their own AI/big data-assisted products,such as Sugar IQ and IQcast by Medtronic cooperated with IBM’s Watson Health,Control-IQ (approved by FDA in December 2019) and Basal-IQ by Tandem Diabetes Care,“Rosie” AI assistant of Lenovo,and AI driven Predictive Insights for Apple’s One Drop and Apple Watch products.In addition,companies such as Livongo,mySugar,Solgo,Cecelia Health,Virta Health are also developing AI driven systems.It is reasonable to believe that in the foreseeable future,huge amounts of data (such as energy intake in diet,sleep time and duration,calories consumed in exercise,heart rate,blood pressure,blood glucose value,weight,mood,and external temperature and humidity) collected from BAN will be uploaded to big data analysis platform.After big data analysis,the platform evaluates or calculates the user’s physical status,the type and quantity of appropriate drugs,whether the diet or exercise should be adjusted,and then feedbacks to the user in time.At the same time,through AI’s long-term learning of the relationship between the user’s blood glucose value and other parameters such as diet and exercise,the lag of glucose concentration in biomarker solution compared with the blood glucose value can be offset through the way of prediction in advance,and more importantly,it is expected to achieve accurate estimation of blood glucose value through other parameters,so as to reduce the dependence of patients on glucose sensors to a great extent.
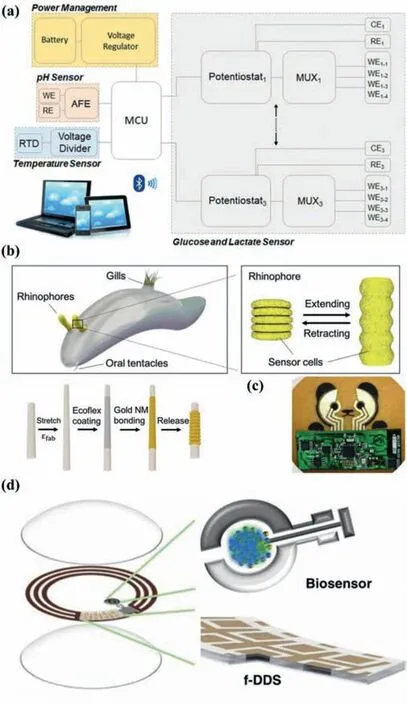
Fig.8.The development trend of minimally-/non-invasive wearable glucose meters in the future.(a) Integrated glucose and other analyte sensing platform,combined with temperature and pH sensors to calibrate enzyme activity.Reprinted with permission [79].Copyright 2020,Elsevier.(b) Utilizing biomaterials or bioinspired structure.Here a glucose sensor inspired by the structure of rhinophore is taken as an example.The stretchable rhinophore is covered with sensing cells (up),and the bionicsensor also coated the sensing material on the stretchable substrate (down).Reprinted with permission [138].Copyright 2020,Elsevier.(c) Fashionable appearance,such as a glucose sensor with a panda exterior.Reprinted with permission[65].Copyright 2018,Wiley-VCH Verlag GmbH &Co.(d) Glucose sensors collaborate with other health monitoring/treatment systems such as intelligent drug delivery systems.For example,an SCL made by Bao’s research group can automatically release drugs to the eyeball surface under the control of Joule heat of electric current.Reprinted with permission [106].Copyright 2020,Wiley-VCH Verlag GmbH &Co.For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was financially supported by the National Key R&D Program of China (No.2018YFA0703200),the National Natural Science Foundation of China (Nos.61890940,51903051),and the Natural Science Foundation of Shanghai (No.19ZR1404400).
杂志排行
Chinese Chemical Letters的其它文章
- Long-wavelength (red to near-infrared) emissive carbon dots:Key factors for synthesis,fluorescence mechanism,and applications in biosensing and cancer theranostics
- Nanotechnology combining photoacoustic kinetics and chemical kinetics for thrombosis diagnosis and treatment
- The point-of-care-testing of nucleic acids by chip,cartridge and paper sensors
- Sodium bicarbonate,an inorganic salt and a potential active agent for cancer therapy
- New advances in gated materials of mesoporous silica for drug controlled release
- Recent advances in the synthesis of non-carbon two-dimensional electrode materials for the aqueous electrolyte-based supercapacitors
