Nanotechnology combining photoacoustic kinetics and chemical kinetics for thrombosis diagnosis and treatment
2021-03-14HoTinLinLinZhojingFngchoXueYnzhoLiWenZeng
Ho Tin,Lin Lin,Zhojing B,Fngcho Xue,Ynzho Li,Wen Zeng,,c,∗
a Department of Cell Biology,Army Medical University,Chongqing 400038,China
b Department of Anatomy,Army Medical University,Chongqing 400038,China
c Departments of Neurology,Southwest Hospital,Army Medical University,Chongqing 400038,China
Keywords:Nanotechnology Photoacoustic kinetics Chemical kinetics Drug delivery and release Thrombosis disease
ABSTRACT Thrombotic disease is a major problem that endangers human health.At present,MRI and CT are commonly used clinically to diagnose thrombosis,and thrombolytic drugs are used for treatment),but the diagnosis time is lagging,the utilization of drugs is low,and the resulting systemic toxicity problems such as side effects lead to poor treatment effects.Nanotechnology combining photoacoustic dynamics and chemical dynamics has shown great application value in tumor targeting,diagnosis,detection and treatment.It has also become a new direction in the diagnosis and treatment of thrombotic diseases,and has created new applications in the field of nanomaterials.This review summarizes the new progress of this combination in the diagnosis and treatment of thrombotic diseases according to the differences in the construction of the nanotherapy system,at the same time,we put forward some new problems and prospects for the integration of thrombosis diagnosis and treatment.
1.Introduction
According to statistics from the World Health Organization(WHO),cardiovascular disease is currently the world’s leading cause of death.Thrombosis can cause vascular obstruction,which is the main pathogenesis of acute ischemic cardiovascular disease and the main cause of catastrophic events such as pulmonary embolism,myocardial infarction and stroke [1].Early diagnosis and treatment can effectively reduce morbidity and mortality [2].At present,the commonly used clinical treatment methods are the infusion of fibrinolytic agents,such as urokinase,streptokinase and tissue plasminogen activator (tPA),they can convert plasminogen into plasmin,which triggers fibrin lysis to dissolve thro mbus [3].However,there is a therapeutic time window when thrombosis occurs.While the half-life of these thrombolytic drugs in the body is usually only a few minutes,and the dense structure of thrombus hinders the effectiveness of thrombolytic drugs.What is more,some drugs can pass through the blood-brain barrier and cause neurotoxicity and bleeding risks,which causes great difficulties in clinical treatment [4,5].Therefore,local thrombolytic therapy has become a new direction to explore,this method is mainly to load thrombolytic drugs into the carrier system,and use a variety of targeted methods to transport them systemically to the thrombosis site to induce targeted thrombolysis,thereby reducing the amounts of thrombolytic drugs required and the complications of bleeding and re-embolization caused by drugs.At present,the mainly used diagnostic methods are CT,MRI,etc.It is almost impossible to accurately locate thrombus in the early stage,and often miss the best treatment time.Therefore,there is an urgent need for clinical methods that can accurately locate and qualitatively diagnose thrombus in the early stage.What is more,it is necessary to develop new methods to improve the current status of thrombosis treatment.
The rapid development of nanotechnology in recent years has shown great application value in the targeting,diagnosis,detection and treatment of diseases such as tumors and cardiovascular systems (Fig.1) [6–8].The unique physical and chemical properties of nanomaterials (NMs) provide the possibility for its functional modification and combination with enzymes,proteins,receptors and antibodies (Abs) for specific diagnosis and treatment,for example,as a contrast agent in MRI.There are also a large number of reports on photodynamic therapy,photothermal therapy (PTT) and photoacoustic imaging (PAI) diagnostic and therapeutic agents,such as liposomes,porous NMs,magnetic nanoparticles (MNPs),gold nanoparticles (GNP),quantum dots (QDs),silica nanoparticles,Carbon-based NMs and upconversion nanoparticles(UCNPs) [9–14].At the same time,the continuous development of photoacoustic kinetics and chemical kinetics brings new possibilities to the diagnosis and treatment of diseases.Chemical kinetics is widely used in physics and chemical sciences to study reaction mechanisms and processes at the molecular level.This kind of research is particularly valuable in formulating treatment strategies to fight disease.Indeed,information about the microscopic processes underlying changes in macroscopic variables are crucial for understanding the mechanism of action of a given drug as well as for identifying strategies to change both the thermodynamics and the kinetics of disease-associated processes.The acid-base changes in the local microenvironment and the over-expression of H2O2act as reaction conditions or catalysts to induce physiological reactions and drug effects at the diseased site.This method presents certain advantages in the treatment of enzymatic changes and local microenvironmental changes in cancers and other diseases [15,16].Photoacoustic dynamics includes photodynamics and acoustic dynamics.It is an effective method for researching,diagnosing and treating diseases using specific light sources or ultrasound.The advantages of photoacoustic physical properties,especially the ability to penetrate biological tissues,can be used to adjusting cell viability,drug delivery and release as well as other fields,and it has the advantages of non-invasiveness,high precision,simple operation and few side effects [15,17].Therefore,the current research hotspots of thrombotic diseases are focused on the diagnosis and treatment based on nanoparticles and photoacoustic and chemical dynamics,such as the use of different imaging methods to locate the thrombusin vivoandin vitro,designing targeted drugs by targeting activated platelets and fibrin gathered at the thrombus formation site,constructing a drug reaction system for the special microenvironment (pH,H2O2,shear stress,etc.) and Promoting the release of drugs in specific areas through external stimulation or directly act on thrombus to achieve therapeutic purposes,etc.This article will review the applications of nanoparticles,photoacoustic and chemical kinetics in the diagnosis and treatment of thrombus from these aspects,discuss and prospect the existing problems.
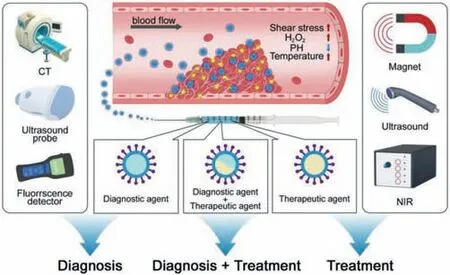
Fig.1.The role of nanotechnology in the diagnosis and treatment of thrombotic diseases.Nanotechnology combining photoacoustic kinetics and chemical kinetics are used in thrombosis diagnosis and treatment.In diagnosis,it mainly consists of nano-related optical imaging,nano-related optical imaging,nano-related radioisotope labeling,nano-related radioisotope labeling, etc.In treatment,there are mainly three kinds of nano therapeutic systems,including nano therapeutic system based on targeted modification,nano therapeutic system based on internal microenvironmental response and nano therapy system based on external stimulation.Besides,integrating diagnosis with treatment of thrombosis is also a vital part.
2.Diagnosis of thrombosis with different imaging modes
Currently,the imaging methods used to detect thrombosis mainly include ultrasound,invasive thrombus imaging catheterization,computer tomography (CT) and magnetic resonance angiography.Although they play a huge role in the diagnosis of thrombus,these imaging methods lack the sensitivity and specificity of acute thrombus detection [18].At the same time,it is impossible to make an accurate diagnosis for incompletely occluded blood vessels and cannot judge the size and nature of thrombus [19].The key to thrombosis diagnosis is early diagnosis,but the existing methods still cannot meet this premise.Therefore,new imaging techniques for thrombosis diagnosis have been further developed.Detection methods based on photoacoustic kinetics,including photothermal imaging (PTI),Thermoacoustic imaging,upconver-sion luminescence imaging (UCL),and PAI,have gradually become methods for detecting early thrombosis [20,21].
2.1.Nano-related optical imaging
Optical imaging is currently a relatively mature imaging method with the advantages of fast feedback and non-ionizing radiation[22].Although the penetration depth of optical imaging is limited,continuous monitoring of dynamic physical processes in small animals helps to broaden the understanding of thrombosis and dissolution processes [23].At the same time,the development of nearinfrared light has promoted the role of optical imaging in the diagnosis of thrombotic diseases.The second near-infrared window)(NIR-II) has the advantages of high resolution,and can provide anatomical and hemodynamic information at the same time in optical bioimaging,so it can effectively mediate thrombus imaging[24].Liet al.constructed a small molecule fluorophore LZ-1105 based on NIR-II as a probe for long-termin vivoimaging of dynamic vascular structural changes and thrombosis in small animals.The half-life of this fluorophore exceeds three hours,prolonging dynamic monitoring time for thrombosis [25].
2.2.Nano-related photoacoustic imaging
PAI is a new biomedical imaging method based on the photoacoustic (PA) effect,which can provide functional information related to the cellular and molecular characteristics of tissues by using endogenous and exogenous contrast agents [23].It combines light excitation and ultrasound detection.Compared with fluorescence imaging,PAI has higher spatial resolution and deeper imaging depth;compared with ordinary ultrasound imaging,PAI is not limited by the mechanical properties of biological tissues,so it has better tissue contrast.Compared with computed tomography and PET-CT,PAI does not have ionizing radiation,so it is safer [24,25].In summary,PAI can acquire the anatomical,functional,and cellular molecular information of the lesion in real time with high spatial resolution and high tissue contrast without ionizing radiation.This imaging mode can also be enhanced by exogenous contrast agents [26].Up to now,PAI has been used for multi-scale visualization of biological structure imaging from organelles,cells to organs,and has been applied to many disciplines such as cardiology,oncology,and neurology [27].Therefore,PAI is a potential method for the diagnosis of thrombosis.Recently,people have explored gold nanoparticles as an exogenous contrast agent for the diagnosis of thrombosis.When atherosclerosis or thrombosis occurs,macrophages are recruited to the thrombus site [28].Therefore,macrophages are potential delivery targets for exogenous contrast agents.AuNS targeting macrophages and then performing intravascular PA (IVPA) imaging under ultrasound guidance has been confirmedin vivoandin vitromodels and isolated arterial samples(Fig.2) [29].In addition to macrophages,ICAM-1 and E-selectin are also effective targets for AuNS in thrombus imaging [30].In addition to gold nanoparticles,semiconducting polymer nanoparticles(SPN) as a contrast agent are also a new approach currently being explored.SPN has high photostability,optical activity,and a variety of chemical functions.Therefore,SPN is an ideal molecular imaging agent and has been widely used for cell labeling,tumor imaging,and monitoring of reactive oxygen species [31,32].Recent studies have shown that SPN can further enhance PAI while enhancing the contrast of molecular imagingin vivo,making it possible to assemble molecules which sensitive to the thrombus site into nanoparticles to construct imaging probes specifically activated by thrombus to detect thrombus formation.In addition,SPN also can be used for real-time monitoring of drug delivery and accumulation in diseased sites,and can effectively improve the efficiency of thrombosis diagnosis and monitoring [33].

Fig.2.Darkfield reflectance optical images of intact murine macrophages.(A)Murine macrophages loaded with gold nanoparticles (B).The diagram (C) and the IVUS image (F) of the tissue mimicking phantom.The dynamic ranges of IVUS and IVPA images were 50 and 17 dB,respectively.The IVPA images of the same crosssection of the phantom were taken at 532 nm (D) and 680 nm (G) wavelength.The combined IVUS and IVPA images of the phantom (E:532 nm wavelength,H:680 nm wavelength) indicate the origin of the PA responses in IVPA images.Reproduced with permission [29].Copyright 2015,Future Science Group.
2.3.Nano-related radioisotope labeling
In addition to the types of probes used in PAI,radioisotopelabeled nanoparticles targeting platelets,fibrin and other components also play a role in molecular imaging of thrombus,and even visualize the progression of thrombus [34].For example,Adroveret al.designed a thrombus probe synthesizedin vivobased on the principle of fast pre-targeted imaging (f-PreI) (Figs.3A and B).The probe is composed of two parts:biomolecular thrombus specific TCO-antiCD41 and imaging probe [68Ga]Ga-IONP-TZ.After being injected into the body,TCO-antiCD41 accumulates at the thrombus site (Fig.3C).[68Ga]Ga-IONP-TZ reacts after recognizing TCOantiCD41.Through PET imaging,the thrombus can be identified within a few minutes and the change process of the thrombus site can be obtained within 30–60 min.This method accurately locates the thrombus position while providing images of thrombus changes over a period of time,and the probe miss rate is also significantly reduced [35].The combination of radioisotope labeling and optics is also an effective method of imaging.For example,Liuet al.designed an optical and PET radiotracer ENC2015 for factor XIIIa.ENC2015 is labeled with PET radioisotope fluorine.This tracer is both an optical near-infrared fluorescent probe and an 18F positron emission radioactive tracer.The dual optional-PET labeled probe integratesin vivodiagnosis and histological verification with a single chemical part,and improves the specificity of detection while achieving early diagnosis [36].In addition,64Cu,Fe,etc.are also used as trace elements [37].In addition to MRI,CT,etc.,nanoparticles or microbubbles targeting thrombus components also enhance the accuracy of ultrasound in the diagnosis of thrombus [38].Wanget al.conjugated microbubbles (MBs) with a single-chain antibody specific for activated glycoprotein IIb/IIIa,and then used contrast-enhanced ultrasound to detect thrombus in the carotid artery of mice,realizing real-time monitoring of thrombus without invasiveness and radiation [39].
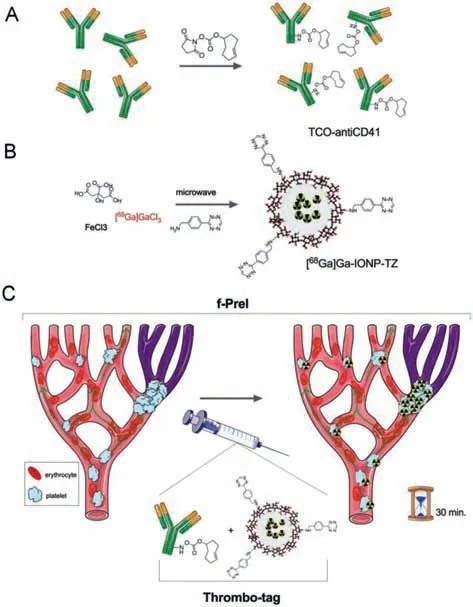
Fig.3.Experimental design for the in vivo detection of thrombi by fast pretargeted imaging:(A) modification of antiCD41 with transcyclooctene (TCO) groups;(B) synthesis of nanoparticles modified with tetrazine (TZ),[68Ga]Ga-IONP-TZ;(C) in vivo use of thrombo-tag for the detection of platelets thrombi.Reproduced with permission [35].Copyright 2020,The Royal Society of Chemistry.
2.4.Nano-related MRI
MRI is an important method for thrombus detection.The commonly used contrast agents are iron oxide and gadolinium,which improve the accuracy of clinical diagnosis.Among them,iron oxide nanoparticles are T2 contrast agents,which can provide negative image contrast,and can detect microthrombi by targeting platelets[40].Gadolinium-based materials are T1-weighted contrast agents that can provide information on the tissue interface of the thrombus site [41].In addition,Taet al.developed an MRI T1/T2 switchable nanosensor based on iron oxide nanoparticles.The nanosensor functionalized with fibrin-binding peptide exhibits T2 effect without thrombin (dark signal).In the presence of thrombin (bright signal),the T1 effect is shown,so it can locate the thrombus and provide information about its stage at the same time.This method uses a single imaging agent to identify and classify systemic thrombi,which is more helpful to treat different blood clots[42].
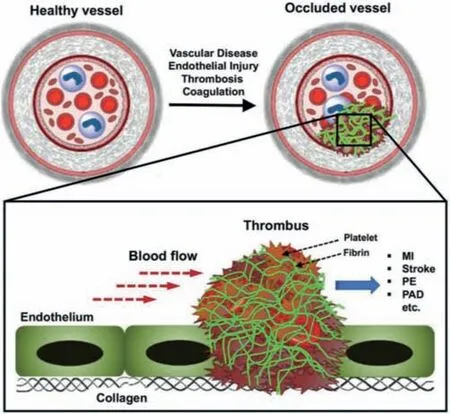
Fig.4.Schematic illustration of occlusive thrombus formation in blood vessels leading to vascular diseases,with platelets and fibrin as the major components of the thrombus.Reproduced with permission [50].Copyright 2020,The Royal Society of Chemistry.
I n addition,thermoacoustic imaging and PTI are also playing an increasing role in the diagnosis and treatment of diseases [43,44].However,despite the rapid development of thrombus imaging methods,each imaging technology has certain limitations.A single imaging method is not enough to meet the increasing challenges in practical applications.Therefore,many scientists are devoted to the study of the fusion of multiple imaging modes [45,46],and multiple experimental results have also proved that multi-mode imaging can effectively solve the problems of short half-life of contrast agents,poor targeting,and poor sensitivity.It is a potential development direction for timely diagnosis vascular diseases such as thrombus in the future [47–49].
3.Nano therapeutic system based on different condition response in thrombolysis
3.1.Nano therapeutic system based on targeted modification
The main components of thrombus include platelets,fibrin and red blood cells (RBC) (Fig.4) [50].Its formation and growth are mainly dependent on three mechanisms:(1) Platelet adhesion to vascular lesions (such as endothelium in inflammation sites,deposition and exposed collagen);(2) Through fibrinogen (Fg) binds to active platelet surface integrinαIIbβ3and aggregates activated platelets at this site;(3) Thrombin is amplified on the surface of active platelets rich in phosphatidylserine (PS),thereby converting the blood Fg into fibrin.The fibrin undergoes self-assembly and FXIIIa-mediated cross-linking to form an insoluble biopolymer network,making the blood clot firmer [51,52].In view of these mechanisms,the currently commonly used clinical methods for the treatment of thrombotic diseases are mainly antiplatelet drugs and fibrinolytic agents [53].However,these methods are difficult to avoid problems such as varying degrees of systemic side effects,low drug utilization,and short half-life [54].The current research hotspots to solve these problems are mainly focused on drug nanomodification,targeting the process of thrombosis,such as platelets,fibrin,RBC,exposed endothelium and collagen,etc.However,due to the coverage of thrombus,the endothelium and collagen are generally difficult to achieve direct contact with drugs [55–57].Many experiments have shown that nanoparticle-based targeted treatment of diseases can improve the utilization and efficacy of drugs in specific areas,while reducing the systemic side effects of drugs,which has become a new approach for disease treatment[58].
3.1.1.Target platelets
In resting platelets,GPIIb-IIIa (αIIbβ3) integrin is inactive,while in the process of thrombosis,platelets are activated to express and activate GPIIb-IIIa (αIIbβ3) integrin in large quantities,using this target point,Huanget al.constructed a nanoliposome tPA-PEGcRGD-lip that can target activated platelets [59,60].Its key component is cRGD peptide,which can specifically bind toαIIbβ3integrin to make the lipid accurately recognizes and binds the activated platelets at the thrombus formation site,and controls the accumulation and release of tissue-type plasminogen activator (tPA)at the thrombus site to achieve the purpose of targeting thrombus to release thrombolytic drugs [1].Both flow cytometry and CLSM measurement in the experiment showed that the liposome specifically binds to activated platelets,and at the same time,through the destabilization of the liposome membrane under physiological conditions,tPA is effectively released and thrombolytic effect is achieved at the thrombus site,which improves treatment effi-ciency also reduces systemic adverse reactions [60].
As another target for platelet targeting,unlike GPIIb/IIIa,P-selectin is an adhesion molecule that is not expressed on platelets in a resting state in normal circulation,but is mainly expressed on the surface of activated platelets.When activated through the mediation of thrombin,histamine,and tumor necrosis factor,P-selectin is rapidly expressed in large quantities,which mediates the interaction between activated platelets and related inflammatory factors,and participates in the pathophysiological process of thrombosis and other cardiovascular diseases [61].At the same time,activated endothelial cells in ischemic tissues also express a large amount of P-selectin.This mediating effect can be blocked by P-selectin antibodies and related drugs.Therefore,Pselectin may be an important target for the treatment of thrombosis or acute and chronic cardiovascular diseases [62,63].For example,Juenetet al.developed a nanocarrier based on fucoidan functionalization and loaded with rt-PA to achieve the purpose of targeted positioning and thrombolysis (Figs.5A and B) [64,65].Prior to this,it has been demonstrated that fucoidan-functionalized nanoparticles targeting P-selectin can be used to diagnose endothelial activation and intravascular thrombosis,as well as tumor selective drug delivery [66,67].Fucoidan has a similar structure to P-selectin glycoprotein ligand 1 (PSGL-1),the main ligand for Pselectin,and has a high affinity for P-selectin [68].Combined with rt-PA,in a mouse model of venous thrombosis,they observed the accumulation of drugs at the thrombus site and the enhancement of thrombus dissolution efficiency (Fig.5C).
3.1.2.Target fibrin
Fibrin is also a research hotspot in targeted drug therapy.Fibrin is a plasma protein produced by thrombin cleaving Fg and is one of the main components of thrombus.In the process of thrombus formation,under the catalysis of thrombin and coagulation factors,Fg in the circulation is locally activated to fibrin,and is crosslinked and deposited on the inside or surface of the thrombus in a network form to stabilize the thrombus and promote activated platelets raise [69].Therefore,fibrin has a similar role as platelets as a target for drug-specific delivery [57].CREKA (Cys-Arg-Glu-Lys-Ala) is a short peptide that can specifically bind to blood clots,and it has good targeting properties to fibrin.Yodsanitet al.developed a tirofiban nanoparticle (T-RBC-DTC NPs) that can target thrombus and regulate local H2O2content.They used RBC membrane coating and combined with CREKA.The results showed that compared with the control group,in the experimental group,T-RBC-DTC NPs gathered in the thrombus site in a large amount,and thrombus formation was significantly inhibited [2].
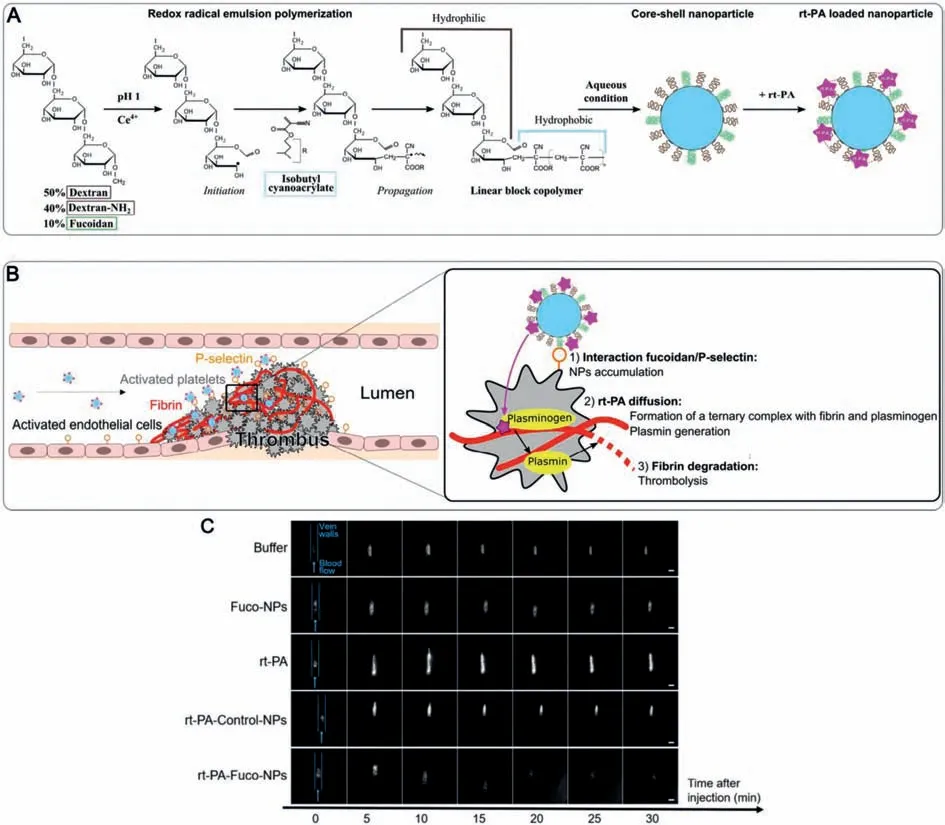
Fig.5.(A) Nanoparticle formulation.Polysaccharide chains are oxidized by cerium(IV) ions.The free radical initiates the radical emulsion polymerization of isobutylcyanoacrylate monomers,which creates amphiphilic linear blocks copolymers.In aqueous conditions,they assemble into core-shell nanoparticles.rt-PA is mixed with the suspension and interacts with the aminated nanoparticles by electrostatic interactions.(B) Nanoparticle interaction with the thrombus:rt-PA-Fuco-NPs shows good thrombolytic effect.(C) Fluorescence imaging of rhodamine 6G-labeled platelets at site of thrombosis over time.Representative examples of thrombus evolution as determined by platelet accumulation at different times after injection of buffer,Fuco-NPS,rt-PA,rt-PA-Control-NPs and rt-PA-Fuco-NPs,respectively (scale bar=200 μm).Prior to injection,circulating platelets were fluorescently labeled with rhodamine 6G and thrombosis was generated on a mesenteric vein by applying a FeCl3-soaked paper for 1 min.Reproduced with permission [64].Copyright 2017,Elsevier Ltd.
However,studies have shown that using platelets or fibrin as a single target does not allow the drug to fully accumulate at the thrombus site,and combining platelets and fibrin as a target may be a potential way to improve targeted drug accumulation.Recently,Sunet al.designed “fibrin + platelet” targeting liposomes)as model nanoparticles,and the liposome is modified by plateletbinding peptides (PBP) and fibrin-binding peptides (FBP) to achieve targeting.By comparison,this method significantly enhances the targeted accumulation of liposomes on thrombus sites [70].Although there are not many studies on the combined targeting of"platelet + fibrin",this result also fully suggests that the combination of multiple targeting mechanisms may achieve better targeted drug delivery.
3.1.3.Target red blood cells
As another component of thrombus,RBC plays an extremely important role in the circulatory system.It is mainly involved in the delivery of oxygen and carbon dioxide.At the same time,it also plays an important role in coagulation and thrombosis.Especially in veins,RBC and fibrin aggregate to form a red thrombus.Many studies have shown that RBC can promote thrombosis and enhance its stability.Therefore,RBC has become a potential strategy for thrombolytic therapy [71].Previous experiments have coupled tPA with RBC and then re-infused the RBC/tPA conjugate in animals.The results have shown that this method can inhibit arterial and venous thrombosis [72,73].Zaitsevet al.fused a pro-urokinase mutant (uPA-T) that can be activated by thrombin and a single-chain antibody (scFv) targeting the proenzyme to RBC into a scFv/uPA-T complex.The complex can target circulating RBC and circulate with the blood to the thrombosis site.After being specifically activated by thrombin,urokinase is released.Under the premise of ensuring the biocompatibility of the complex and the activity of urokinase,the half-life of uPA-T is prolonged and the fibrin in the thrombus is dissolved,and a good thrombolytic effect is achievedin vivoandin vitro.In addition,this method will not produce toxic side effects on RBC [74–76].Therefore,it is a promising strategy to prevent and dissolve thrombus by targeting RBC.
3.2.Nano therapeutic system based on internal microenvironmental response
In the process of thrombosis,the H2O2,temperature,pH,etc.change in the local microenvironment.At the same time,due to thromboembolism,local shear stress increases,which increase the specificity of the thrombus site and become an effective way to find the treatment for thrombotic diseases.
The vascular fluid shear stress at the site of stenosis and thrombosis can increase to 10–100 times than that in normal blood vessels,which can be used to distinguish normal blood vessels and thrombotic blood vessels [77].Under physiological conditions,platelets are activated by high shear through the stenosis to attach to the tube wall [78].Inspired by this,Korinet al.used the high shear stress caused by blood vessel stenosis during thrombosis as a response signal to study shear-activated nanotherapeutics(SA-NTs),Successfully delivered the drug to the blocked blood vessel site and released a large amount of thrombolytic drug at the thrombus site to dissolve the thrombus [79].SA-NTs are nanoparticles formed by concentrating and drying polylactic acid-glycolic acid with good biocompatibility and biodegradability.Due to its hydrophobicity,it can remain stable in the blood and can be dispersed when subjected to stress stimulation [80].They firstly immobilized the drug on the nanoparticles,and then used tissue plasminogen activator to embed the nanoparticle aggregates.This complex remains intact under physiological conditions and circulates with the blood system.When receiving local high-shear stress stimulation,it quickly decomposes into multiple components and effectively adheres to the blood vessel wall and the surface of the thrombus.The drug releases the thrombus to dissolve and the blood vessel recanalizes.In addition,SA-NTs equipped with fluorescent dyes also provide new ideas for real-time thrombus localization.
3.2.2.H2O2 response
After endothelial injury,platelets will adhere,activate and aggregate,and the activation of platelets is closely related to H2O2burst.H2O2is more stable than other reactive oxygen species(ROS) and,therefore,is the main signal transduction medium in the vasculature [60].There is evidence that thrombosis increases oxidative stress,which plays a causal role in platelet pathophysiology [81].H2O2is derived from activated platelets and is also a key mediator of platelet activation and aggregation,which is also a major cause of thrombosis [82].Therefore,the consumption of H2O2will become an important way to block platelet activation and thrombosis.On the one hand,the accumulation of H2O2in the thrombosis site promotes the development of the disease,on the other hand,it provides the possibility of thrombolytic therapy.Zhaoet al.recently designed an H2O2-responsive nanoparticle carrier for delivery of thrombus-targeted antithrombotic agents (such as Tirofiban) [2].The carrier is composed of Dextran nanonucleus and RBC membrane shell combined with tirofiban.Tirofiban is coupled to dextranviaphenyl borate bond,which can be rapidly oxidized and cracked by H2O2,thus,H2O2can be removed during the oxidation process,When the nano-carrier circulates to the thrombus site,H2O2responds quickly,lyses the carrier and releases the drug,thus achieving the release of the drug in the thrombus site and promoting the consumption of H2O2,dissolving the thrombus and inhibiting the thrombus formation.

Fig.6.Design of t-PA-installed polyion complex (PIC) nanoparticles possessing nitroxide radicals (t-PA@iRNP).(A) t-PA@iRNP was formed based on a PIC micelle composed of three elements:cationic PEG-b-PMNT diblock amphiphilic copolymers possessing ROS-scavenging moieties as side chains,anionic poly(acrylic acid) (PAAc)and t-PA.(B) Graphical illustration of collapsed t-PA@iRNP after intravenous delivery within the acidic ischemic penumbra region.Reproduced with permission [85].Copyright 2019,Elsevier Ltd.
3.2.3.pH response
In addition,the local pH of the ischemic region is significantly reduced during thrombosis due to acidosis.Therefore,local low PH microenvironment has become a potential therapeutic target for thrombolytic therapy.pH-responsive nanocarriers have shown tremendous advantages in the field of tumor therapy and acute stroke therapy [83,84].Recently,Meiet al.have designed a selfassembled polyionic composite nanoparticle (t-PA@iRNP) with a nitrogen-oxygen radical-containing t-PA.They encapsulated t-PA in the core of iRNP,thus inhibiting the enzymatic degradation activity of t-PA and the excretion of systemic circulation (Fig.6) [85].Because of the inclusion of iRNP,the activity of TPA was significantly inhibited,at the same time,t-PA@iRNP tends to disintegrate due to strong protonation and charge repulsion between polycations in the acidic microenvironment of the thrombus site,and t-PA activity is restored by acid-triggered breakdown of the complex.The half-life of t-PA was prolonged from 8.2 min to 71.2 min by t-PA@iRNP treatment,and a large amount of t-PA could be effectively released from the target site by acidic disintegration of the complex.The method not only reduces the toxic and side effects of the medicine,but also enhances the circulation time in the body and improves the utilization rate of the medicine [85,86].In vivoandin vitroexperiments in different animal models showed that the nanoparticles based on pH response had good safety [87–89].Zhanget al.combined the acidic microenvironment and reactive oxygen species (ROS) to construct a pH/ROS dual response nanocarrier,and further combined with molecular targeting characteristics to demonstrate the precise delivery and release of drugs under vascular inflammation conditions,providing a new direction for thrombolytic therapy [90].
3.3.Nano therapy system based on external stimulation
Although nano therapeutic system platforms based on internal microenvironmental response have great potential in personalized medicine,their selectivity and specificity for different parts and types of thrombus is still a huge challenge.The structural conformation or physical and chemical properties of the response platform can be changed according to the corresponding environment,which may overcome this challenge [91,92].The design of the stimulus response nanoplatform is not limited to being sensitive to the pathophysiological changes of the thrombus site,but also to the response to external stimuli such as temperature,light,magnetic field,and ultrasound [92–94].External stimulus can play a remote controlling role in biomedicine and body activities,and has attracted many attention and research in recent years [95,96].Various external stimuli such as magnetic field,ultrasound,light and heat have been widely used to regulate the biological activity of specific locations in the body,including cell function,protein activity and tissue regeneration,etc.,showing good application prospects [97–100].At the same time,external stimulation such as sound,light,magnetic also has the function of treating tumor,stone and other diseases,so it is possible to use external stimulation to directly act on the thrombus or to control the release of drugs to dissolve thrombus indirectly.
3.3.1.Magnetic stimulation
Bettelheim supposes that the drops of blood symbolize63 sexual maturity66, a special bond forged by a mother who is preparing her daughter to become sexually active (Bettelheim 1975, 139).
Magnetism is an accepted targeting strategy,and magnetic nanoparticles (MNP) are Biocompatibility,biodegradable,easily controlled in size and controlled by an external magnetic field,it has been widely used in targeted drug delivery systems to deliver therapeutics and diagnostics locally and efficiently [101].For example,Liet al.developed a platelet membrane (PLT)-coated biomimetic nanoparticle carrier containing L-arginine andγ-Fe2O3magnetic nanoparticles (PAMN) in the early diagnosis and treatment of acute ischemic stroke (Fig.7A),PLT can target and adhere to damaged blood vessels after being activated,so it has great potential in thrombus targeting and drug delivery [101–103].γ-Fe2O3magnetic nanoparticles (MNs) have excellent magnetic field responsiveness [104].This carrier has been proven to possess the natural characteristics of PLT film.With the guidance of an external magnetic field,the carrier can quickly target to Ischemic stroke lesions (Fig.7B),and release arginine in the lesions to promote endothelial cells to produce NO,promote vasodilation and destroy local PLT aggregation,so as to achieve the effect of blood vessel expansion and thrombolysis (Fig.7C).In addition,based on the convection-enhanced transport theory of a rotating magnetic nanomotor,Chenget al.used a rotating magnetic nanomotor to enhance the local transport of t-PA molecules at the blood clot interface for the treatment of ischemic stroke.Its effectiveness and the efficiency are fully confirmed in the rat embolization model[105].
3.3.2.Ultrasound stimulation
The use of ultrasound to construct a treatment system is another strategy to respond to external stimuli.Ultrasound is a mechanical wave propagating in the air that can cause material vibrations with a frequency above 20 kHz.It often causes physical changes in acoustic cavitation and microstreaming (acoustic cavitation and microstreaming).Cavitation refers to the formation and activation of gas-filled bubbles in the medium induced by ultrasound.The rapid burst of gas-filled bubbles will produce shock waves and high-speed fluid micro-jets [106].Using this feature,Uesugiet al.designed a new type of t-PA ultrasonic responsive delivery system [107].The activity of t-PA is inhibited by gelatin embedding,and ultrasound stimulation is given to the thrombus site to loosen and dissociate the interaction between t-PA and gelatin complex.The acoustic steaming of ultrasound effect under the action of ultrasound promotes the penetration of t-PA molecules into the thrombus,and the radiation force promotes the re-formation and opening of the fibrin matrix,and promotes the diffusion of drugs,making fibrin and drugs the surface area increased [108],and ultrasonic irradiation changes the molecular size and activity of t-PA and finally achieves the purpose of thrombolysis.This is particularly important for the rescue of stroke and acute myocardial infarction [109,110].At the same time,experiments show that the appropriate frequency and intensity are also particularly important.Low-frequency and highintensity ultrasound directly transmits energy and acoustic cavitation breaks the thrombus into small fragments,which can easily lead to distal embolism.However,ultrasound with a frequency of 1 MHz and an intensity of 0.75 W/cm2for 5 min will not directly lyse the thrombus and increase the activity of t-PA by 2.2 times[107,111].
3.3.3.Light stimulation
Compared with magnetic field and ultrasonic response,light has the inherent advantages of non-invasiveness,high temporal and spatial resolution,and easy control of intensity and wavelength [112].At present,the model based on the response of light mainly promotes the opening of ion channels,the unbinding of photosensitive proteins stimulated by light,and the photocrosslinking of tissues by PTT and PDT,the utility model can be applied to a plurality of fields,such as tumor,thrombolytic therapy [113,114].Among them,near infrared (NIR) light is widely used due to its lower tissue absorption and toxicity,less photon scattering and deeper tissue penetration [115].At the same time,nano-alkylene oxide,carbon nanotubes and metal nanoparticles can convert NIR into heat to achieve certain therapeutic purposes [116].In addition,under NIR irradiation,photosensitizerbased nanostructures have the ability to generate reactive oxygen species (ROS),which has an impact on treatment [117].Recently,Zhanget al.constructed a mesoporous carbon nanospheres containing porphyrin-like metal centers (RGD-PMCS) based on Arg-Gly-Asp (RGD) modification,Zhanget al.constructed a mesoporous carbon nanospheres containing porphyrin-like metal centers (RGDpmcs) modified with Arg-Gly-Asp (RGD) to induce thrombolysis at specific sites by hyperthermia and ROS under a NIR laser irradiation [118].This is a new exploration of PTT and PDT.Mesophilic carbon nanospheres locate to the thrombus by targeting the GPIIb-IIIa (αIIbβ3),and then convert light energy into thermal energy rapidly under NIR illumination.At the same time,under NIR irradiation,RGD-PMCS produced ROS,which destroyed the platelet factor 3 (PF3) in lecithin by lipid peroxidation and inhibit the recurrence of thrombus [119].PTT/PDT dual mode improves the efficiency of thrombus rupture and prevents secondary embolism of larger fragments.It provides a rapid,safe and effective thrombolytic method [120].The heat generated by NIR irradiation can quickly promote drug release,and the release rate can be achieved by controlling the light intensity.Therefore,light-triggered drug release is also a way of treatment [121].
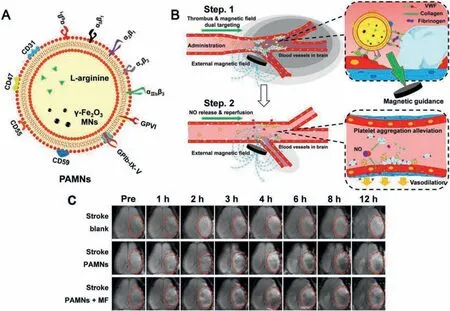
Fig.7.Schematic diagram of PAMN structure and in vivo targeting modalities.(A) As a PLT membrane biomimetic nanocarrier,PAMNs inherit the natural characteristics of the PLT membrane that carry specific binding integrins (α6β1, α5β1, α2β1, αvβ3 and αIIbβ3) glycoproteins GPVI and GPIb-IX-V,and complementary regulatory proteins such as CD59,CD55,CD47,and CD31,which impart the PAMNs with damaged blood vessel adhesive and immune escape capabilities.(B) Due to the maintenance of the mimetic properties of the PLT membrane and the application of a magnetic field gradient,the PAMNs reach the stroke lesion more quickly to achieve rapid targeted delivery of L-arginine.The in-situ generation of NO induces vasodilation and reduces PLT aggregation.(C) In vivo T2MRI before (pre) and after saline and PAMN injection (1,2,3,4,6,8,and 12 h).Reproduced with permission [102].Copyright 2020,American Chemical Society.
Although external stimulation is more effective in thrombolytic therapy,there are still some problems to be solved urgently.For example,how to control the damage to surrounding tissues caused by the thermal effects of external magnetic fields and light sources[122];how to enhance the tissue targeting of ultrasound and avoid possible metastatic diffusion due to enhanced vascular permeability [123];how to control the magnetic field and ultrasonic intensity to avoid the direct rupture of thrombus into larger thrombus masses and form secondary blockage [124];how to strengthen the precise positioning of external stimulation in time and space [125].Therefore,it is necessary to further explore these problems in the future to be able to truly realize its application in thrombolytic therapy.
Three nano therapy systems have important application value,reducing systemic side effects,and there are also some key problems to be solved.The targeted modified nano-therapy system constructed based on the components of thrombus improves the utilization of drugs,but due to the coverage of other components,drugs often cannot directly contact the target component.The nano therapy system based on the local microenvironment has shown great advantages in personalized medicine,and can accurately treat disease according to the special physiological changes of the thrombus site,but it has poor selectivity for different types of thrombus.The nano therapy system based on external stimuli can realize external remote control and realize chemotherapy while taking into account physical therapy.However,external stimuli such as magnetic and ultrasound are damaging,the penetration depth also needs more accurate control.In summary,the nano therapy system has made great progress in thrombotic diseases,but it also has certain side effects and limitations,which requires more in-depth research.
4.Integrating diagnosis with treatment of thrombosis
Since there is a time window for thrombosis treatment,the treatment time limit is closely related to the prognosis.If diagnosis and treatment can be synchronized,it will have a good impact on its prognosis.Therefore,there is great interest in strategies that can detect and dissolve thrombus [126,127].A contrast agent that can specifically target thrombus is used for non-invasive PAI of thrombus.The contrast agent can perform imaging and treatment functions at the same time,which is of great significance for the treatment of thrombotic diseases.Kanget al.designed a fibrin targeted nanocarrier loaded with the Antiplatelet drug Tirofiban,called FTIAN,FTIAN can be used as a contrast agent for PAI to specially image the thrombus,and it can also treat the thrombus by releasing drugs [57].FTIAN is made of borate antioxidant polymer (fBAP) coupled with near infrared (NIR) fluorescent dyes and lipopeptides targeting fibrin.fBAP and lipopeptides can selfassemble under aqueous conditions to form nanoparticles with fibrin targeting peptides on their surfaces,and inhibit platelet activation and inflammation by inhibiting the generation of H2O2at the thrombus site.Experimental results prove that the carrier effectively dissolves thrombus.
In addition,the new molecular probe is another solution for thrombosis diagnosis and treatment.MR molecular imaging is an implementation of molecular probes.Because of its advantages of not using ionizing radiation,deep tissue penetration and high resolution,MR molecular imaging is another imaging method with great clinical application value besides radionuclides [128].Combining MR contrast agents,targeting agents and drugs with special carriers through different physical and chemical methods can obtain multifunctional MR molecular probes,which can be used for early non-invasive molecular level diagnosis and treatment of various diseasesin vivo[129,130].In addition to its role in the early detection and dynamic monitoring of thrombosis,the thrombolytic treatment cannot be underestimated.Recently,Zhouet al.constructed Fe3O4-based polylactic acid-glycolic acid copolymer(PLGA) nanoparticles for detection,monitoring and targeted thrombolysis [131].The system embeds rt-PA,and the outer layer is covered with cyclic arginine glycine-aspartate peptide (cRGD) and chitosan (CS) to form Fe3O4-PLGA-rt-PA/CS-cRGD nanocarriers,Fe3O4acting as a contrast agent,rt-PA is used as a targeted thrombolytic drug,and cRGD is used to target activated platelets.Experimental results show that the carrier can play multiple functions in early detection of thrombus,dynamic monitoring of thrombolytic effi-ciency and thrombolytic therapy using MRI at the molecular level.
5.Summary and prospect
In the past 20 years,great progress has been made in the diagnosis and treatment of thrombotic diseases.In particular,nanotechnology has promoted a qualitative leap in diagnosis and treatment.Compared with traditional methods,the advantages of combining nanotechnology are reflected in the following aspects:(1)By modifying the nanocarrier,the half-life of the drug is increased,and the long-term circulation and continuous detection and treatment of the drug in the body can be realized;(2) By loading specific binding molecules of each component of thrombus,targeted drug delivery can be achieved,and the accuracy and specificity of detection and treatment can be achieved;(3) Most nano-carriers are biodegradable,reducing the toxic and side effects brought to the whole body;(4) The nanomaterials used are biocompatible,reducing body rejection.At the same time,combined photoacoustic and chemical kinetics are currently emerging diagnosis and treatment methods,especially in terms of diagnosis,non-invasive and specific,can achieve visual monitoring of blood vessels,and achieve targeted precision in treatment through energy delivery and stimulus response platform construction.Shortens the treatment time limit and plays a positive role in the prognosis.
But despite a series of breakthroughs,there are still some problems in the diagnosis and treatment of thrombosis.Because thrombus often blocks blood vessels and often causes irreversible damage to the body,although the diagnosis and treatment methods based on nanotechnology and greatly shorten the treatment response time,they still cannot meet the timely treatment of thrombus.In addition,there have not been many reports on the diagnosis of different types of thrombus in different parts.Therefore,how to further increase the specificity and accuracy of early diagnosis and enhance drug targeting to improve the therapeutic effect of drugs is still the focus of future exploration.Although a large number of experiments have explored the safety of nanomedicine,they still cannot fully prove that nanoparticles are completely safe for the diagnosis and treatment of thrombosis.Due to internal circulation and accumulation in the liver and other organs,they are likely to cause liver toxicity and nephrotoxicity.What is more,certain nanoparticles have been proved to have a damaging effect on the fetal cardiovascular system by some experiments.Therefore,security is still a key concern for nanotechnology [132].And the general direction shows that only combined applications including conventional treatment,nanotechnology,photoacoustic kinetics and other technical means can achieve early diagnosis and early treatment of thrombosis,reduce thrombotic complications and improve prognosis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Key Research and Development Plan Young Scientists Program (No.2017YFA0106000),the National Science Fund for Outstanding Young Scholars (No.31822021),the National Natural Science Foundation of China (No.31771057),and the National Key Research and Development Plan(No.2016YFC1101100).
杂志排行
Chinese Chemical Letters的其它文章
- Long-wavelength (red to near-infrared) emissive carbon dots:Key factors for synthesis,fluorescence mechanism,and applications in biosensing and cancer theranostics
- The point-of-care-testing of nucleic acids by chip,cartridge and paper sensors
- Sodium bicarbonate,an inorganic salt and a potential active agent for cancer therapy
- New advances in gated materials of mesoporous silica for drug controlled release
- Current development in wearable glucose meters
- Recent advances in the synthesis of non-carbon two-dimensional electrode materials for the aqueous electrolyte-based supercapacitors
