Sodium bicarbonate,an inorganic salt and a potential active agent for cancer therapy
2021-03-14YueWngXueerZhouWenxunWngYongyoWuZhiyongQinQingPeng
Yue Wng,Xueer Zhou,Wenxun Wng,Yongyo Wu,Zhiyong Qin,Qing Peng,∗
a State Key Laboratory of Oral Diseases,National Clinical Research Center for Oral Diseases,West China Hospital of Stomatology,Sichuan University,Chengdu 610041,China
b State Key Laboratory of Biotherapy and Cancer Center,West China Hospital,Sichuan University and Collaborative Innovation Center,Chengdu 610041,China
Keywords:Sodium bicarbonate Cancer Tumor microenvironment Acidosis Pharmacology
ABSTRACT Cancer is a serious threat to humans due to its high mortality.The efforts to fully understand cancer and to fight against it have never been stopped.The traditional therapies,such as surgery,radiotherapy and chemotherapy,are useful but cannot meet the increasing demands of patients.As such,novel approaches against cancer are urgently required.It has been found that the acidic tumor microenvironment plays important roles in promoting the cancer progression.In recent years,sodium bicarbonate (NaHCO3),a simple inorganic salt,has been found to be able to reverse the pH of tumor microenvironment and inhibit the invasion,metastasis,immune evasion,drug resistance and hypoxia of tumor cells.Thus,NaHCO3-based therapy is a potential approach for the treatment of cancer,and the related studies have been increasingly reported.Herein,we aim to provide a comprehensive understanding of the acidic tumor microenvironment and summarize the applications and mechanisms of NaHCO3 in cancer therapy.The combination of NaHCO3 with chemotherapy,immunotherapy or nanoparticles systems is discussed.In addition,the concerns of NaHCO3 in clinical use and the potential ways to use NaHCO3 for cancer therapy are also discussed.
1.Introduction
Cancer,a serious threat to human health,is considered as the second leading cause of death worldwide [1].It is estimated that 20% people develop cancer during their lifetime,and 1/8 men and 1/11 women die from caner (10 million total cancer deaths in 2020) [2].With the rapid population growth and aging tendency,the incidence of malignant tumors is on the rise [3].Although significant progresses have been made in cancer therapy,it is still of the greatest challenge to treat cancer in a satisfying manner.
To date,surgery,radiotherapy and chemotherapy are still the most common cancer therapies in clinic.In recent years,some novel approaches,such as targeted therapy,gene therapy,immunotherapy and phototherapy,are (being) developed [4,5].However,their applications are largely limited by the undesired side effects,such as drug resistance,cardiotoxicity and immune disorders [6–8].As such,much attention has been paid to seek novel,safe and effective approaches for cancer therapy.
It has long been known that cancer is a single-cell disease in which mutations in proto-oncogenes and tumor suppressor genes determine the carcinogenesis and tumor progression [9].Although Stephen Paget proposed the "seed and soil" hypothesis emphasizing the influence of tumor microenvironment on its development as early as 1889,researchers lacked enthusiasm and innovation in studying tumor microenvironment until the 1980s [10].The change in attitudes began with the discovery that tumor microenvironment can reprogram the phenotype of tumor cells to facilitate their growth,invasion,and metastasis [11].
Cells together with the physical conditions in the tumor microenvironment promote the tumor development.The anaerobic glycolysis caused by hypoxia (Pasteur effect) produces a large amount of lactate [12].In addition,under aerobic or moderate hypoxia conditions,tumor cells undergo aerobic respiration and produce carbon dioxide (CO2).Lactate fermentation and decomposition of hydrated CO2produce a large number of protons,which are transported into the tumor microenvironment through various transporters,ultimately resulting in the extracellular acidosis [13].The extracellular pH (pHe) in tumor tissues is between 6.5 and 7.0 while pHein normal tissues is between 7.2 and 7.5 [14,15].Meanwhile,the intracellular pH (pHi) in cancer cells is usually higher(pHi>7.4) than that in normal cells (pHi~7.2) [16].Tumor acidosis can cause drug resistance,immune evasion and chronic autophagy,promote the decomposition of tumor matrix and the epithelial mesenchymal transformation,and thus accelerate tumor invasion and metastasis [17,18].In this regard,regulating the tumor pHeis a potential approach for cancer treatment.
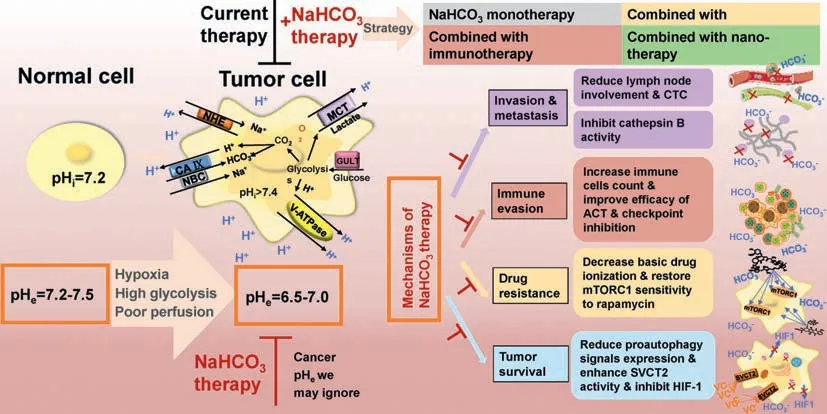
Fig.1.Schematic illustration of acidic tumor microenvironment and the applications and mechanisms of NaHCO3-based cancer therapy.
Sodium bicarbonate (NaHCO3),a simple and readily available inorganic salt,has been used to correct the acidosis of tumor microenvironment and shown significant and positive effects on cancer therapy [19].Basically,NaHCO3can be used alone or combined with other therapies,such as chemotherapy [20].It is undoubted that NaHCO3can play significant roles in regulating tumor microenvironment and in the ultimate cancer therapy.In this review,therefore,we aim to provide a comprehensive understanding of the acidic tumor microenvironment (tumor acidosis),summarize the applications and mechanisms of NaHCO3in cancer therapy,and discuss the potential combination of NaHCO3with chemotherapy,immunotherapy or nano-therapy (Fig.1).In addition,the concerns of NaHCO3in clinical use and the potential ways to use NaHCO3for cancer therapy are also discussed.
2.Tumor acidosis
Tumor acidosis has been experimentally proven and widely recognized.The sources of acid are mainly associated with lactate produced by glycolysis and CO2produced by respiration [21].Acidosis can not only promote tumor cell proliferation,invasion,migration and other malignant biological behaviors,but also make tumor cells resistant to certain chemotherapeutic drugs,evade host immune surveillance,and promote autophagy and hypoxia [22].
2.1.Biogenesis of tumor acidosis
2.1.1.Lactate-derived acidosis
Due to the heterogeneity of tumors,hypoxia caused by insuffi-cient perfusion and increased oxygen consumption is widely accepted [23].Hypoxia leads to an increase in the expression of HIF1α,which leads to an increase in the expression of glucose transporters,thereby accelerating the uptake of glucose by tumor cells [24].At the same time,HIF1αpromotes the expression of the enzyme that converts glucose to pyruvate and the enzyme that converts pyruvate to lactate,such as lactate dehydrogenase A (LDHA),which accelerates the production of pyruvate and lactate in cells [25].Under the stimulation of hypoxia and acidic pHi,the expression of the monocarboxylate transporter 4 (MCT4) that transports lactate and H+ions to the outside of the cell increases,resulting in an increase in the concentration of extracellular H+ions [26].In addition to MCT4,there are many highly expressed H+extruders on the tumor cell membranes for H+ions efflux,including vacuolar H+-ATPase (V-ATPase),sodium-hydrogen exchangers(NHEs),sodium-bicarbonate cotransporters (NBCs),monocarboxylate transporters (MCTs),carbonic anhydrases (CAs) [27].V-ATPase and NHEs use ATP hydrolysis and energy stored in Na+gradient respectively to actively transport H+ions against their gradient [28].Tumor cells may also undergo glycolysis under aerobic conditions although it is still controversial [29].
2.1.2.CO2-derived acidosis
In the case of aerobic or moderate hypoxia,tumor cells not only produce lactate,but also produce CO2through aerobic respiration using lactate and fatty acids as substrates [30].CO2is hydrated into HCO3-and H+ions by CAs,including CAIV,CAIX and CAXII,which are bound through the membrane [31].Among them,CAIX is still active at very low pH and is considered to be a "pH regulator" responsible for acidifying tumor pHe,whose high expression has been considered as a negative prognostic indicator in breast cancers [32].The H+ions are transported outside the cell through H+extruders described above.In addition,because CO2can diffuse freely,when the concentration gradient inside and outside cells is sufficient,it can also directly diffuse outside cells to affect tumor pHe,which is still controversial [33].
2.2.Consequences of tumor acidosis

Fig.2.Tumor acidosis will result in the following results:Facilitating tumor invasion and metastasis by promoting EMT and matrix degradation;affecting immune cells function to promote immune escape;reducing drug entry into cells and increasing drug efflux by secreting exosomes to acquire drug resistance;chronic autophagy producing amino acids and ATP to promote tumor survival.
The acidic tumor extracellular microenvironment is toxic to the peritumoral normal tissues,including promoting cell necrosis or apoptosis through a p53-dependent pathway initiated by caspase activation,and reducing genomic stability through chromosome fragmentation and translocations [34].Interestingly,tumor cells not only adapted to the acidosis,but also used it for further growth and invasion [35].Epithelial mesenchymal transformation (EMT)and extracellular matrix decomposition play an important role in acidosis-induced tumor invasion and metastasis.Furthermore,tumor acidosis also promotes immune evasion,drug resistance,autophagy and hypoxia (Fig.2).
2.2.1.Tumor invasion and metastasis
Tumor acidosis favors tumor invasion and metastasis.The exact mechanism has not been clarified,but several existing evidences suggest its relation with EMT and extracellular matrix degradation,as described below.
Epithelial cells lose epithelial properties and gain mesenchymal features during the process of EMT,which facilitates cancer cells migration [36].The present study shows that tumor cells undergo phenotypic transformation during reverse switch from EMT to mesenchymal-epithelial transition (MET) to adapt to acidosis,favoring tumor cell proliferation [37].In addition,recent findings show that acid-sensing ion channels 1 (ASIC1) and ASIC3 increase the intracellular Ca2+concentration and RhoA activity under acidosis conditions,thus initiating EMT of pancreatic cancer cells,which can provide a new target for tumor invasion inhibition [38].
Tumor acidosis also induces extracellular matrix degradation by enhancing activity of proteolytic enzymes and release of exosomes.Acidosis promotes proteolytic enzymes release of nearby normal cells (fibroblasts and macrophages),such as cathepsin B[39].Moreover,acidosis can increase exosomes secretion,which is also strongly associated with tumor invasion [40].For example,acidosis-induced exosomal miR-21 and miR-10b favor cancer cells proliferation and metastasis,thus considered as therapeutic targets for liver cancer [41].In addition,acidosis will also promote the release of vascular endothelial growth factor (VEGF) to accelerate angiogenesis and facilitate tumor invasion [42].
2.2.2.Immune evasion
Tumor acidosis plays an important role in immune evasion [43].Acidosis suppresses T-cell receptor activation [44],inhibits interferon gamma (IFN-γ) production by T cells and NK cells [45],impairs monocyte-derived tumor necrosis factor (TNF) [46]and induces dendritic cell (DC) phenotypic transition to a tumorassociated DC phenotype [47].Meanwhile,there is evidence that acidosis influences other components of immune system,such as myeloid-derived suppressor cells and macrophages [48,49].
2.2.3.Drug resistance
Drug resistance is a great challenge to chemotherapy and the development of chemicals with low or slowly-developed drug resistance is of great interest [50].Acidosis may contribute to drug resistanceviainterference with drug entry and drug retention in tumor cells.A large number of anticancer drugs are weak bases that are quickly inactivated through neutralization by acidic tumor microenvironment or sequestrated into intracellular acidic vesicles,such as lysosomes [51].Acidosis can also make the P-glycoprotein overexpressed on tumor cell surface,thus increasing drug efflux[52].A possible strategy to reduce drug resistance is inhibition of ion pumps from acid effusions,such as proton pump inhibitors(PPI) [53].Recently,there has been a self-assembled carbonic anhydrase inhibitor of nanofiber structures whose size increases with increased acidosis,resulting in damage to intracellular acid vesicles,against drug resistance [54].
2.2.4.Tumor survival
Tumor acidosis is associated with nutrient deficiency and poor perfusion,thus inducing autophagy.Through autophagy,cells break down cytoplasmic components into amino acids and ATP for tumor cells survival [55,56].Therefore,autophagy allows tumor cells to survive in a highly acidic environment.Autophagy regulation can be used in conjunction with conventional cancer treatments to improve therapy efficacy,such as the use of the lysosomal inhibitor ketone [57].
3.Common therapies for cancer
As mentioned above,cancer is particularly dangerous.According to the 2020 global cancer report released by the world health organization (WHO),about one in five people will develop cancer during their lifetime,and one in eight men and one in ten women will die from cancer [2].Nowadays,there are diverse treatments for different tumor types,including traditional therapies(surgery,chemotherapy and radiotherapy) and novel therapies (targeted therapy,immunotherapy,gene therapy,nano-therapy,sonodynamic therapy and multimodal therapy).
3.1.Traditional therapies
3.1.1.Surgy
Although tumor therapies have made great progress,surgical resection is still the most effective method for most solid tumors.However,surgery can also cause damage to normal tissues and organs,and postoperative complications or dysfunction may occur,affecting the quality of patient’s life [58].Rapid development of surgical techniques and equipment has translated into better outcomes for cancer patients,such as Da Vinci surgical robot and the application of liquid nitrogen during surgery [59,60].Due to more accurate preoperative staging and comprehensive treatment models,tumor surgery has entered the era of precise and microinvasive treatment.
3.1.2.Radiotherapy
Radiotherapy is used to treat malignant tumors and certain benign diseases by ionizing radiation to eliminate and eradicate local primary tumors or metastases [61].It can cure at least 40%of cancers [60].In recent years,assisted with improved radiotherapy equipment and technology,indications for radiotherapy have become wider and curative effect has been correspondingly more satisfactory,such as stereotactic body radiotherapy,carbon ion radiation treatment and proton beam therapy [62–65].However,normal tissues are also irradiated inevitably in radiotherapy,leading to undesired side effects to surrounding tissues [66].
3.1.3.Chemotherapy
Chemotherapy uses cytotoxic drugs to kill tumor cells by affecting the structure and function of nucleic acids and proteins in cells,which is one of the main topics in anticancer research [67–69].Following the availability of novel technologies that enable accurate delivery of drugs to targeted tumor or immune cells,the combination of chemotherapy,immunotherapy and targeted therapy has become increasingly prevalent for tumor treatment [70,71].Nowadays,the two greatest concerns regarding chemotherapy are drug resistance and damage to normal cells,such as gastrointestinal reactions and hair loss [72,73].
3.2.Novel therapies
3.2.1.Gene therapy
Gene therapy transfers exogenous genes into human body,repairing and correcting structural and functional defects of tumorrelated genes,or enhancing the host’s defense mechanisms so as to kill tumors [74,75].The advent of CRISPER/Cas9 technology has improved the accuracy of targeted gene editing [76].However,there are still many problems to be solved.For example,transferred genes cannot successfully enter target cells because they are degraded by enzymes during circulation.Correspondingly,nanoparticles are being developed to address this problem [77].
3.2.2.Immunotherapy
Immunotherapy is regarded as the therapeutic approach that achieves enhanced immunity by activating the host’s immune defense mechanism,including immune checkpoint inhibition,adoptive cell transfer (ACT),immune modulator therapy and vaccination [78,79].The most common checkpoint proteins and their corresponding checkpoint inhibitors are PD-L1 and ipilimumab [80],CDLA-4 and nivolumab [81].However,immunotherapy also has many side effects,such as immune-related adverse effects (irAEs)and cytokine release syndrome (CRS) [82].These side effects often require special treatment,such as steroids and immunoregulatory therapy [83].
3.2.3.Targeted therapy
Molecular targeted therapy targets key molecules in tumor genesis and development,and uses effective blockers to interfere with signal transduction pathway and tumor microenvironment to achieve the purpose of treatment [84].It is one of the most widely used and fastest growing tumor treatments.Some typical targeted therapies include cetuximab for BRAF-mutant colorectal cancer [85]and crizotinib for NSCLC with MET exon 14 alterations[86].
3.2.4.Nano-therapy
Nanotechnology is one of the fastest growing technologies,and an increasing number of nanomaterials are used for advanced drug delivery due to their great potentials in improving water solubility,enhancing targeting capacity,prolonging drug circulation time,and reducing drug resistance and side effects [87–95].About a dozen anticancer nano-therapies have been approved,such as PEGylated liposomal doxorubicin (Caelyx®,Doxil®) [96].In addition to the approved nano-therapeutics,some novel nanotechnologies and functionalized nanoparticles-based drug delivery systems have been used for regulating tumor microenvironment,realizing targeted radiotherapy and enhancing immunotherapy [97–100].
3.2.5.Sonodynamic therapy
Sonodynamic therapy (SDT) is a novel non-invasive and safe therapeutic technique involving the combination of low-intensity ultrasound and sonosensitizer,which avoids light damage in phototherapy and provides deeper penetration depth in soft tissue.Novel nanoparticle technologies are used in SDT to achieve targeted therapy with excellent drug loading,improved bioavailability and controlled release.For example,the multi-responsive nanoriceball NGR@DDP can significantly inhibit the growth of hepatocellular carcinoma with negligible side effects [101].
3.2.6.Multimodal therapy
As mentioned above,monotherapy has numerous side effects and often cannot cure cancer.Therefore,multimodal therapy based on the synergy of two or more therapies has become a promising novel therapeutic method.Multimodal therapy not only enhances the anti-cancer efficacy,but also reduces the side effects caused by high-dose drugs in monotherapy.Notably,nano-platforms play an important role in multimodal therapy.Wanget al.combined nanoplatforms and photothermal therapy with the construction of titanium nitride nanoparticles,thus allowing complete tumor ablation using photothermal ablation in deeper regions [102].
4.NaHCO3-based therapy
The continuous progress of the above-mentioned therapies and their combination has significantly improved the efficacy of cancer therapy.But the outcome in many cases is still unsatisfying.In the past decades,NaHCO3-based therapy targeting the acidic pH of tumor microenvironment has been developed and has shown a certain degree of promise in cancer therapy[103–105].
4.1.NaHCO3 monotherapy
As an inorganic salt with alkalescence,NaHCO3is able to neutralize the weakly acidic pH in tumor microenvironment and thus can be used for cancer therapy directly.Robey and colleagues found that oral NaHCO3therapy in mice had no effect on primary tumor growth,but that NaHCO3therapy resulted in a significant reduction in the number and size of lung,bowel,and diaphragmatic tumor metastases [103].They showed that in the experiment of 30-day period,the mice treated with NaHCO3had a total of 147 metastatic lung lesions (vs.326 in control group),with an average lesion diameter of 4.5 mm (vs.5.2 mm in control group).In the 60-day experiment,the treatment of NaHCO3reduced the average number of lesion pixels in mice to 74 (81% reduction),and half of the mice in the control group had more than 240 lesion pixels.In addition,the survival rate of mice in the NaHCO3group was greatly improved.In another work,Banerjee and colleagues achieved the initial burst release of NaHCO3with polycaprolactone coating in the calcium phosphate delivery system [106].They found that the sharp increase in local pHereduced the proliferation capacity of osteosarcoma cells and the density of osteosarcoma,suggesting that NaHCO3could be used to increase the tumor pHeafter bone tumor surgery to reduce tumor invasion and metastasis.
4.2.Combined with immunotherapy
Immunotherapies for tumors include checkpoint inhibition and ACT of tumor-specific T cells [107].However,tumor acidosis inhibits the activity of cancer-killing immune cells,such as T cells,dendritic cells and nature killer cells,and blocks the secretion of IFNγand TNFα,resulting in immune escape [108].It has been found that tumor acidosis promoted the polarization of tumorassociated macrophages into a non-inflammatory phenotype,thus promoting angiogenesis and supporting tumor growth and metastasis in the mouse models of melanoma [109].Therefore,it is promising to improve the efficacy of immunotherapy by modulating pHewith NaHCO3.
Pilon-Thomaset al.showed that the addition of NaHCO3significantly inhibited tumor growth and improved the efficacy of immunotherapy [110].They found that the efficacy of combining NaHCO3with anti-CTLA4 or anti-PD1 antibodies was equivalent to that of combining two checkpoint inhibitors in the B16 melanoma models.They also found that NaHCO3had a more significant inhibitory effect in the Panc02 pancreatic cancer model.NaHCO3or anti-PD1 antibody monotherapy had no effect on tumor growth,but their combination showed a significantly enhanced tumor growth inhibition rate (50% enhanced).Although there are no more relevant studies on the combination therapy of NaHCO3with immunotherapy,it is commonly recognized that tumor acidosis promotes the immune escape.Hence,the application of NaHCO3may be a promising approach to suppress immune escape and improve the immunotherapy.
4.3.Combined with chemotherapy
Combination of NaHCO3with anticancer drugs may be greatly promising to enhance the anticancer efficacy since pH directly affects the cellular uptake of ionizable drug molecules,especially the alkalescent drugs.Mitoxantrone,an alkalescent anticancer drug,is ionized by the acidic tumor microenvironment and thus is hard to be taken up into tumor cells [111].It was found that mitoxantrone had a higher uptake by tumor cells and showed the higher cytotoxicity in alkaline (pH 7.4) environment than in acidic one (pH 6.6).In another work,Raghunandet al.showed that pre-administration of NaHCO3in tumor-bearing mice increased the tumor pHeby 0.6 pH units and enhanced the therapeutic index of mitoxantrone by 3.3 folds [112].
Doxorubicin is also an alkaline drug and its anticancer efficacy can also be enhanced by NaHCO3.Hananet al.combined liposomal NaHCO3and doxorubicin and found that the viability of triplenegative breast cancer cells treated with single doxorubicin in an acidic pHecondition was 48% and decreased to 12% when combined with liposomal NaHCO3[113].Fluorescent microscopy and quantitative analysis showed that the uptake of doxorubicin by cancer cells was enhanced by two times in the presence of NaHCO3(Fig.3).Interestingly,the NaHCO3-induced efficacy enhancement of mitoxantrone is slightly less than that of doxorubicin in the MCF-7 tumor/SCID mouse models [114].This suggests that the effect of NaHCO3on the efficacy of chemotherapy may be associated with the acidity coefficient (pKa) of drugs.
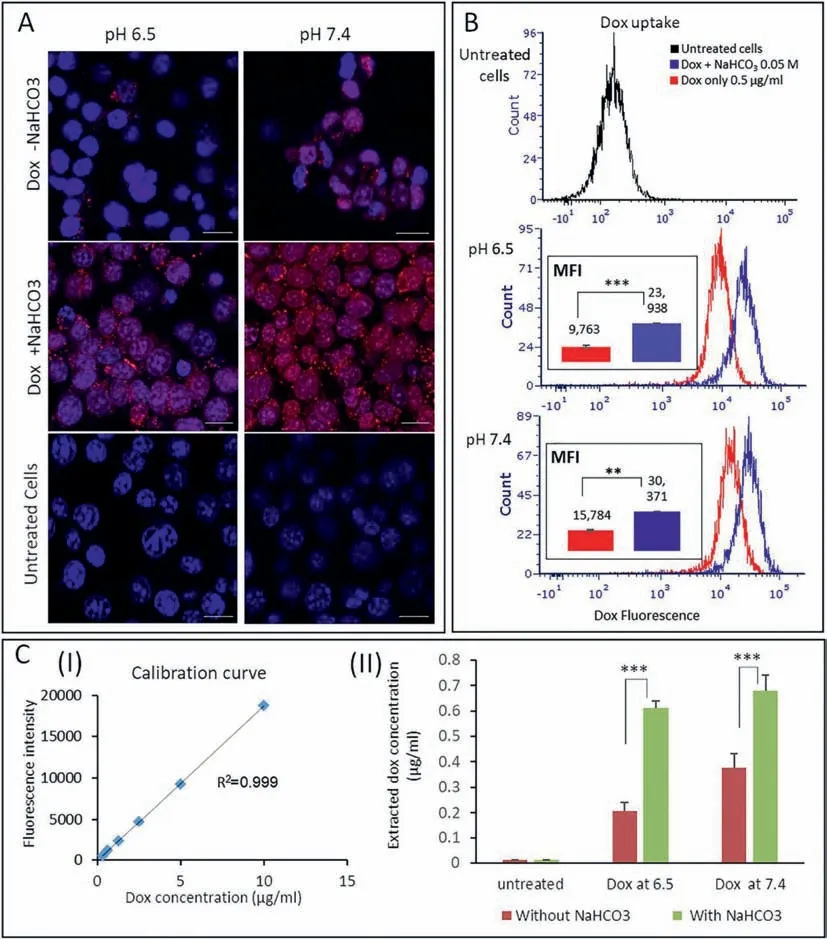
Fig.3.Cellular uptake of doxorubicin in the presence or absence of NaHCO3,examined by (A) fluorescent microscopy (scale bar:20 μm),(B) flow cytometry and (C)quantifying intracellular doxorubicin.Reproduced with permission [113].Copyright 2020,Elsevier Publishing Group.
In a clinic trial,Hamaguchiet al.found that combining NaHCO3with epidermal growth factor receptor tyrosine kinase inhibitor could significantly increase the urine pH of patients with advanced pancreatic cancer and expanded the median overall survival (OS) to 16.1 months,an 11.4-month increase compared to the non-alkaline urine group [115].Although the study did not measure the tumor pHechanges,it still strongly proves that NaHCO3can enhance the efficacy of chemotherapy and prolong the survival of patients,even no less than efficacy of the standard cancer treatment for advanced pancreas cancer.A recent case-control study has also shown the similar results [116].
The role of NaHCO3in inhibiting drug resistance is also reflected in the enhancement of rapamycin efficacy.The complex 1 of the mechanistic target of rapamycin (mTORC1) is activated in cancer cells,ensuring unlimited proliferation of tumor cells [117].Rapamycin is an mTORC1 inhibitor that inhibits tumor development by targeting mTORC1 [118].Rapamycin significantly reduced the proliferation of HT29 colon cancer cells by 37.1% in physiological pH,but the inhibitory effect disappeared under acidic conditions,indicating that the acidic pHediminished the rapamycin inhibitory effect on mTORC1 [119].Therefore,modulating pHeis a very promising method to inhibit drug resistance.Compared with rapamycin monotherapy in mice bearing MC-38 tumors,the combination treatment of NaHCO3and rapamycin increased tumor pHe,enhanced tumor growth inhibition by 26.4%,and improved the incidence of tumor cell apoptosis by 30% [104].These results demonstrate that NaHCO3effectively increases the tumor pHe,thereby enhancing the rapamycin anti-tumor effect and inhibiting drug resistance.
4.4.Combined with nano-therapy
Nanotechnology is one of the fastest growing technologies due to its excellent targeting properties,high bioavailability,and reduced side effects [120].Combination of NaHCO3with nanomaterials has shown great potentials in cancer therapy.Basically,there are two ways to combine NaHCO3with nanotechnologies.One is delivering NaHCO3by nanocarriers to tumor extracellular microenvironment and thus alkalinize the acidosis,and the other is preparing pH-responsive nanocarriers with NaHCO3,which can target the acidic tumor environment and release anti-tumor drugs [105,121].
The targeted delivery of NaHCO3viananotechnology will further enhance its alkalization ability,prolong the alkalization time,increase the immune cells number,inhibit tumor growth,and thereby inhibit acid-induced drug resistance.Abumanhal-Masarwehet al.showed that the liposomal NaHCO3could achieve an extracellular NaHCO3concentration of 3.7%,significantly higher than that of 0.17% achieved by free NaHCO3,indicating a better tumor targeting capacity of liposomal NaHCO3[113].In addition,the liposomal NaHCO3could further increase the number of CD45+immune cells,T cells,B cells and macrophages.
NaHCO3can also be added to the nanoparticles to generate carbon dioxide gas in an acidic environment to destroy the nanoparticle shell and release the drug quickly.Kimet al.added NaHCO3to poly(lactide-co-glycolide) (PLGA) nanoparticles loaded with vaccine adjuvants to obtain the pH-responsive function [105].Compared with conventional PLGA nanoparticles,the properties and efficacy of pH-responsive PLGA nanoparticles have been significantly improved,including the increase in the loading capacity,the number of CD4 T cells,NK cells and IFN-γ+ NK cells,the antigen-specific CD8 T cell and natural killer (NK) cell responses,and the tumor volume inhibition rate.In summary,pH-responsive nanoparticles enhanced the anti-tumor effect of vaccine adjuvants,providing a promising delivery platform for cancer immunotherapy.
5.Mechanisms of NaHCO3-based therapy
The target of NaHCO3-based therapy is the acidic tumor environment and its anticancer mechanisms may include inhibition of tumor invasion and metastasis,inhibition of immune evasion,inhibition of drug resistance,and inhibition of hypoxia and autophagy(Fig.4).

Fig.4.Schematics of the anticancer mechanisms of NaHCO3-based therapy.(1) Inhibition of tumor invasion and metastasis:reduced lymph node involvement and circulating tumor cells (CTC),and inhibited cathepsin B activity;(2) inhibition of immune evasion:increased immune cells number,and improved ACT efficacy and checkpoint inhibition;(3) inhibition of drug resistance:decreased weak-base drug ionization,and restored mTORC1 sensitivity to rapamycin;(4) inhibition of hypoxia and autophagy:reduced expression of pro-autophagy signals,and enhanced SVCT2 activity to inhibit the activity of HIF-1α.
5.1.Inhibition of tumor invasion and metastasis
Tumor acidosis has been shown to induce the expression and activity of many systems involved in matrix remodeling,including matrix metalloproteinases (MMP1,MMP2 and MMP9) and lysosomal proteases (cathepsin B,D or L) [122].Robey and colleagues found that oral NaHCO3increased the tumor pHeand inhibited the release and activity of cathepsin B which is mainly responsible for the metastasis of tumor cells [103].This result explains why oral NaHCO3therapy in this experiment had no effect on primary tumor growth,but resulted in a significant reduction in the number and size of lung,bowel,and diaphragmatic tumor metastases.
The inhibition effect of NaHCO3on tumor metastasis also includes the reduction of the number of lymph node involvement and circulating tumor cells (CTC).It was found that 9 of 12 mice showed almost no tumor metastasis in the lymph nodes in the NaHCO3group,while 7 of 11 mice had a large number of metastatic tumors in their lymph nodes in the control group [123].Accordingly,the average number of CTC was 5 ± 1 cells/mL in the 14 mice treated with NaHCO3,but 13 ± 3 cells/mL in the 12 mice in the control group.
5.2.Inhibition of immune evasion
Tumor acidosis has been shown to inhibit the antitumor activity of immune cells,such as neutrophils,monocytes,macrophages,NK cells,CD4 T cells,CD8 T cells and myeloid dendritic cells [124].The combination of NaHCO3therapy and immunotherapy has been reported to enhance the anticancer efficacy.It was found that ACT combined with NaHCO3reduced tumor growth rates and increased T-Cell persistence significantly [110].In addition,NaHCO3therapy can lead to an increase in the number of immune cells.For example,NaHCO3resulted in a significant increase in the number of CD8βT cells infiltrating into Yumm 1.1 tumors [104].The injection of liposomal NaHCO3in mice with breast cancer also resulted in an increase in the number of T cells,B cells and macrophages [113].
5.3.Inhibition of drug resistance
The tumor acidic pH ionizes the weak-base anticancer drugs and reduces their uptake by tumor cells,resulting in the development of drug resistance.NaHCO3is an alkaline salt and thus can neutralize the acidic pH and inhibit drug resistance.It has been shown that the slight increase in pHesignificantly reduced doxorubicin ionization by 76%,thus increasing its uptake by tumor cells and improving the anticancer efficacy [125].Similarly,the liposomal NaHCO3was found to increase the tumor pHeby about 0.25 pH units,thereby enhancing the activity of doxorubicin in 4T1 breast cancer cells [113].The NaHCO3-caused enhancement of antitumor activity of doxorubicin may be related to the pHeinduced changes in subcellular distribution and topoisomerase activity [112].
Tumor acidosis can also attenuate the rapamycin inhibition on mTORC1,resulting in drug resistance.NaHCO3is able to reduce the activity of mTORC1 through alkalization of the acidic pH,thus reducing drug resistance.It has been shown that NaHCO3can induce the alkalization of tumor pH and thus enhance the activity of rapamycin and also promote its uptake by tumor cells [104].
5.4.Inhibition of hypoxia and autophagy
As mentioned above,there is a bidirectional promotion relationship between HIF-1 and acidic tumor microenvironment.It is reported that tumor microenvironment acidity reduces the transport of ascorbic acid into cells by suppressing the activity of sodium vitamin C cotransporter 2 (SVCT2) and causes the suboptimal intracellular ascorbic acid content [126].McCartyet al.pointed out that NaHCO3has the potential to enhance the activity of SVCT2 and increase the uptake of ascorbic acid into cells,thus achieving the optimal intracellular concentration of ascorbic acid [127].Optimal intracellular ascorbic acid content can further inhibit the transcriptional activity and expression of HIF-1 and suppress NFκB activity to slow tumor invasion and metastasis.
In addition,autophagy is an important means for tumor cells to adapt to acidic environment [128].Oral NaHCO3was found to reduce the expression of pro-autophagy signals in MDA-MB-231 xenograft tumor-bearing mice,but the role of NaHCO3in autophagy has not been fully disclosed [129].
As discussed above,NaHCO3can increase extracellular pH to inhibit tumor progression.The mechanisms include the inhibition of protease activity,immune escape,drug resistance,hypoxia and autophagy.In addition to NaHCO3,some other buffers with pKa of~7 may also have comparable or better results than NaHCO3,such as cholamine chloride,BES and TES [130].
6.Advances and concerns of NaHCO3-based therapy
Basically,the antitumor therapies focus on tumor itself aiming at killing tumor cells directly [131].However,the associated problems,such as drug resistance and immune escape,largely limit the efficacy.One of the reasons for these problems may be the tumor heterogeneity [132].In contrast,NaHCO3-based therapy targets the tumor acidic microenvironment,which is a common phenotype of tumors,and inhibits tumor growth by increasing the tumor pHe.In this regard,NaHCO3-based therapy may be more versatile than the others [133].It is worth mentioning that NaHCO3has an obvious effect in inhibiting early metastasis of primary tumors,but not in non-highly glycolytic sensitive tumors treatment because their metastasis is considered to be pH independent [134].It is estimated that the non-highly glycolytic sensitive tumor accounts for only~10% in all kinds of tumor [135].Thus,most patients with glycolytic tumor types can be benefit from NaHCO3-based therapy.In addition,NaHCO3is readily available and non-toxic,which ensures the NaHCO3-based therapy affordable and safe [136].
However,there are also some limitations in the clinical use of NaHCO3.First,since it does not fully neutralize the large amount of acid produced by large tumors,NaHCO3-based therapy may be ineffective for large solid tumor [137].Second,NaHCO3should be avoided in combination with weakly acidic or neutral anticancer drugs,because their combination will reduce the anticancer effi-cacy,such as 5-fluouracil and cisplatin [138].Also,there has been a case of metabolic alkalosis and hypernatremia caused by overdose and carelessness in use of NaHCO3[139].In addition,the unsatisfying taste of NaHCO3may lead to poor patient compliance [140].
7.Conclusion and perspectives
Tumor acidosis-mediated tumor metastasis,drug resistance,immune invasion,aggravated hypoxia and increased autophagy are important reasons for the unsatisfying prognosis and high mortality.NaHCO3has shown great potentials in cancer treatment due to its ready availability,safety and ability to neutralize the tumor acidic microenvironment.The anticancer mechanisms of NaHCO3include (1) inhibition of tumor invasion and metastasis;(2) inhibition of immune evasion;(3) inhibition of drug resistance;and (4)inhibition of hypoxia and autophagy.
Despite the great potentials and progress of NaHCO3in cancer treatment,many aspects of NaHCO3-based therapy need to be further explored.First of all,the mechanisms of the antitumor effect caused by the alkalization of the tumor acidosis by NaHCO3need to be further disclosed.For example,it can be further confirmed whether NaHCO3inhibits exosomes secretion and reduces the activation of the multidrug transporter P-glycoprotein.Second,the combination of drugs that target the production of acids with NaHCO3may be a promising strategy to improve efficacy and avoid deficiencies.Third,the enhanced efficacy of radiotherapy is related to the increase in oxygen partial pressure.Therefore,NaHCO3may be combined with radiotherapy since it can inhibit the tumor hypoxia.In addition,the possibility of combining NaHCO3with phototherapy,such as photothermal therapy and photodynamic therapy,may be a direction in the future.The delivery of NaHCO3,including the delivery routes and formulations,is also of great importance for deep investigation.
NaHCO3can be used alone and also in combination with other therapies,such as immunotherapy and chemotherapy.The practical use of NaHCO3is simple since there are several potential approaches for using it in clinic.The general administration routes may include oral delivery,intravenous injection and intratumor injection.NaHCO3can be used in the form of solution directly or in the form of advanced delivery systems,such as nanoparticles.In summary,NaHCO3is much more than a simple inorganic salt and has great potentials in cancer therapy as an active agent.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.81973261),the Foundation of West China Hospital of Stomatology (No.RD-02–201903),and the Research Funding from West China School/Hospital of Stomatology,Sichuan University (No.RCDWJS2020–7).
杂志排行
Chinese Chemical Letters的其它文章
- Progress in mechanochromic luminescence of gold(I) complexes
- Spinel-type bimetal sulfides derived from Prussian blue analogues as efficient polysulfides mediators for lithium-sulfur batteries
- Alopecuroidines A-C,three matrine-derived alkaloids from the seeds of Sophora alopecuroides
- Boronic acid-containing diarylpyrimidine derivatives as novel HIV-1 NNRTIs:Design,synthesis and biological evaluation
- Diaminodiacid bridge improves enzymatic and in vivo inhibitory activity of peptide CPI-1 against botulinum toxin serotype A
- Peptide stapling with the retention of double native side-chains
