Host-guest interactions directed the morphology transformation of a charge-transfer complex of a naphthalene-tailored amphiphile/methyl viologen:From thin-films into diamond-like assemblies
2021-03-14QingtianJiLijunFanShuaishuaiLiuHaojieYeShuzhenXiangPeiyiWang
Qingtian Ji,Lijun Fan,Shuaishuai Liu,Haojie Ye,Shuzhen Xiang,Peiyi Wang
State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Key Laboratory of Green Pesticide and Agricultural Bioengineering,Ministry of Education, Center for R&D of Fine Chemicals of Guizhou University, Guiyang 550025, China
Keywords:Cucurbit[8]uril Amphiphile Methyl viologen Assemblies Host-guest interactions
ABSTRACT By introducing a host molecule cucurbit[8]uril (CB[8]) into a charge transfer system containing an amphiphile 1-[11-(naphthalene-2-ylmethoxy)-11-oxoundecyl]pyridinium (NP) and an electron-deficient molecule methyl viologen (MV),a novel and anisotropic ternary building block was constructed by hostguest interactions,thereby leading to the morphology transformation of the final assemblies from thinfilms (NP/MV complexes) into diamond-like structures (NP/MV/CB[8]complexes).These intriguing assemblies were firstly discovered and were similar with the shape of well-known metal organic frameworks(MOFs),but just comprised three small organic molecules without metal ions.This finding can enrich the shape of current supramolecular assemblies and thus contributing to more potential applications in material science.
Exploration of novel morphologies and topologies assembled from discrete building blocks driven by non-covalent bonds has aroused keen interests by chemists and biochemists on account of their promising applications in material science [1–5].In the past decades,enormous architectures with various features and morphologies were fabricated and designed,such as fibres,sheets,tubules,vesicles,micelles,ribbons [6–11].Because of the final applications of an assembly are determined and restricted by the structural shapes and features,continuous studies and attentions intensively focused on the exploration and development of novel assemblies with tunable morphologies [12–18].Till now,many non-covalent synthetic strategies employing hydrogen bonds,electrostatic interactions,charge-transfer (CT),and host–guest interactions have been used to manipulate the assembly behavior for systematically joining and assembling the building elements to yield all sorts of architectures [19–25].Among them,host–guest interactions,which can encapsulate two or more molecules or ions,have exerted a significant role in the construction of advanced materials owning to its high selectivity,efficiency,binding affinity,and stimuli-responsiveness [26–29].Therefore,employing hostguest interactions to create new assemblies is attractive and available.
In the host-guest systems,many scaffolds including calixarenes,cyclodextrins,and crown ethers,have been certified exerting the ability to encapsulate guest molecules [30–32].Currently,a significant class of cucurbiturils has attracted great attentions for their widely applications in various fields such as drug delivery,stimuliresponsive devices,supramolecular polymers,supramolecular organic frameworks (SOFs) [33–41].Among them,cucurbit[8]uril(CB[8]) formed by eight glycolurilunits,is a preferred host scaffold to be selected in creating new host-guest complexes because of its hydrophobic cavity is accessible through two identical carbonyl laced portals [42–49].For example,Kim and co-workers have fabricated a ternary complex by employing CB[8]to encapsulate a charge transfer complex of the electron-rich 2,6-dihydroxynaphthalene and the electron-deficient methyl viologen (MV),which consequently assembled into vesicles [50].Scherman and co-workers have constructed robust supramolecular hydrogelsvialoading a range of guest molecules within the macrocyclic host CB[8]directed by host–guest interactions [51].Li and coworkers have prepared water-soluble three-dimensional porous SOFs by mixing 4-phenylpyridinium-tailored tetraphenylmethane derivatives with the host CB[8].And these SOFs could load the therapeutic agent pemetrexed disodium with high drug delivery efficiency and improved efficiencies for cancer therapy [52].Cao and co-workers have constructed two kinds of stimuli-responsive fluorescent and shape-controllable SOFs (cuboid or spheroid) by rationally introducing CB[8]into pyridinium-tailored tetraphenylethylene derivatives through host–guest interactions.And these two fluorescent SOFs displayed varied photophysical properties (red-shifts can reach 82 nm) and could be applied in cellular imaging by adding a competitive guest 3,5-dimethyl-1-adamantylamine hydrochloride [53].Obviously,CB[8]can served as a favored candidate directing the formation of new assemblies.
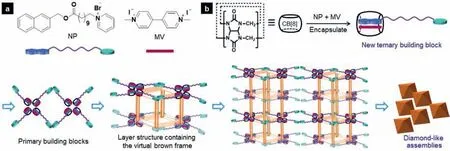
Fig.1.(a) The chemical structures of NP and MV.(b) The formation of new ternary building blocks directed by CB[8]encapsulation and the correlative schematic diagram for the assembly process of diamond-like assemblies.
Inspired by those reports,it is suggested that more interesting hierarchical architectures with various functional properties should be constructed by tuning the host-guest interactions.In our previous study,we have fabricated various micro/nano-structures with tunable shapes and morphologies by manipulating different non-covalent forces [54–57].Herein,host-guest interactions were employed to explore and create new assemblies.As shown in Fig.1,a typical amphiphile NP (1-[11-(naphthalene-2-ylmethoxy)-11-oxoundecyl]pyridinium bromide) containing the electron-rich naphthalene group and the pyridinium cation linked by a flexible alkyl arm was designed and synthesized [58],in which the electron-rich naphthalene group was exploited to form CT complex with another electron-deficient molecule MV.By encapsulating the CT complex into CB[8],a new ternary building block can be constructed by host-guest interactions,which may consequently assemble into new architectures.
The morphology assembled from NP and MV was firstly investigated.As a general procedure,the assembly behavior of NP and MV was carried out as follows:a water solution of 2-NP (400 μL,10 mmol/L) and MV (400 μL,10 mmol/L) were added into the deionized water (1.2 mL),respectively.After mixing the two components completely,the aggregates formed,which were characterized by scanning electron microscopy (SEM) and atomic force microscopy (AFM).In our previous study,we know that NP afforded microsheets with an average size of 65,9,and 1 μm in length,width,and thickness,respectively [58].As shown in Fig.2,the morphology of the assemblies was changed into thin-films with an average size of 50 nm in thickness after adding 1 equiv.MV into NP system.And the related height profile (Fig.2d) certified these thin-films had flat surfaces.
In order to elucidate the driving forces and molecular packing pattern of thin-films,UV–vis,fluorescence,1H NMR spectroscopy,and X-ray powder diffraction (XRPD) experiments were performed.A red shift was observed from UV–vis (Fig.3a),suggesting the formation of “J”-type aggregates between the electron-rich naphthalene group and the electron-deficient molecule MV driven by CT interactions.Quenching effect of NP in emission spectra (Fig.3b)further confirmed that the formation of these thin-films was directed by CT interactions.In the1H NMR spectra,the proton resonances of the naphthalene ring of NP shifted to the upfield after mixing with MV (Fig.3c),indicating the formation of CT complex.The sharp reflection peak from XRPD results revealed that the semicrystalline nature of the thin-films and a highly ordered layer packing arrangement with a distance around 2.38 nm (Fig.3d).
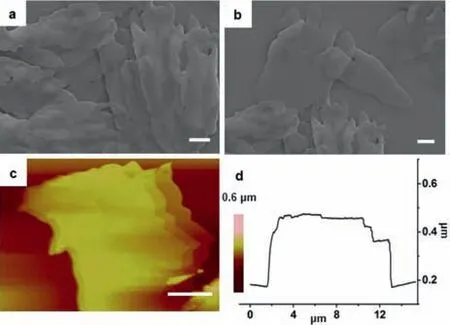
Fig.2.SEM (a,b),AFM (c),and height profile (d) of NP/MV (conc.2.0×10-3 mol/L,molar ratio=1:1).Scale bars are 4 μm for (a,b,and c).
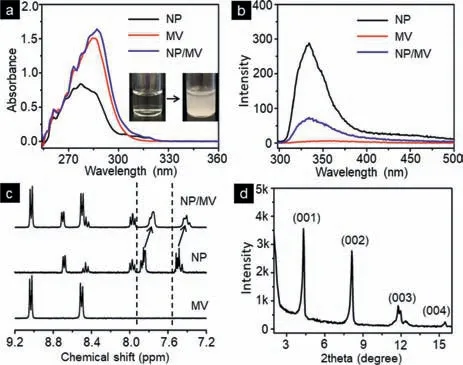
Fig.3.(a) UV–vis and (b) florescence spectra of NP,MV,and NP/MV (conc.0.25 mmol/L,molar ratio=1:1);(c) 1H NMR spectra of MV,NP,and NP/MV (conc.2.0 mmol/L,molar ratio=1:1,D2O:CD3OD=3:1);(d) X-ray powder diffraction patterns of the assemblies of NP/MV.Insert:photography of NP/MV solution,the colourless solution of NP/MV became cloudy quickly in 10 min.
Given the above results,a possible assembly mechanism was proposed as follows:a primary binary building block was established from NP and MV by CT interactions between the electronrich naphthalene group and the electron-deficient molecule MV,which consequently assembled into layer structures.With the help of hydrogen bonding formed by water and bromide connected all the layers together [54,55,58]to give the final assemblies (Fig.4).
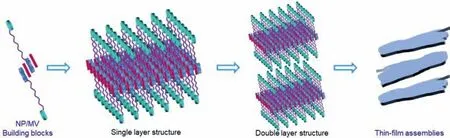
Fig.4.The relative schematic diagram for the assembly process of thin-film assemblies form the NP/MV building blocks.
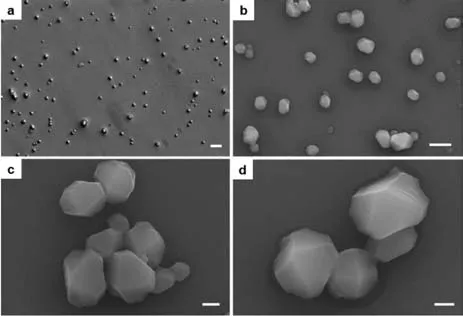
Fig.5.OM (a),SEM (b-d) of CB[8]/NP/MV (conc.2.5×10-4 mol/L,molar ratio=1:1:1).Scale bars are 10 μm for (a),4 μm for (b),1 μm for (c,d).
OM and SEM were employed to investigate the new structures promoted by host-guest interactions.Two approaches could be used to prepare the assembly,one protocol was that:CB[8](2 mg) and MV (150 μL,10 mmol/L) were dissolved in 1.2 mL deionized water;Then a clear yellow solution was observed after adding NP (150 μL,10 mmol/L) into the mixture,which subsequently yielded the assemblies.Another protocol was that:MV and NP solutions were premixed firstly,which would quickly assemble into the precipitates (thin-films);after the addition of the host CB[8],an operation of heating the mixture was to adequately make the CT complex (NP/MV) enter to the cavity of CB[8]to form the ternary building block,during which an obtained clear yellow solution then assembled into the final assemblies.Both the two approaches afforded the similar shape of the assembly.As proposed,the morphologies were dramatically transformed from thin-films into diamond-like assemblies after the addition of CB[8](Fig.5).All the structures are composed of four surfaces from an observable perspective with the hierarchical skeletons,indicating that rationally constructing a new ternary complex as the new building block directed by host-guest interactions can create new architectures.
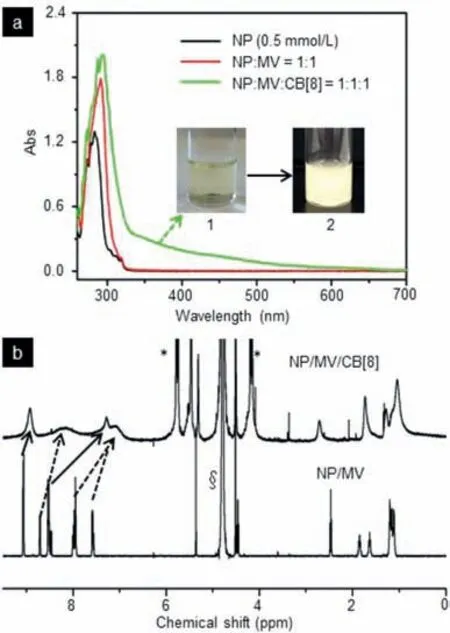
Fig.6.(a) UV–vis spectra of NP,NP/MV,and NP/MV/CB[8](conc.5.0×10-4 mol/L,molar ratio=1:1:1);(b) 1H NMR spectra of NP/MV and NP/MV/CB[8](conc.2.0×10-4 mol/L,molar ratio,1:1:1,solvent,D2O),the solid arrows point MV protons,the dotted arrows point naphthalene protons.Insert:photography of 1 (NP/MV/CB[8]),the yellow solution of NP/MV/CB[8]became cloudy (2) quickly.§ D2O;∗protons of CB[8].
In order to prove the formation of diamond-like assemblies was promoted by host-guest interactions,UV–vis and the correlative1H NMR were investigated.As shown in Fig.6a,a broad new peak from 330 nm to 550 nm was observed (for the yellow transparent solution in Fig.6a),which attributed to the encapsulation of the CT complex of NP and MV directed by the host molecule CB[8].This phenomenon was in accordance with the colorific change in the solution from colorless (NP/MV) to yellow after adding CB[8],further suggesting the formation of ternary complexes was guided by host enhanced CT interactions [59].1H NMR spectra revealed that the proton resonances of the MV shifted to upfield after mixing with CB[8],as well as the proton resonances of the naphthalene ring of NP,indicating that the formation of ternary complex was promoted by host-guest interactions (Fig.6b).A possible assembly process was proposed as follows:the anisotropic ternary building block NP/MV/CB[8]was formed by the enhanced CT complex directed by host-guest interactions,which then could form the layer structures containing a similar metal organic framework unit (the virtual brown frame from Fig.1b) [60,61];With the assistance of hydrogen bonding formed by water and bromide joined all the layers together [54,55,58]to yield the diamond-like assemblies (Fig.1b).A known competitive guest (1-adamantanamine hydrochloride) was introduced into the CB[8]/NP/MV assemblies,then the diamond-like assemblies were initially disassembled (Figs.S1a and S1b in Supporting information) and finally yielded some thin-films (Figs.S1c and d in Supporting information).
In conclusion,we have constructed a new ternary building block through encapsulating a charge transfer complex into a host molecule CB[8]promoted by host-guest interactions,which consequently directed the morphology transformation from thin-films into diamond-like assemblies.This strategy can be used for the construction of advanced materials containing more functional elements,thus leading to more potential applications in material science.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgments
We acknowledge the financial supports from the National Natural Science Foundation of China (Nos.31860516,21662009,21702037),Frontiers Science Center for Asymmetric Synthesis and Medicinal Molecules,Department of Education,Guizhou Province[Qianjiaohe KY No.(2020)004],and Program of Introducing Talents of Discipline to Universities of China (111 Program,No.D20023).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.05.036.
杂志排行
Chinese Chemical Letters的其它文章
- Long-wavelength (red to near-infrared) emissive carbon dots:Key factors for synthesis,fluorescence mechanism,and applications in biosensing and cancer theranostics
- Nanotechnology combining photoacoustic kinetics and chemical kinetics for thrombosis diagnosis and treatment
- The point-of-care-testing of nucleic acids by chip,cartridge and paper sensors
- Sodium bicarbonate,an inorganic salt and a potential active agent for cancer therapy
- New advances in gated materials of mesoporous silica for drug controlled release
- Current development in wearable glucose meters
