Blue light-promoted cyclopropenizations of N-tosylhydrazones in water
2021-03-14KaichuanYanHuaHeJianglianLiYiLuoRuizhiLaiLiGuoYongWu
Kaichuan Yan,Hua He,Jianglian Li,Yi Luo,Ruizhi Lai,Li Guo,Yong Wu
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Department of Medicinal Chemistry,Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology,West China School of Pharmacy,Sichuan University,Chengdu 610041,China
Keywords:N-Tosylhydrazone Carbene Metal-free Cyclopropenization Cyclopropanation
ABSTRACT Carbene transfer reactions play an important role in the field of organic synthesis because of their ability to construct a variety of molecules.Herein,we reported on blue light-induced cyclopropenizations of N-tosylhydrazones in water,which avoids the use of expensive metal-based catalysts and toxic organic solvents.This metal-free and operationally simple methodology enable highly efficient cyclopropenizations,X-H insertion reactions,and cyclopropanation under mild reaction conditions.
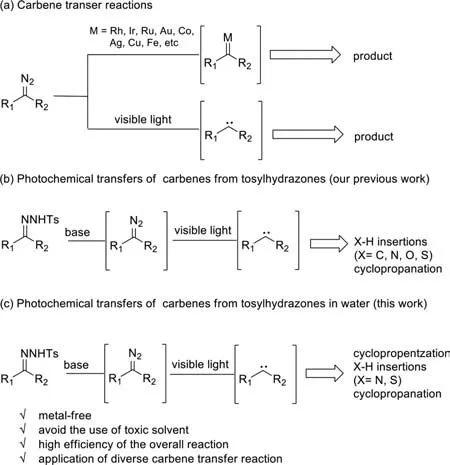
Carbene transfer reactions are powerful synthetic tools in organic synthesis and open up a new window of reactivity patterns ranging from cross-coupling reactions [1–3];rearrangement reactions [4–11],X-H insertion reactions [12–18],cycloadditions [19–24]to C–H activations [8,25–29](Scheme 1a).In the past few decades,although remarkable achievements have been made in carbene transfer reactions,the presence of expensive metal-based catalysts and toxic organic solvent limited their further development [7,29–31].In this context,the development of environmentally benign transformations remains highly desirable.
Only recently,the visible light photolysis of diazoalkanes has emerged as an important alternative to traditional metalcatalyzed carbene-transfer reactions (Scheme 1a) [32–37].Furthermore,identifying sustainable and safe carbene precursors has also become one of the major tasks in the field of carbene transfer reactions.In this regard,hydrazones have become especially important carbene precursors throughin situgenerated diazo intermediates.Compared with diazo compounds,hydrazones are more stable,less explosive,and they can be easily prepared from aldehydes or ketones [31].Consequently,we reasoned the possibility that carbene transfer reactions initiated by the visible light photolysis of hydrazones,which has recently been verified by the works of Koenigset al.and ourselves,respectively (Scheme 1b) [34,38–42].
Scheme 1.Carbene transfer reactions.
On the other hand,cyclopropenes,as strained carbocyclic compounds,are widely used synthons in organic synthesis due to their high reactivity [43,44].A particularly practical reaction to access cyclopropylenes is the cyclopropenization reaction of alkynes,for which a variety of expensive,precious metal catalysts,based on AuII,RhII,IrI,and AgIhave been reported [45–48].Also,a metalfree cyclopropenization reaction using tosylhydrazones has been reported,despite the shortcomings of low yields and elevated temperature [34,40,49–51].In the context of the development of environmentally benign methods for the synthesis of cyclopropylenes and our previous work,herein we developed a blue lightinduced cyclopropenization reaction ofN-tosylhydrazones with alkynes (Scheme 1c).Notably,this is the first report on lightinduced metal-free cyclopropenizations ofN-tosylhydrazones in water.
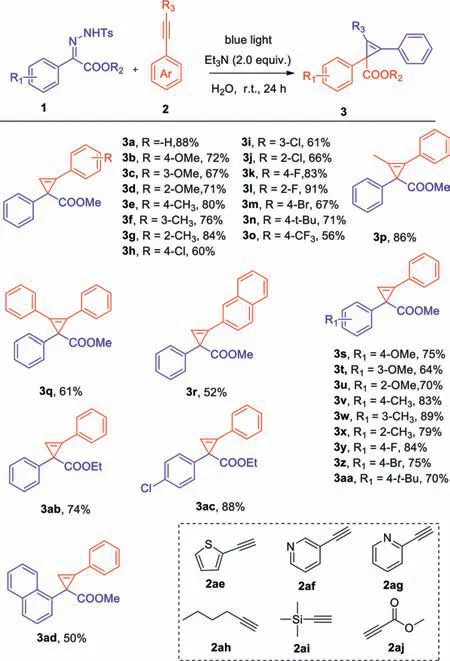
Scheme 2.Substrate scope of N-tosylhydrazones and alkynes.Reaction conditions:1 (0.1 mmol),2 (1.0 mmol) and Et3N (2.0 equiv.) were dissolved in 1.0 mL of H2O and were irradiated at room temperature with blue LEDs (10 W,470 nm) for 24 h.Isolated yield by chromatography on silica gel.
Furthermore,other carbene transfer reactions involving the visible light photolysis ofN-tosylhydrazones,such as X-H insertions and cyclopropanations were proved to compatible with these water-mediated conditions.
Initially,the blue-light-mediated cyclopropenization ofNtosylhydrazone 1a (0.1 mmol) and phenylacetylene 2a (10 equiv.)was examined in dichloromethane (DCM) at 25 °C without the base.Unfortunately,no desired product was detected (Table 1,entry 1).Surprisingly,required methyl 1,2-diphenylcycloprop-2-ene-1-carboxylate (3a) was obtained in yields ranging from 30% to 55%when using KOH,t-BuOK,K2CO3,Et3N,and DBU as bases,and Et3N gave the best result (entries 2–6).After that,we further screened the solvent (entries 6–11),and the results showed that the reaction proceeded smoothly in water delivering the desired product in 66%isolated yield (entry 11).Besides,we investigated the equivalents of phenylacetylene and found that adding 10 equiv.of phenylacetylene significantly increased the yield of the target product (entry 13).Finally,through a detailed investigation of the amount of base and the reaction concentration,we have obtained the optimal reaction conditions.
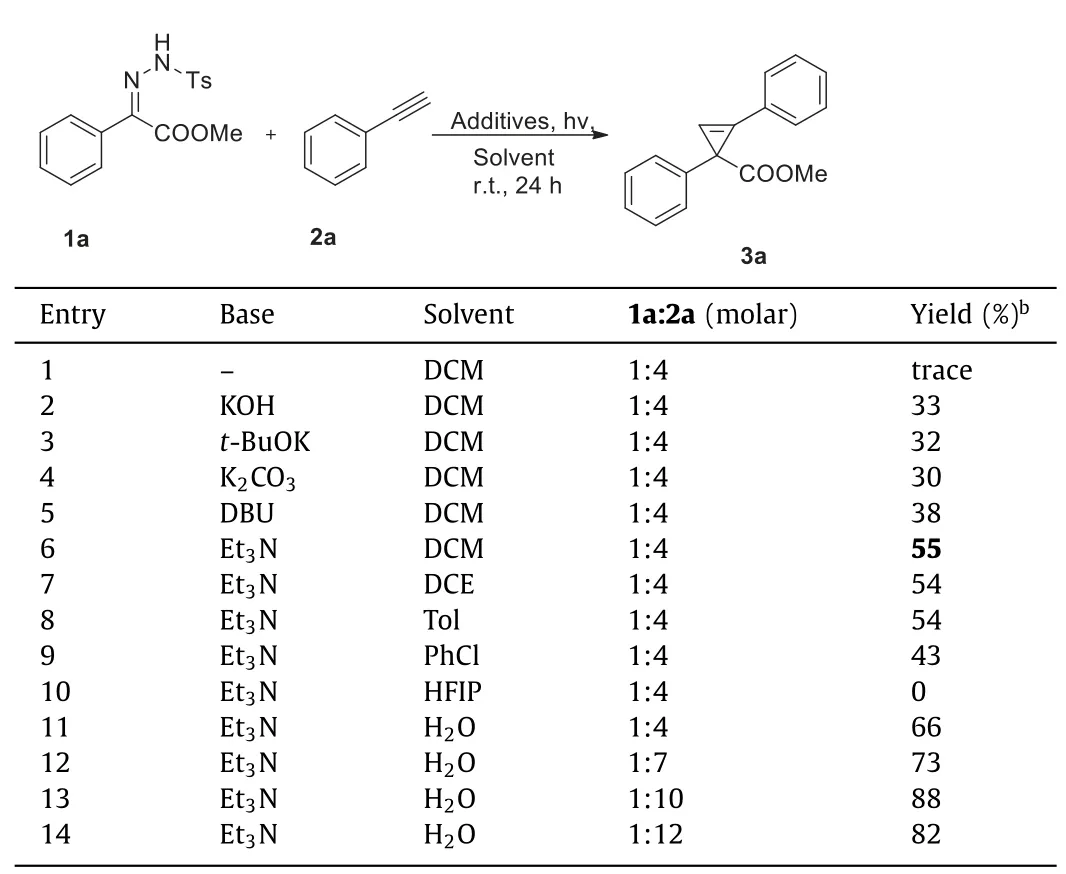
Table 1 Optimization of the reaction conditions.a
With the optimized conditions in hand (Table 1,entry 13),we next explored the functional group tolerance of both theNtosylhydrazones 1 and alkynes 2 (Scheme 2).In general,substrates with different substituents were compatible with the optimized reaction conditions,and the corresponding cyclopropylenes were obtained with moderate to excellent yields (3a-3aa).Among them,the electronic effect of substituted functional groups had a strong influence on the yields of target compounds,which mainly manifested that the transformation efficiency of the electron-withdrawing substituents was generally lower than that of electron-donating substituents.Surprisingly,styrenes possessing fluorine at the benzene ring were exceptions and afforded the corresponding cyclopropenes in 83% and 91% yields (3k,3l),respectively.It is worth mentioning thatN-tosylhydrazone and alkyne substituted by naphthalene had poor compatibility with optimal conditions,and their corresponding cyclopropylenes were achieved in yields of 52% and 52% (3r,3ad).Besides,we explored other substrates such as heterocyclic terminal alkynes,alkynes bearing electron-withdrawing groups,and non-aromatic terminal alkynes.Unfortunately,they were not suitable for this reaction condition,and no expected cyclopropenes were obtained (2ae-2aj).
Based on the success of the light-mediated cyclopropenizations ofN-tosylhydrazones in water,we further studied the applicability of this optimal condition to other carbene transfer reactions.First,we investigated the insertion reactions betweenNtosylhydrazones and X-H bonds (Scheme 3).Primarily,we focused on testingN-tosylhydrazones and a variety of aromatic amines.The results showed that under the optimal conditions,both monosubstituted and disubstituted aromatic amines reacted smoothly withN-tosylhydrazones,and all the expected amino acid ester products were obtained in good to excellent yields (5a-5n).However,naphthalamine reacted withN-tosylhydrazons and afforded the desired product in 66% yield under these conditions (5o).Unfortunately,other aliphatic thiols,alcohols,phenols and amines did not provide the expected products.(4r-4v).Besides,we investigated the reaction ofN-tosylhydrazons with monosubstituted aryl thiophenols,and the corresponding products were obtained with moderate yields (5p,5q).
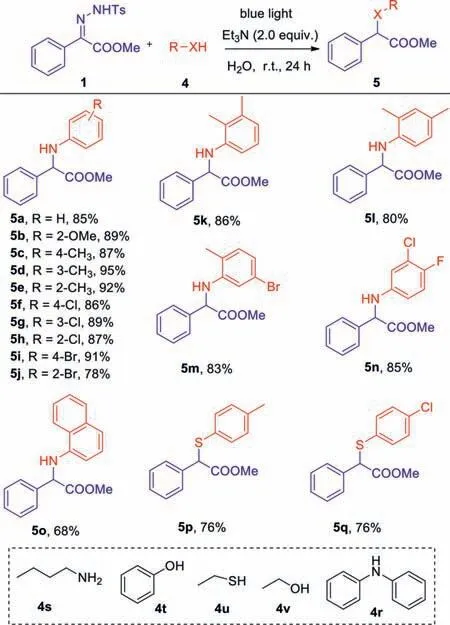
Scheme 3.Substrate scope of N-tosylhydrazones and X-H bonds.
Reaction conditions:1 (0.1 mmol),4 (0.4 mmol),and Et3N (2.0 equiv.) were dissolved in 1.0 mL of H2O and were irradiated at room temperature with blue LEDs (10 W,470 nm) for 24 h.Isolated yield by chromatography on silica gel.
Besides,we tested the cyclopropane reactions betweenNtosylhydrazones and aryl olefins in water (Scheme 4).The results show that the reaction ofN-tosylhydrazones and aryl olefins can be successfully achieved under optimal conditions.Target products can be obtained more efficiently than we previously reported in DCM (7a-7n).Unfortunately,alkenes bearing following functional groups,such as methyl acrylate,acrolein,acrylonitrile,did not provide the expected products (6o-6q).
To further study the efficiency and practicality of this transformation,a 10-fold scale-up of the reaction was conducted in 50 mL of H2O.The desired product 3a was isolated with 74% yield(Scheme 5).
According to the existing literatures [30,39,52,53],The mechanism proposed for this reaction comprises the following steps(Scheme 6):1) decomposition of the hydrazone in the presence of the base to form the diazo compound,2) and then formation of a carbene intermediate under blue light irradiation,3) C–C bond formation by carbene migration insertion of the alkyne,alkene or other X-H bonds with loss of nitrogen to give the final product.
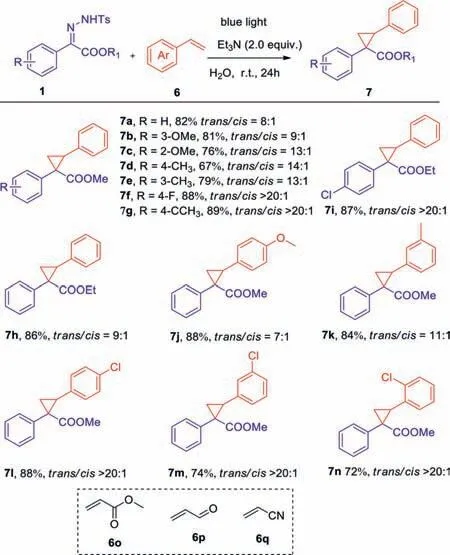
Scheme 4.Substrate scope of N-tosylhydrazones and aryl olefins.Reaction conditions:1 (0.1 mmol),6 (1.0 mmol),and Et3N (2.0 equiv.) were dissolved in 1.0 mL of H2O and were irradiated at room temperature with blue LEDs (10 W,470 nm) for 24 h.Isolated yield by chromatography on silica gel.
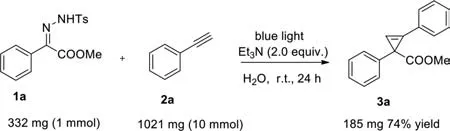
Scheme 5.10-Fold scale-up reaction.
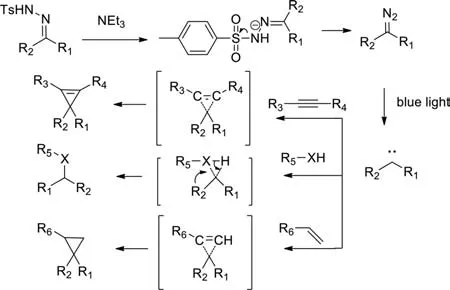
Scheme 6.Proposed mechanism.
In summary,we reported on metal-free carbene transfer reactions ofN-tosylhydrazones in water,which were induced by low energy blue light.Notably,this methodology allowed efficient cyclopropenizations,X-H insertion reactions (X=N,S),and cyclopropanations in the context of environmental harmony.We firmly believe that this water-mediated photolysis ofN-tosylhydrazones would contribute to broadening the applications of free carbene in organic synthesis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (Nos.81373259;81573286,81773577;81602954).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.05.031.
杂志排行
Chinese Chemical Letters的其它文章
- Long-wavelength (red to near-infrared) emissive carbon dots:Key factors for synthesis,fluorescence mechanism,and applications in biosensing and cancer theranostics
- Nanotechnology combining photoacoustic kinetics and chemical kinetics for thrombosis diagnosis and treatment
- The point-of-care-testing of nucleic acids by chip,cartridge and paper sensors
- Sodium bicarbonate,an inorganic salt and a potential active agent for cancer therapy
- New advances in gated materials of mesoporous silica for drug controlled release
- Current development in wearable glucose meters
