The chain-shaped coordination polymers based on the bowl-like Ln18Ni24(23.5) clusters exhibiting favorable low-field magnetocaloric effect
2021-03-14NingfngLiQingfngLinYeminHnZeyuDuYnXu
Ningfng Li,Qingfng Lin,Yemin Hn,Zeyu Du,Yn Xu,∗
a College of Chemical Engineering,State Key Laboratory of Materials-Oriented Chemical Engineering,Nanjing Tech University,Nanjing 210093,China
b Department of Chemistry,Bengbu Medical College,Bengbu 233030,China
Keywords:3d-4f Anionic templets Chain-like structure High-nuclearity clusters Magnetic refrigeration Mixed-ligand method
ABSTRACT The design of assembling high-nuclearity transition-lanthanide (3d-4f) clusters along with excellent magnetocaloric effect (MCE) is one of the most prominent fields but is extremely challenging.Herein,two heterometallic metal coordination polymers are constructed via the “carbonatetemplate” method,formulated as {[Gd18Ni24(IDA)22(CO3)7(μ3-OH)32(μ2-OH)3(H2O)5Cl]·Cl8·(H2O)14}n and{[Eu18Ni23.5(IDA)22(CO3)7(μ3-OH)32(H2O)5(IN)(CH3COO)2(NH2CH2COO)Cl]·Cl6·(H2O)17}n [abbreviated as 1-(Gd18Ni24)n and 2-(Eu18Ni23.5)n respectively;H2IDA=iminodiacetic acid;HIN=isonicotinic acid].Concerning the structures,compounds 1-(Gd18Ni24)n and 2-(Eu18Ni23.5)n both feature the one-dimensional(1D) chain-like structure which is rarely reported in high-nuclearity metal complexes.Meanwhile,the large presences of Gd3+ ions in compound 1-(Gd18Ni24)n are conducive to the fantastic MCE,and the value of-ΔSm is 35.30 J kg-1 K-1 at 3.0 K and ΔH=7.0 T.And more significantly,compound 1-(Gd18Ni24)n shows the large low-field magnetic entropy change (-ΔSm=20.95 J kg-1 K-1 at 2.0 K and ΔH=2.0 T)among the published 3d-4f mixed metal clusters.
High-nuclearity metal complexes have flourished in an increasing number of fields and inspired researchers to assemble plentiful polymers or give an insight into the extensive properties,due to the aggregation of abundant metal ions resulting in the multiple pleasing structures and diversified properties [1–10].As one kind of significant metal clusters,heterometallic transition-lanthanide(3d-4f) high-nuclearity clusters have acquired comprehensive attention on account of multiple structures,and smart applications,such as photocatalytic CO2reduction,gas separation and magnetocaloric effect (MCE) [11–15].
More specially,numerous efforts have been encouraged by 3d-4f compounds as the promising molecule-based magnetic cooling materials which have the chance to replace rare and costly He-3 in the ultra-low-temperature scope [16].And compared with pure 3d-or 4f-based compounds,3d-4f mixed metal complexes with the virtues of different spin centers can provide more opportunities to build magnetic materials [16–23].In order to achieve the remarkable MCE,high-nuclearity 3d-Gd compounds are chosen as the ideal magnetic materials,because a high-spin ground state,isotropic electron orbit and negligible magnetic anisotropy of Gd3+ions are beneficial to the outstanding MCE and the large low-field magnetic entropy change [15,24–29].Moreover,it is generally believed that the correlation of structure-property is presented in nanoscale materials [21,30].For example,a certain number of explorations demonstrate that multidimensional metal polymers are powerful to the brilliant MCE with large magnetic density[3,31].Nevertheless,it is worth paying consideration to the explorations demonstrate that multidimensional metal polymers challenge of assembling 3d-4f complexes as distinguished magnetic coolants,resulting from the different coordination modes of transition and lanthanide ions and the indissolubility engendered by the over-aggregation of metal ions [32–34].So far,although a host of high-nuclearity 3d-4f heterometallic metal clusters have been assembled and published [15,35,36],most of them presented isolated structure [27,37].In other words,constructing multidimensional 3d-4f mixed-metal high-nuclearity clusters manifesting favorable MCE is more challenging but pregnant.
Based on reasonable experimental design,if the isolated structures could be bridged by ligands to form the multidimensional structures? With the purpose of obtaining objective 3d-4f clusters coupled with aesthetically enjoyable structures and large MCE,anionic templates have been efficiently utilized and widely accepted in designing of fabricating metal clusters [12,30,35].Additionally,the CO32-anions as the common template have been successfully used to induce 3d and 4f ions into pure metal clusters or mixed-metal compounds because of its versatile coordination modes and the relatively strong coordination bonding between CO32-and metal ions [29].However,among publicly reported high-nuclearity clusters,most of CO32-anionic templates came from the adsorption of carbon dioxide in the atmosphere or the decomposition of ligands,which were all of high serendipity[30,32].Therefore,we look forward to building novel and tempting 3d-4f high-nuclearity clustersviaquantitatively introducing CO32-anions from carbonate.
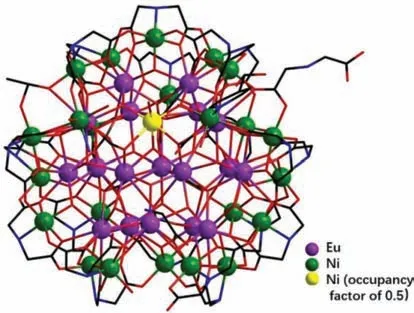
Fig.1.Ball and stick pattern of compound 2-(Eu18Ni23.5)n.
To put the above considerations into practice,two captivating chain-like coordination polymers based on Ln18Ni24(23.5)highnuclearity 3d-4f clusters,1-(Gd18Ni24)nand 2-(Eu18Ni23.5)n,are isolated through CO32-anionic templates and solvothermal treatment.In terms of the structures,compounds 1-(Gd18Ni24)nand 2-(Eu18Ni23.5)nare both linked by IDA2-ligands to constitute the scarce 3d-4f chain-shaped metal clusters up to now.Additionally,products in this work are both templated by CO32-anions that are quantitatively introduced by the adding of carbonate.More importantly,compound 1-(Gd18Ni24)nmakes a new breakthrough in MCE,with-ΔSm=35.30 J kg-1K-1(at 3.0 K with ΔH=7.0 T)and-ΔSm=20.95 J kg-1K-1(at 2.0 K with ΔH=2.0 T).
Single-crystal X-ray diffraction studies expose that compounds 1-(Gd18Ni24)nand 2-(Eu18Ni23.5)nboth crystallize in the monoclinic space groupP21/c.As shown in Fig.1,in compound 2-(Eu18Ni23.5)n,[Eu18Ni23.5(IDA)22(IN)(CH3COO)2(NH2CH2COO)(μ3-OH)32(CO3)7(H2O)5Cl]·Cl6·(H2O)17,contains 18 Eu3+ions,24 Ni2+ions (Ni24 with occupancy factor of 0.5),7 CO32-ions,22 IDA2-ligands,one glycine ligand (NH2CH2COO-) from the decomposition of H2IDA [18],one IN-ligand,two CH3COO-,7 Cl-ions,32μ3-OH-,5 coordination water molecules and 17 lattice water molecules.And compound 1-(Gd18Ni24)n,[Gd18Ni24(CO3)7(IDA)22(μ2-OH)3(μ3-OH)32(H2O)5Cl]·Cl8·(H2O)14,contains 18 Gd3+ions,24 Ni2+ions,7 CO32-ions,22 IDA2-ligands,9 Cl-ions,32μ3-OH-,3μ2-OH-,5 coordination water molecules and 14 lattice water molecules (Figs.S6 and S7 in Supporting information).
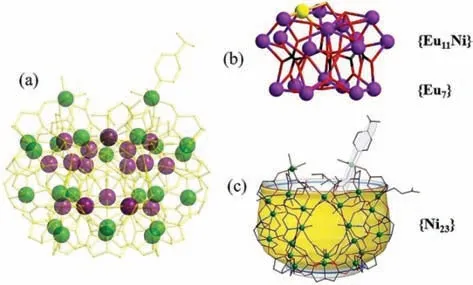
Fig.2.(a) The 2-(Eu18Ni23.5)n cluster.(b) The diagramming of inner {Eu18Ni} core building unit.(c) The outer {Ni23} shell.
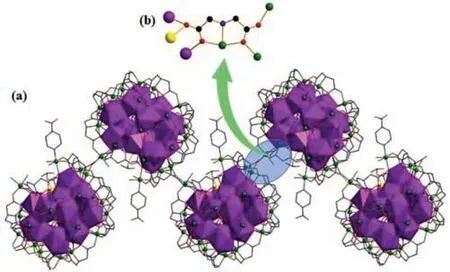
Fig.3.(a) The 1D zigzag chain of (Eu18Ni23.5)n in 2;(b) The coordination mode of connector (IDA2-) between adjacent {Eu18Ni24} units (Color code:yellow,Ni24 with occupancy factor of 0.5).
In regard to structures of two complexes,here only compound 2-(Eu18Ni23.5)nas the example is discussed in detail due to the similarity of two compounds.{[Eu18Ni23.5(IDA)22(CO3)7(μ3-OH)32(H2O)5(IN)(CH3COO)2(NH2CH2COO)]Cl}n6n+cation cluster(Fig.2) of compound 2-(Eu18Ni23.5)ncan be viewed as the coreshell configuration and can be divided into two parts:inter{Eu18Ni} core (Ni with occupancy factor of 0.5,Fig.2b) and outer{Ni23} shell (Fig.2c).Twenty-three Ni2+ions connect with 21 IDA2-ligands and one CH3COO-,leading to a bowl-like {Ni23}shell.In addition,one IN-as the terminal ligand connects with one Ni2+ion.As shown in Fig.2b,inter {Eu18Ni} layer can be divided into {Eu11Ni} (top) and {Eu7} (bottom):in the top,11 Eu3+ions are linked throughμ3-OH-;in the bottom,7 Eu3+ions are interconnected byμ3-OH-.Then {Eu11Ni} and {Eu7} are further connected by 7 CO32-,forming a unique {Eu18Ni} core (Fig.2b).Interestingly,Ni24exists in the core of {Eu11Ni},as shown in Fig.2b.The Ni24ion is connected to adjacent six Eu3+ions by oxygen atoms.
The inter {Eu18Ni} core links with outer {Ni23} transition metal shell throughμ3-OH-,CO32-(Fig.S4 in Supporting information),glycine (Fig.S5c in Supporting information) and IDA2-ligands (Fig.S3 in Supporting information),forming the core-shell {Eu18Ni24}cluster (Fig.2a).The adjacent {Eu18Ni24} clusters interact with each other based on IDA2-ligands (Fig.3b),forming the onedimensional chain (Fig.3).
Although structure of 1-(Gd18Ni24)nis very similar to 2-(Eu18Ni23.5)n,there are still some distinctions between two products:(i) the occupancy factors of all Ni2+ions in 1-(Gd18Ni24)nare one,but Ni24in 2-(Eu18Ni23.5)nis 0.5;(ii)μ2-OH-groups are only connected with metal ions in 1-(Gd18Ni24)n;(iii) IN-,CH3COO-and NH2CH2COO-groups are only coordinated with metal ions in 2-(Eu18Ni23.5)n;(iv) 9 Cl-and 14 free H2O groups are in 1-(Gd18Ni24)n,but 7 Cl-and 17 free H2O groups are in 2-(Eu18Ni23.5)n;(v) the chain-like structures of compounds 1-(Gd18Ni24)nand 2-(Eu18Ni23.5)nare both constructed by IDA2-ligand,but the coordination modes of IDA2-ligands are different (as depicted in Figs.3 and 4)
Meanwhile,CO32-anions act not only as the counter anions to balance the valence,but also as the anion templates to induce the synthesis of metal skeletons.As shown in Figs.S8 and S9 (Supporting information),CO32-anions are distributed in the middle of {Eu18Ni24} and {Gd18Ni24} building blocks as well display multidentate coordination patterns to link the neighbor subunits ({Eu11Ni} and {Eu7},{Gd11Ni} and {Gd7}).
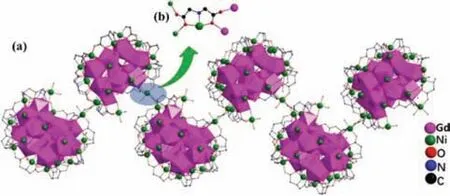
Fig.4.(a) The 1D zigzag chain configuration of 1-(Gd18Ni24)n.(b) The coordination mode of connector (IDA2-) between adjacent {Gd18Ni24} units.
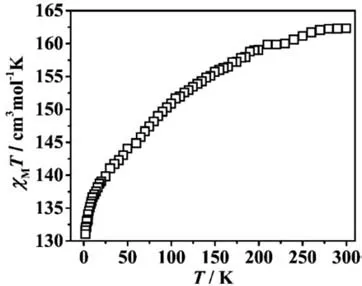
Fig.5.Temperature-dependent magnetic susceptibilities for compound 1-(Gd18Ni24)n.
A great number of Gd3+ions in compound 1-(Gd18Ni24)nencourage us to investigate the MCE.The plot ofχMT-Tfor 1-(Gd18Ni24)nwas studied with the scope of 1.8–300 K and under the appropriate magnetic field of 1000 Oe (Fig.5).The value ofχMTwas 162.31 cm3K/mol at room temperature,which was slightly inferior to the calculated result (165.75 cm3K/mol;for 18 Gd3+ions,S=7/2,g=2;and 24 Ni2+ionsS=1,g=2)[16].Along with the cooling,χMTdecreased in the scale ofT=1.8–300 K,and attained the minimum (131.06 cm3K/mol).This behavior implied the existence of antiferromagnetic interactions in 1-(Gd18Ni24)n.The Curie–Weiss Law was studied to fit theχM-1vs.Tbetween 1.8 and 300 K,with two parametersC=162.14 cm3K/mol andθ=-4.10 K (Fig.S18 in Supporting information) [28].The negative ofθindicated that weak antiferromagnetic interactions are presented in compound 1-(Gd18Ni24)n[30].The research ofM-Hfor 1-(Gd18Ni24)nwas studied (T=1.8–15 K,H=0–7 T;Fig.S19 in Supporting information).AsHincreased,theMgradually rose,and attained the saturation value of 142.07 NμBat 7.0 T.The experimental value ofMwas smaller than the calculated result (174 NμB),which can be attributed to the antiferromagnetic exchanges of the metal ions [38,39].
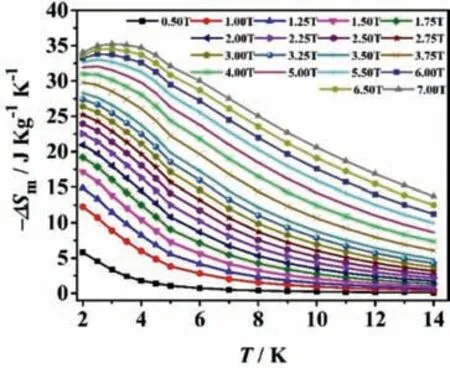
Fig.6.Values of-ΔSm for 1-(Gd18Ni24)n with varieties of temperatures (2.0–14.0 K) along with fields (0.5–7.0 T).
The magnetization studies of 1-(Gd18Ni24)nwere taken based on the scope of temperature (2.0–14.0 K) and the field (0.5–7.0 T),which were performed by the Maxwell relation for ΔSm(T)=∫[∂M(T,H)/∂T]HdH[12].The largest value of-ΔSmfor 1-(Gd18Ni24)nwas 35.3 J kg-1K-1at roughly 3.0 K with a field change of ΔH=7.0 T (as exhibited in Fig.6),which was inferior to the theoretical result (60.27 J kg-1K-1).And the theoretical calculation formula is-ΔSm=nNiRln (2SNi+1)/Mr+nGdRln (2SGd+1)/Mr[35].The discrepancy between theoretical value and experimental value may be ascribed to the antiferromagnetic behavior in 1-(Gd18Ni24)n[31].Besides,the value of-ΔSmis relatively large in reported 3d-4f clusters,manifesting that 1-(Gd18Ni24)nis the potential magnetic cooling material (Table S4 in Supporting information).It is worth noting that the large values of-ΔSmat low fields come up to 12.25 J kg-1K-1(ΔH=1.0 T) and 20.95 J kg-1K-1(ΔH=2.0 T) at 2.0 K (Table S5 in Supporting information).
In summary,two attracting high-nuclearity 3d-4f mixed-metal clusters [1-(Gd18Ni24)nand 2-(Eu18Ni23.5)n]were isolated in the existence of CO32-anionic templates and solvothermal treatment.Single-crystal X-ray diffraction studies suggested that products in this work are both bridgedviaIDA2-ligands to display the infrequent one-dimensional chain shapes.In addition,magnetic investigations indicated that 1-(Gd18Ni24)nis the potential magnetic coolant with-ΔSm=35.3 J kg-1K-1at 3.0 K and ΔH=7.0 T.Especially,the low-field magnetic entropy change of compound 1-(Gd18Ni24)nis remarkable (-ΔSm=20.95 J kg-1K-1at 2.0 T and 2.0 K).This work offers a consultant for assembling novel metal clusters together with the large MCE.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Natural Science Foundation of China (No.21571103) and Jiangsu (No.BK20191359);the Project of Natural Science Foundation of the Higher Education Institutions of Anhui Province,China (No.KJ2019A0350).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.04.042.
杂志排行
Chinese Chemical Letters的其它文章
- Long-wavelength (red to near-infrared) emissive carbon dots:Key factors for synthesis,fluorescence mechanism,and applications in biosensing and cancer theranostics
- Nanotechnology combining photoacoustic kinetics and chemical kinetics for thrombosis diagnosis and treatment
- The point-of-care-testing of nucleic acids by chip,cartridge and paper sensors
- Sodium bicarbonate,an inorganic salt and a potential active agent for cancer therapy
- New advances in gated materials of mesoporous silica for drug controlled release
- Current development in wearable glucose meters
