Microstructure and property modifications in surface layers of a Mg-4Sm-2Al-0.5Mn alloy induced by pulsed electron beam treatments
2021-03-10YingruiLiuKeminZhngJinxinZouPingYnXuZhngLuxiSong
Yingrui Liu,Kemin Zhng,∗,Jinxin Zou,Ping Yn,Xu Zhng,Luxi Song
a School of Materials Engineering,Shanghai University of Engineering Science,Shanghai 201620,China
b National Engineering Research Center of Light Alloys Net Forming & School of Materials Science and Engineering,Shanghai Jiao Tong University,Shanghai 200240,China
Received 18 August 2019;received in revised form 27 November 2019;accepted 6 February 2020
Available online 15 August 2020
Abstract In this work,surface modification of a Mg-4Sm-2Al-0.5Mn alloy with high current pulse electron beam(HCPEB)under different number of pulses were investigated.The evolution in microstructure,composition and phase components and properties in the surface layer before and after HCPEB treatment were characterized.It was found that the Al11Sm3 and Al2Sm phases in the surface layer were gradually dissolved during HCPEB treatment,leading to the formation of a chemical homogeneous melted layers.Besides,deformation bands were formed in the treated layer due to the thermal stress generated during treatment.After 15 pulses treatment,the surface hardness increases to the maximum value of about 62.2 HV,about 61.2% higher than that of the untreated state.Electrochemical results show that the 15 pulses treated sample presents the best corrosion resistance in the 3.5wt% NaCl water solution by showing the highest corrosion potential(Ecorr)of-1.339VSEC and the lowest corrosion current density(Icorr)of 1.48×10−6 A·cm−2.The results prove that the surface properties of the Mg-4Sm-2Al-0.5Mn alloy can be significantly improved by the HCPEB treatments under proper conditions.
Keywords:High current pulsed electron beam(HCPEB);Surface modification;Mg rare earth alloy;Microstructures;Corrosion-resistance.
1.Introduction
Known for a series of advantages such as high specific strength,high stiffness,excellent machinability and good castability[1],Magnesium(Mg)alloys,as promising structural materials are attractive for applications in the fields of automobile,aerospace and 3C industries[2].However,the poor high-temperature mechanical properties and corrosion resistance limit their further applications.Therefore,various methods were adopted to ameliorate the properties of Mg alloys to meet the requirements for applications.The alloying method has been proved to be an effective way to improve the properties of Mg alloys both at ambient and high temperatures(higher than 120 °C)[3],such as AZ and AM series.These alloys contain elements of aluminum(Al)and manganese(Mn),which are most widely used to fabricate various components owing to their good balance in strength,ductility and creep resistance at elevated temperatures[4].
The solid solubility of rare earth elements is relatively large in Mg at elevated temperatures,which shows good age hardening effects on Mg alloys[5,6].Sun et al.[7]investigated the effect of samarium(Sm)on the grain refinement,microstructure and mechanical properties of the AZ31 Mg alloy.They found that a significant grain refinement effect was achieved when the content of Sm was above 2.17 wt%.This was due to the reaction between Al and Sm and the formation of Al2Sm particles,serving as heterogeneous nucleation sites.As a result,the content ofβ-Mg17Al12phase gradually decreases and the mechanical properties are improved obviously.Huang et al.[8]found that the additions of Sm have a magnificent influence on the tensile properties of Al–7Si–0.7Mg alloys due to the modification of eutectic silicon.These researches have indicated that the properties of Mg alloys,especially the mechanical properties like tensile strength and yield strength,can be effectively enhanced through alloying with rare earth(RE)elements.However,the corrosion resistance of Mg-RE alloys cannot meet the requirements of industrial applications largely owing to the formation of second phases.Therefore,an appropriate surface modification technology is necessary and critical to improve the surface properties of Mg-RE alloys for the practical application.
In the past decades,the energetic beam surface modification methods,such as laser,ion and electron beams,have received much attention due to their high efficiency,low energy consumption and less pollution.Among these methods,high current pulsed electron beam(HCPEB)exhibits many advantages over other methods,such as its high efficiency,low energy consumption and reliability,which has been proved to be an effective technique to enhance the surface properties of the samples instead of changing their bulk compositions[9–13].Gao and its co-author[14]conducted the surface modification on AZ91HP alloy by using HCPEB treatment.The sliding friction,wear and immersion tests results showed that wear and corrosion resistance of the alloy are significantly improved.It was related to a nearly complete dissolution of the intermetallic phase Mg17Al12in the melted layer and formation of a supersaturated solid solution on the surface.The relationship between changes in the microstructure of surface layers of AISI D2-steel after HCPEB treatment and surfacesensitive properties were also investigated[15].
However,there are few researches focusing on the surface modification of Mg-Al-Mn-RE alloy which has a good mechanical property,but presents a decreased property of corrosion resistant because of a large number of second phase particles formed in the process of alloying that will act as a persistent local cathode.As a result,the micro-galvanic corrosion would happen between matrix and second phase particles[16].Therefore,in order to investigate the surface modification on a selected Mg-4Sm-2Al-0.5Mn alloy during the HCPEB treatment,the change of surface microstructures,phase compositions and corrosion resistance were carefully investigated in this research.
2.Experimental procedures
2.1.Sample preparation and experimental equipment
Samples with the size of 12mm×12mm×2mm for HCPEB treatments were cut directly from a cast Mg-4Sm-2Al-0.5Mn alloy,which was prepared in a resistance furnace using a high-purity graphite crucible under a protection environment of mixed CO2and SF6gas.Table 1 lists the chemical composition(in wt%)of the alloy analyzed by inductively Coupled Plasma-Atomic Emission Spectrometry(ICAP-7600).Prior to the HCPEB treatments,all samples were ground with metallographic abrasive papers and polished with 0.5μm diamond paste to produce mirror like smooth sur-faces without scratches.Then,all samples were ultrasonically cleaned in acetone.

Table 1Chemical composition of the Mg-4Sm-2Al-0.5Mn alloy.
The electron beam system used in this work was a“Hope-1”type HCPEB source,which can produce an electron beam with the following parameters:accelerating voltage 20–30kV,peak current~10 kA,pulse duration 0.5–5μs,beam diameter Ф40 mm,and energy density 1–30J/cm2.More details about the HCPEB system can be found in Refs.[9-11].The HCPEB treatment parameters used in present work were as follows:accelerating voltage 23kV,energy density 4J/cm2,pulse duration 1.5μs.Previous researches[17]prove that when the pulse number increases to more than 15 pulses,the top surface is very rough with a lot of defects on it.This is harmful to the corrosion resistance of the sample.In view of the above considerations,number of pulses 5,10 and 15 was selected,off time between each pulse 15s.
2.2.Microstructure characterization and microhardness tests
The phase components in the surface layer were examined using X-ray diffraction(XRD)of a Shimadzu D-6000 X-ray diffractometer equipped with a Cu Kαradiation source.The surface microstructure was observed using VHX-600 optical microscope(OM).The surface and cross-section microstructures were observed by using a S-3400N scanning electron microscope(SEM)equipped with an energy dispersive spectrometer(EDS).Before SEM observation,cross-sectional samples were well polished followed by etching in a 4 vol%nitric acid alcohol solution.
The microstructures in the modified layer were investigated using a JEM-2100F type transmission electron microscope(TEM).For TEM observations,disk shaped samples with a diameter of 3mm and tens of nanometers in thickness at the center were prepared by using the techniques of oneside mechanical thinning from the untreated side,followed by dimpling and ion beam(Ar+)thinning.The surface Vicker’s hardness of the samples were measured under 10g,25g and 50g load,respectively.The testing time was 15s when the indenter loaded on the samples,and in order to obtain more accurate measurement data,an average value of 10 points was taken as one hardness data.
2.3.Electrochemical measurement and corrosion analysis
The electrochemical measurements were carried out in a standard three-electrode cell in the 3.5 wt% NaCl water solution using a PARSTAT 4000 electrochemical workstation,which has a Pt counter electrode and saturated calomel electrode(SCE).Before the measurement,the untreated sample was ground with metallographic abrasive papers and polished with diamond paste to 0.5μm and cleaned in ethanol,while the treated samples were directly measured.All samples were soaked in 3.5 wt% NaCl water solution for 30 min to make sure that the open circuit potentials were stable for all tests.The area exposed to the NaCl water solution was 1.13 cm2.All the tests were carried out at room temperature.
The electrochemical impedance spectroscopy(EIS)measurements were recorded at the open circuit potential in the frequency ranging from 10−2Hz to 105Hz,with a sinusoidal actuating signal of perturbation of 10mV.Potentiodynamic polarization curves were measured with a scanning rate of 2 mV·S−1.The scanning range of cathodic polarization was set from−2.5V(SEC)to−0.8V.For better reproducibility,all above electrochemical measurements were repeated three times.The polarization curves were used to evaluate the corrosion current density(Icorr)and the corrosion potential(Ecorr)by Tafel extrapolation method.
3.Results and discussions
3.1.XRD analysis
Fig.1 shows the XRD patterns and its local enlarged patterns before and after the HCPEB treatments.The untreated sample is mainly composed ofα-Mg,β-Mg17Al12,Al2Sm and Al11Sm3phases.After HCPEB treatments,the diffraction peaks ofβ-phases and Al11Sm3phases become quite weak,reflecting that these two phases were dissolved into the Mg.What’s more,the diffraction peaks of Mg have shifted towards higher angles indicating a decrease in interplanar spacing after the HCPEB treatments.Fig.2 clearly shows the changes in interplanar spacing calculated form the measured XRD data.According to the Bragg’s equation and XRD data,lattice parameters were calculated by using Celref 3.0 software.It can be seen form Fig.3 that both the c value and a value decreased after HCPEB treatment compared with the untreated samples.Such a phenomenon can be attributed to the effect of residual stress in Mg matrix together with the supersaturated solid solution[14].
3.2.Surface morphologies observation
Fig.4 shows the microstructures of the untreated sample surface with the typical OM image and SEM image.Many isometric disk-like particles with the size of 20–50μm present in the untreated sample.Its core exhibits a regular shape,and the disk-like structures are surrounded by the strip eutectics.According to Wang’s research[18],these cores are Al2Sm particles,and the orientation relationship between Al2Sm and α-Mg can be described as follows:
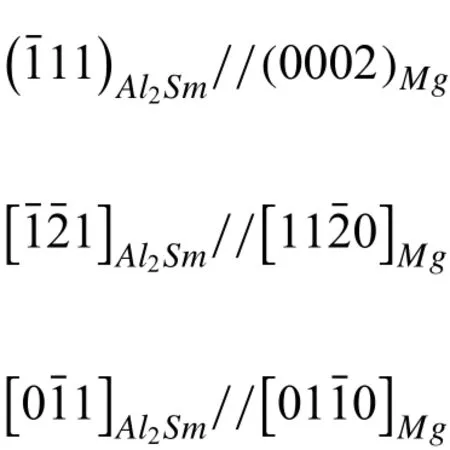
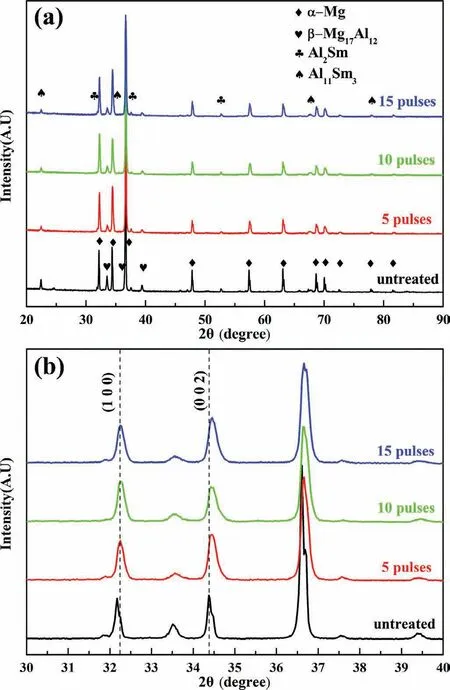
Fig.1.XRD patterns(a)and local enlarged patterns(b)of the Mg-4Sm-2Al-0.5Mn alloy samples before and after the HCPEB treatment.
The above orientation relationships make the Mg atoms adhere to habit planes of the Al2Sm phase during the solidification.The rod-like phases were considered to be Al11Sm3phases,which has been found in Mg-5Al-0.3Mn-2Sm alloy[19].
Fig.5 shows the micrographs of the modified surfaces after HCPEB treatment with different number of pulses.From the OM images(Fig.5(a-c)),the surface of the samples presents wavy contrasts after the HCPEB treatment and,the contrasts are more obscure for the highest number of pulses.After the HCPEB treatment with 5 pulses,many craters show up on the modified surface and at the center of each crater where there was a core,as marked in Fig.5(a).Its formation mechanism has already been established in previous studies[20-22].With the increase of pulses,the modified surface becomes flatter,but massive tiny cavities appear on the surface,as shown in the inset map of Fig.5(b-c).What’s more,deformation bands are formed due to the high thermal stress field generated during HCPEB treatments.From the SEM(Figs.5(d-e))images,it can be illustrated that the density of defects like droplets and holes decreases.The surface becomes compact and homogeneous with the increase of number of pulses.Besides,many white contrast traces distributes on the whole surface and their density gradually decreases with the increase of the pulse number,which can be attributed to the dissolution of second phase particles into the matrix during HCPEB treatments.
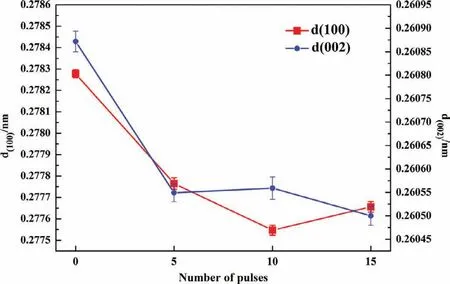
Fig.2.The changes in the values of interplanar spacing d(100)and d(002)of Mg lattices before and after HCPEB treatments under different pulses.
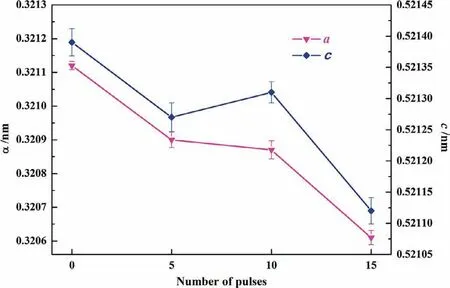
Fig.3.The variation in the lattice parameters of Mg before and after HCPEB treatments under different number of pulses.

Fig.4.Typical surface micrographs of the Mg-4Sm-2Al-0.5Mn alloy samples:(a)OM image,(b)SEM image.
3.3.Element distribution in the surface layer
Fig.6 shows the surface morphology and EDS spectra of the sample treated with 5 pulses.The typical features of melting and eruption induced by the HCPEB treatments can be observed.White particles are well distributed overall treated surface.Furthermore,P1 and P2 positions were selected to detect the local compositions and the results are shown in Figs.6(b-c).The atomic ratio of Al and Sm in the P1(marked by+)position is 1.81:1,close to the stoichiometric ratio of 2:1.Combining with the results of XRD analysis,the white particles appear to be Al2Sm.Meanwhile,it can be seen that the white phase particles distribute independently and are surrounded by the gray phase.The distribution of the Al2Sm phases were to a large degree the same as that in the untreated sample.The EDS spectra detected in P2 position shows that the gray phases contain 98.39%(At%)of Mg element instead of pure Mg for the reason of dissolution of Al and Sm atoms into the Mg matrix in the molten state during the HCPEB treatment,which could be considered as a supersaturated Mg matrix.
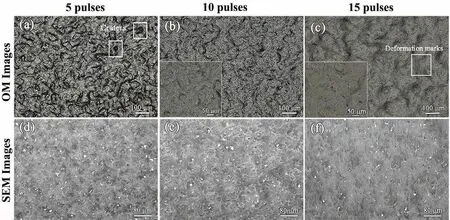
Fig.5.Surface OM images(a),(b),(c)and SEM images(d)(e)(f)after HCPEB treatments with different number of pulses.

Fig.6.SEM image and EDS results from surface(a)of the Mg-4Sm-2Al-0.5Mn alloy samples after 5 pulses of the HCPEB treatment and its EDS spectrum in P1(b)and P2(c)positions.
Fig.7 shows the element distributions on the modified surface.Fig.7(a)and Fig.7(e)show the surface morphologies of the samples treated with 5 and 15 pulses,respectively.Figs.7(b-d)and Figs.7(f-h)show the results of EDS mapping of Mg,Al and Sm elements,respectively.Mn element cannot be conducted because of its low content in the alloy.Just as shown in Fig.7(a),some white particles still exist in the treated surface.Combined with the results of EDS mapping of three elements and the EDS spectra given in Fig.6,it can be confirmed that the positions which contain higher concentration of Al and Sm were Al2Sm phases.Meanwhile,after the HCPEB treatment with 5 pulses,the rod-like Al11Sm3particles were dissolved into the matrix.
However,when the pulse number increased to 15,the distributions of Mg,Al and Sm elements become more uniform in the modified layer,as shown in Figs.7(e-h).It can be due to the repeated melting induced by electron beam treatments.Some residual Al2Sm phases still can be observed in the treated surface,but the size of them changed significantly.It can be illustrated that the more electron beam pulses irradiated on the surface,the less chemical segregation happened on the treated surface.
3.4.Microstructures in modified layer
The cross-section SEM observations in Figs.8(a-c)show the different cross-section morphologies of the samples treated with various number of pulses.It can be seen in the area between the double lines that those rod-like phases and Al2Sm particles disappeared,which reflected the formation of a homogeneous remelted layer on the top surface.Obviously,with the increase of pulse number,the melted layer depth increases as well.The thickness of melted layers for 5,10 and 15 pulsed samples are 6.3μm,9.6μm and 11.2μm,respectively.This mainly lies in the fact that the energy deposited on the surface increases with the number of HCEPB treatment[23-25].It can be interestingly noted that after 15 pulses treatment,as shown in Fig.8(c),the modified layer has an obvious characteristic of melting and erupting in some special locations which contain Al2Sm phases.These Al2Sm phases particles were reserved and acted as the residual crater’s core in the process of the HCPEB treatment.This phenomenon can be explained by the selective melting and erupting mechanism[15].Above changes in microstructure and components reveal that HCPEB treatment can purify the substrate effectively by erupting the inclusions or second phases particles.
Fig.9 shows the TEM images of the untreated and 15 pulsed samples.Fig.9(a)clearly shows that the untreated sample consists of black strip bands and gray matrix,Fig.9(b)is the high-resolution image for the Fig.9(a).The index of the SAED pattern of P1 position given in the inset of Fig.9(b)confirms that the black strip phase and the gray substrate are Al11Sm3and Mg lattice respectively.Fig.9(c)is the HRTEM image of P1 position and shows that these two phases have different orientations.The inverse FFT images selected in P2 and P3 confirm that the black phase is Al11Sm3while the substrate is Mg.

Fig.7.Typical SEM images and corresponding elemental mapping of Mg,Al and Sm taken on the top surface of the Mg-4Sm-2Al-0.5Mn alloy treated by HCPEB with 5 pulses(a-d)and 15 pulses(e–h).
After 15 pulses of HCPEB treatment,it can be seen in Fig.9(d)that the profile of Al11Sm3phases became blurred.The SEAD pattern and inverse FFT images obtained from the selected area of P4 and P5 reveal that only Mg exists in the top surface layer while the other phases were melted and dissolved into the substrate during the HCPEB treatment process.
3.5.Surface microhardness measurements
Fig.10 shows the microhardness values of samples tested under different loads after the HCPEB treatments with 5,10 and 15 pulses,respectively.The untreated sample has a hardness of 35.8 HV,as indicated in Fig.10 with a straight dash line.It’s obvious that the surface hardness increases after HCPEB treatments under different loads.For instance,the microhardness under 50g loading increases from 35.8 HV for the initial sample to 58.7 HV for the 10 pulses and 62.2 HV for the 15 pulses treated sample.Two major mechanisms about surface hardening induced by HCPEB treatments can be explained as follows:First,deep hardening that can extend over 50–300μm is due to the propagation of thermal stress waves.Second,the surface hardened layer in the melted and subsurface layer is the results of phase transformation,grain refinement,structural defects(voids and dislocations)and residual stresses[26].Using different loads for the hardness test is a way to investigate the hardening mechanisms,and it can be deduced that the improvement of the hardening in the HCPEB treatment treated samples in this study comes essentially from the surface layer.
3.6.Electrochemical measurements
The influence of HCPEB treatment on the corrosion resistance was evaluated by potentiodynamic polarization and electrochemical impedance spectrometry(EIS)measurements.Fig.11 presents the polarization curves measured from−2.5V to−0.8V.Generally,the mainly corrosion resistance characteristics include corrosion potential Ecorrand corrosion current density Icorr,which were calculated by using Tafel extrapolation method and listed in Table 1.As shown in Table 1,the sample treated with 5 pulses shows the lowest Ecorrof−1.564VSECand highest Icorrof 5.66×10−5A·cm−2.Meanwhile,for the sample treated 15 pulses,the Ecorrincreases to−1.339VSECand the Icorrdecreases to 1.48×10−6A·cm−2,indicating that the corrosion resistance is improved.These can be explained as follows:the corrosion mechanism of the Mg based alloy in NaCl water solution is galvanic corrosion due to the existence of the second phase particles like Al2Sm or Al11Sm3,which would form a lot of tiny galvanic corrosion cells[27].For 5 pulses treated sample,lots of defects like cavities are formed on the treated surface,which provided many positions for corrosion easily.However,after 15 pulses treatment,the density of defects decrease and the surface becomes flat and compact,which is beneficial to the surface protection in corrosive environment.

Fig.8.Cross-section images of the Mg-4Sm-2Al-0.5Mn alloy samples after HCPEB treatments under different number of pulses.

Fig.9.Typical TEM BF image of the untreated sample(a),the local enlarged image SAED pattern of area P1(b)and the HRTEM image of P2 and P3(c).TEM BF image(d)and its corresponding SAED pattern(e)of the 15 pulses treated sample.HRTEM image of the 15 pulses HCPEB treated sample(f).
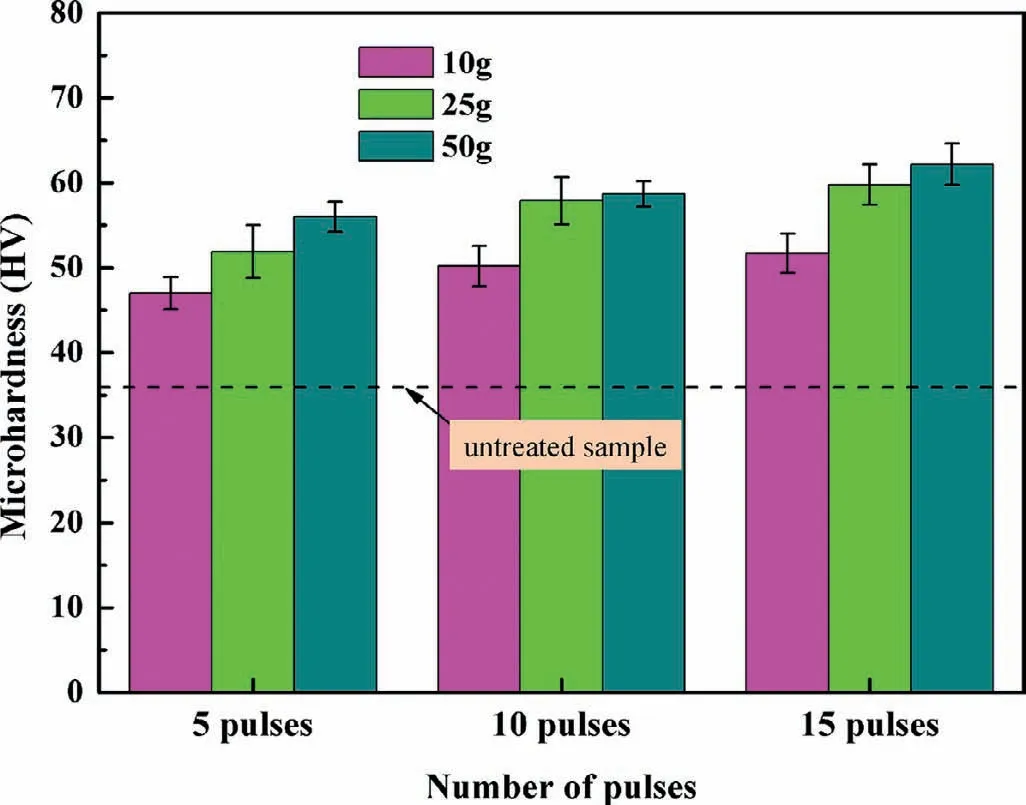
Fig.10.Surface microhardness of the samples before and after HCPEB treatments under different loading conditions.
In order to further analyze the corrosion mechanism,EIS were tested in the 3.5wt% NaCl water solution.As shown in Fig.12.The Nyquist plots of all samples exhibit a single capacitive loop lying in the first quadrant.According to the theory of EIS,the high frequency(HF)capacitive loop is usually related to the charge transfer resistance and the double layer capacitance at the interface of metal/solution(Rct),and the radius of the capacitive loop presents the value of polarization resistance,which is considered to be one of the critical characteristic parameter to estimate the property of corrosion resistance[28].It’s obvious that the value of the radius is different from each other and the sample treated with 15 pulses displays the highest value of Rct(~1996Ω·cm2),indicating that the sample treated by 15 pulses has the best corrosion resistance among all the samples.What’s more,when the sample is treated with 5 pulses,its corrosion resistance becomes even worse than that of untreated sample,which is in accordance with the results of polarization curves.This is related to the existence of many defects and voids in the treated surface layer.

Fig.11.Potentiodynamic polarization curves of the samples before and after HCPEB treatments.
According to the surface condition and corrosion mechanism of the Mg alloy in NaCl water solution,an appropriate equivalent circuit is given in the inset of Fig.12,and the fitting results are given in Table 2.Because all the measured samples present only one capacitance loop in the first quadrant,the equivalent circuit contains one time constant[29-31].In the equivalent circuit,RS,Rctand Cdllrepresent the solution resistance,charge transfer resistance and the double electric layer capacity,respectively.While the constant phase element(CPE)is used to demonstrate the deviation from the pure capacity behavior and compensation for the non-homogeneity of the system[32].Generally,the impedance of a CPE is described as:

Fig.12.Nyquist plots of the samples before and after HCPEB treatments.
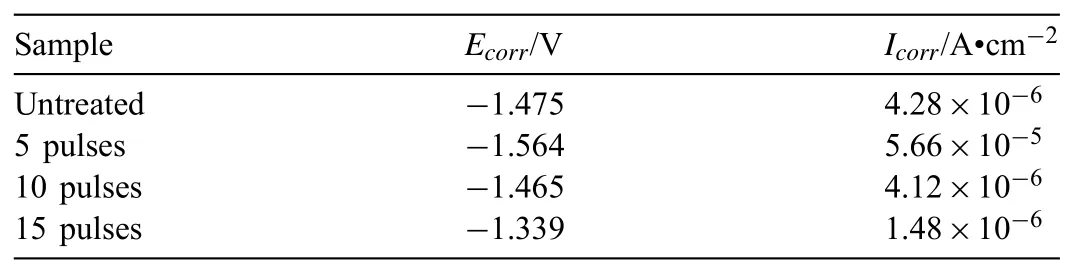
Table 2Corrosion data of the Mg-4Sm-2Al-0.5Mn alloy samples before and after HCPEB treatments.

Table 3EIS fitting data of the Mg-4Sm-2Al-0.5Mn alloy samples before and after HCPEB treatments.
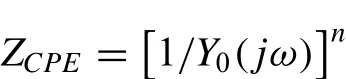
Where j,ωand Y0are the imaginary coefficient,the angular frequency,pseudo capacitance,respectively,and ncloseness is the ideal capacitance process[33].In general,the higher Rctvalue implies the lower dissolution rate of the Mg substrate in corrosive environment[34].According to the fitting results given in Table 2,the values of Rctfirstly decrease and then increase with the increase of pulses,implying that the corrosion rate of the Mg substrate reduced after 15 pulses of HCBEB treatment.This is consistent with the results of polarization tests and is attributed to the homogenization of chemistry and phase components on the 15 pulses treated sample.
4.Conclusions
In this work,the effect of HCPEB treatments on the surface microstructures,phase transformation and corrosion resistance of a Mg-4Sm-2Al-0.5Mn alloy has been investigated.The main results are summarized as follows:
1.The primary Al2Sm and Al11Sm3phases are dissolved gradually in the remelted layer with the increasing number of pulses.A supersaturated solid solution formed on the remelted layer.As a result,the Mg lattice changed noticeable,which is attributed to the solid solution of Al and Sm together with the residual stress in the surface layer.
2.Cross-section analyses reveal that the thickness of the remelted layer increases with the number of pulses.After 15 pulses treatment,the depth of the melted layer increases to 11.2μm.EDS results confirm that the chemical composition of the remelted layers becomes homogeneous after the HCPEB treatment.TEM observations show that the second phase particles gradually dissolved and the profile of Al11Sm3phases become blurred.
3.After 15 pulses treatment,the microhardness under 50g loading increases from 35.8 HV for the initial sample to 62.2 HV for the 15 pulses treated sample.Electrochemical measurements show that the best corrosion resistance is achieved when the pulse number is 15,which have the highest Ecorrof−1.339VSECand the lowest Icorrof 1.48×10−6A·cm−2.However,the corrosion resistance of sample treated with 5 pulses becomes even worse due to the formation of lots of defects and voids together with the non-homogeneous microstructure on the treated surface.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the National Natural Science Foundations of China(No.51271121,51471109).
杂志排行
Journal of Magnesium and Alloys的其它文章
- Recent developments and applications on high-performance cast magnesium rare-earth alloys
- Surface characterization and corrosion behavior of calcium phosphate(Ca-P)base composite layer on Mg and its alloys using plasma electrolytic oxidation(PEO):A review
- Towards developing Mg alloys with simultaneously improved strength and corrosion resistance via RE alloying
- Magnesium matrix composite reinforced by nanoparticles–A review
- The design of Co3S4@MXene heterostructure as sulfur host to promote the electrochemical kinetics for reversible magnesium-sulfur batteries
- A new die-cast magnesium alloy for applications at higher elevated temperatures of 200–300°C
