Surface characterization and corrosion behavior of calcium phosphate(Ca-P)base composite layer on Mg and its alloys using plasma electrolytic oxidation(PEO):A review
2021-03-10RaziehChaharmahaliArashFattahalhosseiniKazemBabaei
Razieh Chaharmahali,Arash Fattah-alhosseini,Kazem Babaei
Department of Materials Engineering,Bu-Ali Sina University,Hamedan 65178-38695,Iran
Received 25 March 2020;received in revised form 4 July 2020;accepted 10 July 2020
Available online 10 August 2020
Abstract Magnesium has been known as an appropriate biological material on account of its good biocompatibility and biodegradability properties in addition to advantageous mechanical properties.Mg and its alloys are of poor corrosion resistance.Its high corrosion rate leads to its quick decomposition in the corrosive ambiance and as a result weakening its mechanical properties and before it is repaired,it will vanish.The corrosion and degradation rate must be controlled in the body to advance the usage of Mg and its alloys as implants.Different techniques have been utilized to boost biological properties.Plasma electrolytic oxidation(PEO)can provide porous and biocompatible coatings for implants among various techniques.Biodegradable implants are generally supposed to show enough corrosion resistance and mechanical integrity in the body environment.Much research has been carried out in order to produce PEO coatings containing calcium phosphate compounds.Calcium phosphates are really similar to bone mineral composition and present great biocompatibility.The present study deals with the usage of calcium phosphates as biocompatible coatings applied on Mg and its alloys to study the properties and control the corrosion rate.
Keywords:Mg alloys;Calcium phosphate(Ca-P);Plasma electrolytic oxidation(PEO);Surface characterization;Corrosion behavior.
1.Introduction
Magnesium(Mg)and its alloys are really functional and useful in many different engineering applications[1–5].Nevertheless,a critical disadvantage of Mg and its alloys is their poor corrosion resistance,which restricts many application fields in comparison to other structural metal materials including Ti and Al alloys and steels[6–11].Coatings or surface treatments are needed to warrant the lifetime of Mg and its alloys function and surface treatments against different corrosive media[12,13].Protective coating is a truly effective method to raise the corrosion resistance of Mg and its alloys.There are many techniques to obtain protective coatings.Amongst many feasible and available coating treatments to amend the corrosion resistance of Mg alloys[14,15],PEO is a plasma chemical and an electrochemical treatment.This surface treatment combines electrochemical oxidation with a spark having high voltage within an alkaline electrolyte resulting in a physically protective oxide layer formation on the metal surface to augment corrosion and wear resistance as well as components long lifetime[16–19].This process can make as tough as corundum-dense and solid coatings on Mg surface and its alloys[4,20–25].It is considerable that the PEO treatment is a multifactor-controlled process being influenced by many parameters whether extrinsic or intrinsic ones[26,27].Three dependent needs specify the bone-forming capability of a bone alternative:growth factor for osteoinduction,scaffolding for osteoconduction and progenitor cells for osteogenesis[28].According to these features,magnesium-PEO coatings can make a controllable surface roughness and constant composition.Nevertheless,inevitable imperfections made by the discharges(resulting in pitting)[29]and little bioactivity(postponing the reproduction and adsorption of osteoblast)[30]is still a concern.Compositions of calcium phosphate(Ca-P),particularly in shape of biphasic calcium phosphates(BCP),hydroxyapatite(HAp)andβ-tricalcium phosphate(TCP),are useful and safe options for implants,because they are the principal components of human bones(37.5 wt%:P 11.6%,Ca 25.9%)[31,32].
The plenty of Ca-P can activate not only implants bioactivity,but also can accelerate the cure procedure of wound within simulated body fluid(SBF)solution.Since HAp has great bioactivity and biocompatibility,it is highly produced as a coating on metallic substrates for bio-application[33].In comparison to ordinary preparation techniques including laser cladding or hot spraying,the structure of formed Ca–P coatings within electrolyte is nearer to that of the bone minerals[34].Furthermore,the electrochemical deposition method can form a steady coating on a porous substrate or one having a complicated form and modify the compositions and morphology of Ca–P coating simply[35].Nevertheless,HAp coating gained from electrochemical deposition possesses a low bonding strength(around 4–6MPa)that might result in peeling off right after implantation[36].
As plasma electrolytic oxidation(PEO)can produce porous ceramic coating having a high adhesion to substrate,it is predicted that this porous coating is able to be utilized as an intermediate film to deposit HAp since it can produce pinning force as HAp is deposited within the pores and boost the corrosion resistance of the porous film.Simultaneously,new progressions in biomineralization possess already indicated that nanosized crystals play a major role in the production of stiff tissues of animals[37].Liu et al.[38]deposited dicalcium phosphate dehydrate(DCPD)and calcium deficient HAp on PEO coating using a chemical transformation technique.They have described the formation of HAp on the coating surface within the incubation in SBF electrolyte and the released H2volume reduction.Gao et al.[39]produced PEO-HAp coating using cathodic deposition.They demonstrated that Mg substrate corrosion resistance was developed by PEO coating and extra HAp film.After immersion analysis in physiological ambience,calcium-phosphorus(Ca/P)ratio augmented for PEO coating but it declined for PEO-HAp coating.
In the present investigation,the common PEO technique has been studied as one of the best procedures to place the Ca-P base composite film on the Mg biomedical grade.This procedure is composed of a high adhesive strength material,favorable biomedical and mechanical properties,and simple setup in addition to controllable chemical reaction techniques.So,the characterization of surface and corrosion behavior of Ca-P base composite films has been measured for this process.
2.Mg and its alloy
2.1.The advantages of Mg alloy as bone implants
The lightest metal among of all the engineering metals is Mg with a density of 1.7g/cm3[40].The mechanicaland physical properties of Mg make it appropriate like a biodegradable metal implant for either orthopedic or stent usages.Table 1 shows a comparison of Mg with the natural bone and other engineering materials that are utilized as implants.The distinction in the other engineering materials elastic modulus agreeing with value for natural bone changes that may result in effect of stress shielding between implant and bone of the injured bone as it will exclude the bone of the normal stress agreeing with the entire cure procedure in addition to the stability of implant[41].Generally,the usage of degradable implants decreases the requirement for another operation for implant removal that saves money for the health system as well as being useful to the patients.Also,degradable implants provoke the hurt tissue because of a slow load transfer from the implant to the tissue[42].Nevertheless,the optimum degradation function of a biodegradable implant is supposed to be controlled in order to confirm the total mechanical integrity of the tissue in addition to biocompatibility through postponing the production of degradation products.As a result,a Mg implant has all of the mentioned benefits that make it a proper material to be used as a biodegradable implant.
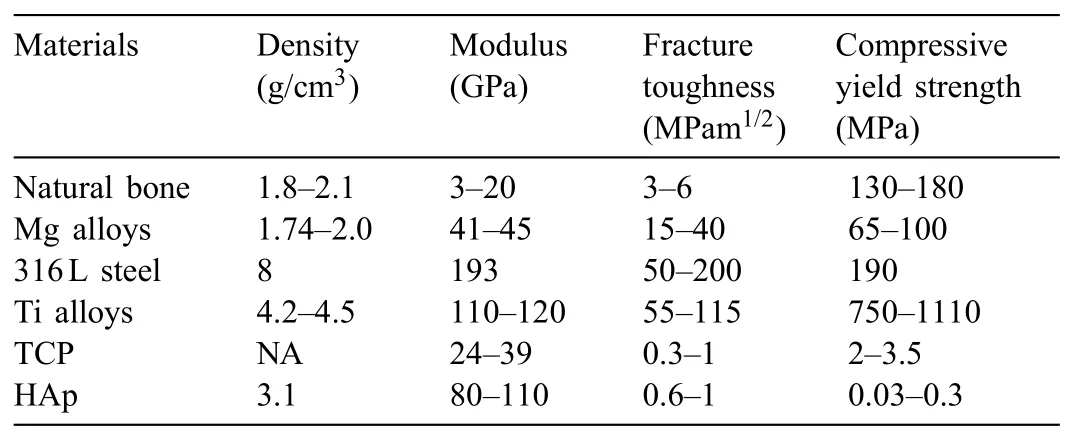
Table 1Summary of the physical and mechanical properties of various implant materials in comparison to natural bone properties[49].
2.2.Application of Mg bone implants
Because of the referred benefits of Mg alloy including bone implants,investigators have allocated much attempt to prosper Mg and its alloys to be used in repair of bone in the beginning 20th century.Device of bone fixation mostly contains bone pin,bone screw,bone plate and so on that plays a major role in consolidation within repairing bone.Many of Mg bone fixation tools are indicated in Fig.1[43].The clinical usage of Mg bone fixation tools can be followed back to the beginning of 20th century and many common case reports were shown in Fig.2[44–47].
2.3.Corrosion of Mg and its alloy
The biodegradable implant corrosion kinetics is stimulated and driven via interactions of its surface with the immediate surroundings and so could be noticed a surface phenomenon.Obviously more propagation or aggravation inside the surface results in the entrance of corrosion in the bulk metal.The overall difficulties related to the weak corrosion resistance of Mg alloys are ascribed to a couple of basic parameters:(1)the much negative potential that lets corrosion go ahead without oxygen and makes H2O decline to H2gas;and(2)the weak Mg surfaces passivation because of the production of soluble hydroxide and oxide layers that slightly coat the substrate metal[48–50].
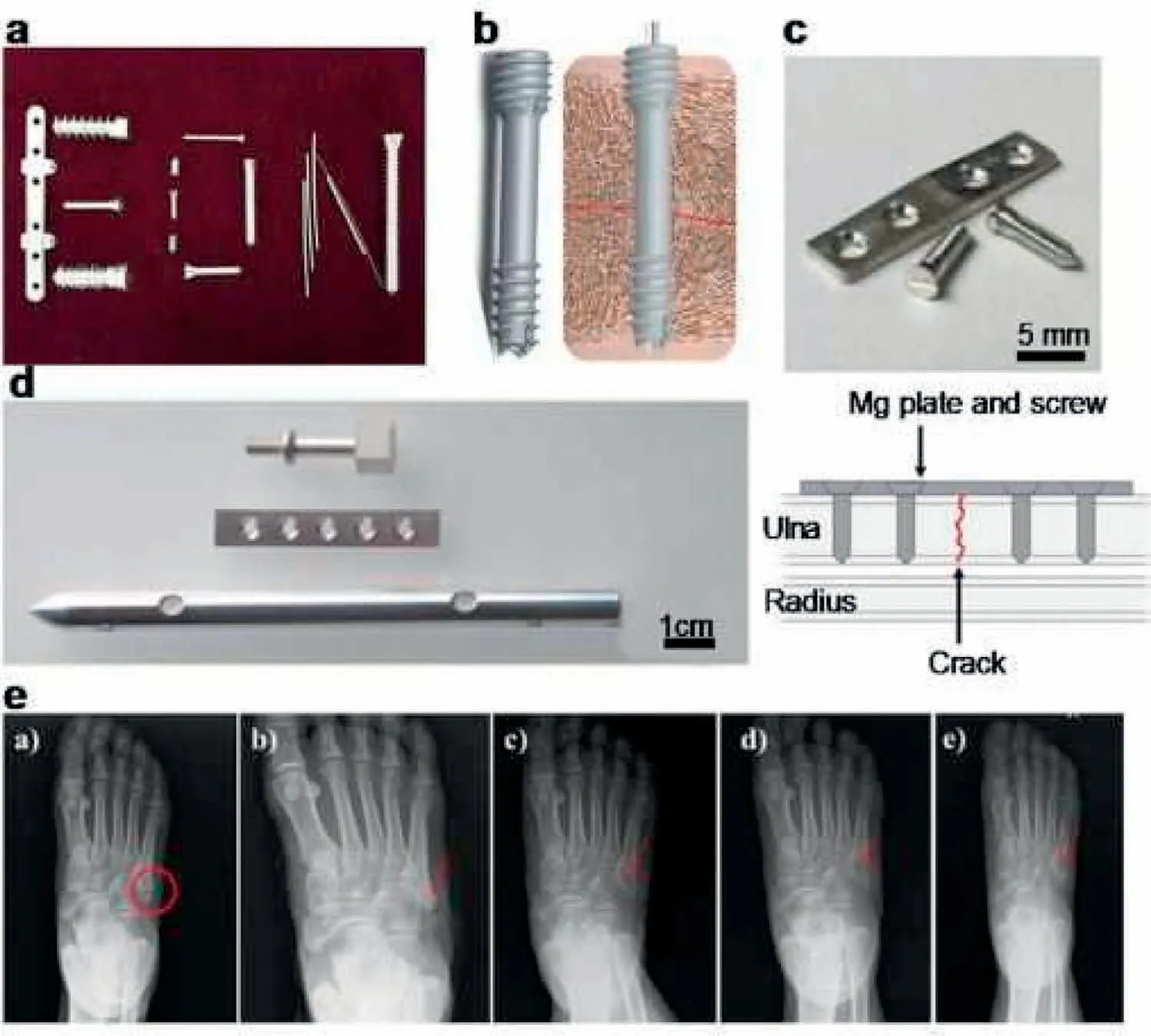
Fig.1.(a–d)Depict the typical bone fixation devices,and(e)reveals one case of their bone repair application[43].
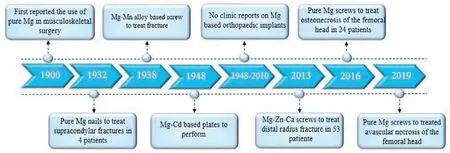
Fig.2.Typical human case reports on Mg bone fixation devices since the early 20th century.
Corrosion of Mg in an aqueous electrolyte depends on the pH and potential of electrode,as shown in the diagram of Pourbaix below[51].Mg2+ions dissolution occurs at the anode that emits its accompanying electrons to decline water at the cathodic site and forming ions of H2and hydroxyl(OH−).The ions of Mg2+and OH−are mixed to produce a porous Mg(OH)2precipitate layer on the surface of metal(Fig.3)[52–55].
2.4.Coating techniques applied on Mg alloys for implant application
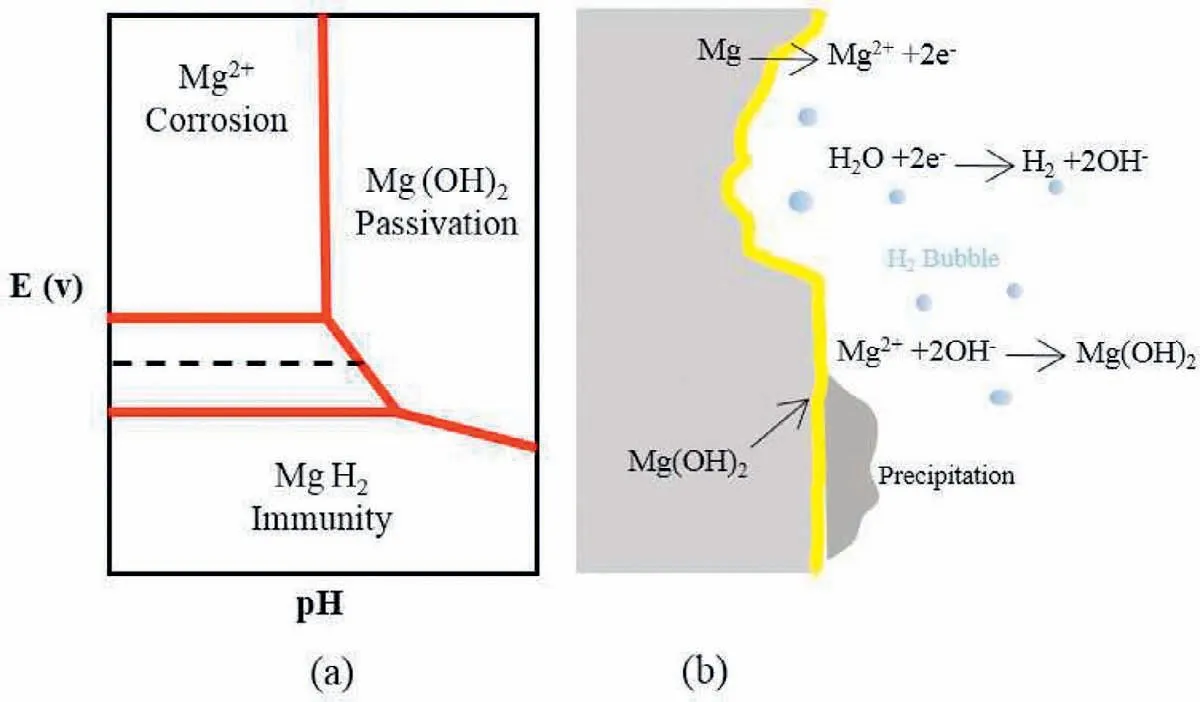
Fig.3.(a)Pourbaix diagram of Mg,and(b)schematic diagram of Mg corrosion in aqueous water involving generation of H2 bubbles.
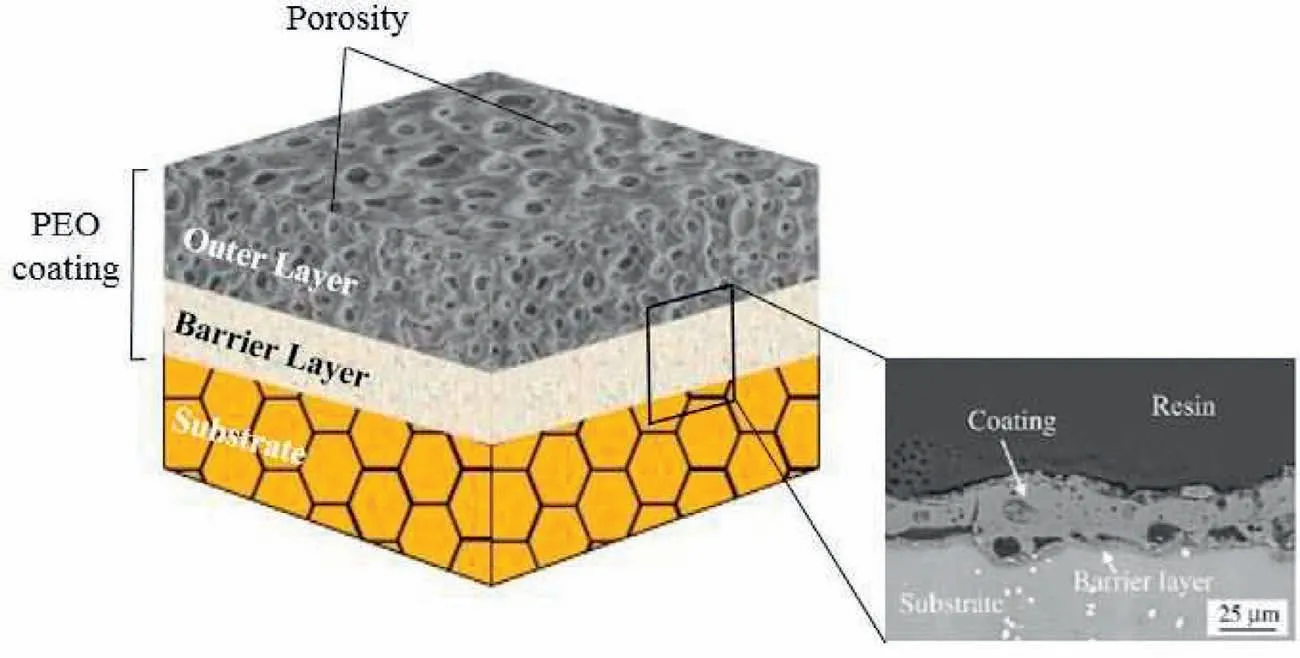
Fig.4.Schematic of different layer of PEO coating[73].(With permission from[73];License Number:4794660106616,Mar 23,2020,Elsevier).
However,the Mg corrosion resistance is assumed as the major disadvantage for numerous engineering usages,its degradation treatment might be a good start for this material to be pondered like a biodegradable implant.In order to prevent rapid surface degradation and maintain the mechanical integrity of magnesium and its alloys,a variety of coating methods have been proposed.Yin et al.[56]examined the coatings formed on magnesium and its alloys.They divided the created coatings into four categories:physical,chemical,mechanical and biological.Among these methods,PEO is one of the most popular techniques for magnesium alloys.The use of Ca-P-based coatings in biological materials has been studied by many researchers to examine the biocompatibility of coatings with the human body[57–59].
3.PEO coatings
PEO is a hopeful procedure obtained from typical anodizing to produce ceramic-like coatings on light metals and also their alloys(Mg,Al,Zr and Ti).[60–65].It is obvious that the PEO treatment is significantly more complicated than anodization treatment[66].One of its reasons is that a PEO coating is persistently remade throughout its thickness by the discharge formation which happens as growing.Furthermore,there is a significant scope to use the electrical and chemical conditions to improve the coating microstructure[26,67–70].
Higher voltages are employed(~250–750V),generally related to an AC electrical supply such that numerous breakdown of dielectric occurs by the increasing thickness of oxide layer,such a big number of microdischarges that are all over surface of the work-piece in PEO process.In fact,PEO is taken from DC or AC polarization of a treated material being in high voltage,producing plasma microdischarges on the electrode surface that has high temperature(up to 4700–6700°C)in addition to high pressure inside its channels[71,72].
Many PEO coatings are composed of a pore band,an outer layer and an inner layer;so,a simplified structure of the cross-section is able to be suggested(Fig.4)helping the later measurements[73–77].Nevertheless,the three zones volume ratios differ amongst the distinct methods.The thickest coating possesses the thickest outer film.The little sized pores sound to be inclusions of gas that have been entrapped within solidification as micro-sparks vanished.Some channels of discharge are obvious for all of the open pores and coatings that are present at the surface and are not quite penetrating[78,79].Another significant feature of the coating morphology is porosity[80,81].Pore size and porosity can influence the coating characteristics including corrosion and thermal resistance;also,it is a major method to decline high pressures and temperature within the coating material crystallization and the surface film growth influenced via dissolved oxygen within the molten oxide.The PEO coatings pores are noticed to possess a helpful usage to utilize in Mg and Ti alloy based substrates in which porosity develops adhesion of cell to the surface[82].
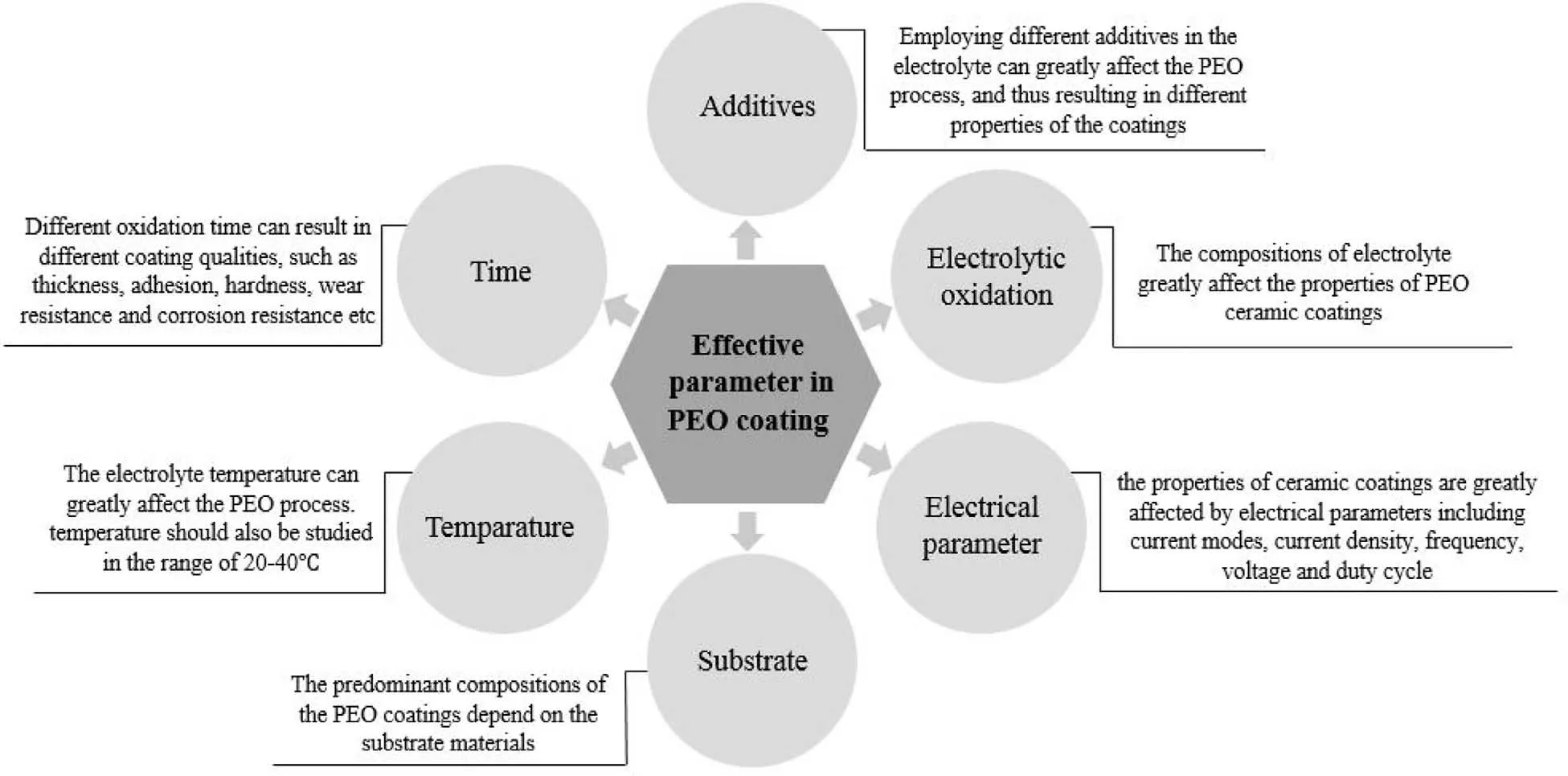
Fig.5.The effective parameters on the PEO coating.
The efficient factors on the properties of PEO coating are indicated in Fig.5.The PEO coatings properties are influenced via a lot of status such as electrolyte composition,electrical parameters,additive electrolyte temperature,the alloy nature,etc.[27,83–89].The systematic alterations to the topography of surface on account of the anodizing factors let different coating characteristics to be acquired.
Fig.6 displays the formation process of PEO coating on alloy of Magnesium.The anodized procedure has four steps:(1)the production of a thin and dense barrier(Fig.6(a));(2)the oxides initiation in the vacant Ca Mg matrix after the existence of sparks;(3)the oxide layer horizontal growth;(4)and at last the thickening of the PEO coating[90–92].
4.Ca-P coatings prepared on Mg by PEO
Phosphorus(P)and Calcium(Ca)are vital elements for human body and salts of Ca-P have been proven efficient to improve biocompatibility,corrosion resistance,bioactivity and metallic implants osseoconduction[93,94].So,Ca-P coatings have been a popular topic in the field of metallic implants.Shi et al.[95]have been reviewed the used calcium salts,the existing difficulties to prepare high calcium content PEO coatings and the corresponding strategies.Ca-containing solutions are categorized into inorganic salts of Ca and organic Ca salts.Organic salts of Ca contain ethylene diamine tetraacetic acid calcium disodium salt(EDTA-CaNa2,)[96],calcium citrate((C6H5O7)2Ca3,4H2O)[97],calcium glycerophosphate(C3H7CaO6P)[98],calcium acetate((CH3COO)2Ca)[99],and calcium gluconate(Ca(C6H11O7)2)[100].Inorganic salts of Ca including calcium hypophosphite(Ca(H2PO2)2)[101],calcium hydroxide(Ca(OH)2)[102,103],calcium fluoride(CaF2)[104],calcium bis(dihydrogenphosphate)(Ca(H2PO4)2)[105,106],and calcium carbonate(CaCO3)[107],have been already utilized as sources of Ca to acquire PEO coatings of Ca doped.Likewise,P-containing solutions can be utilized to produce PEO coatings.Such solutions are normally inorganic phosphates including Na2HPO4[97,99],K3PO4[104],and Na3PO4[99,108].In addition,phytic acid(PA),which originates in most cereal grains,legumes,nuts,oilseeds and has powerful chelating capability with di-and trivalent cations such as Mg2+,Zn2+,Al3+[109].has been used as an organic phosphorus-containing MAO electrolyte on magnesium alloy[110].
The effects of different phosphate salts on formation of coating have been investigated and the results indicate that the utilized phosphate salts specify the formation of coating via conductivity of solution and values of pH.It is hard to produce PEO coatings of high calcium content owing to many reasons.First,most of Ca salts including Ca(OH)2,CaF2,CaCO3,and(C6H5O7)2Ca3show little solubility in aqueous solution.Only a few organic Ca salts,such as EDTACaNa2,C3H7CaO6P,and(CH3COO)2Ca,can have proper aqueous solubility.Second,Ca salts can be mixed with inorganic phosphates to produce calcium phosphate precipitates[97].Third,the utilized PEO solutions can be divided into alkaline and acidic electrolytes.For Mg alloys,alkaline solutions are normally utilized to produce PEO coatings on their surface because of their chemical activity[111].Zhu et al.[105]studied calcium phosphate monobasic(Ca(H2PO4)2)effect in different concentrations(4,6,8 and 10g/l).The results of electrochemical impedance spectroscopy in electrolytes containing sodium hydroxide and sodium phytate showed that the corrosion resistance of the coatings increased by increasing Ca(H2PO4)2concentration.
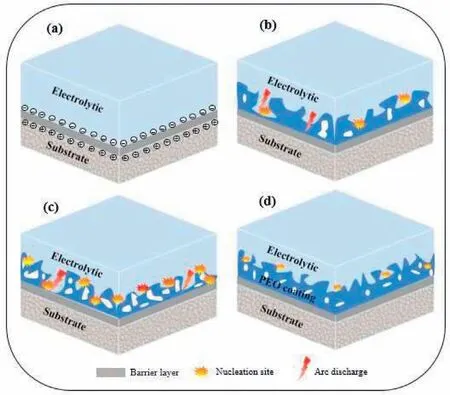
Fig.6.Formation mechanism illustration during PEO.
The production process for calcium phosphate crystals must be influenced via two parameters:calcium phosphate nucleation and Ca and P diffusion from inner film towards surface of film[112].The surface porous structure might play a major role in inducing nucleation of calcium phosphate.The production mechanism of the calcium phosphate coatings depend on the pH of the treatment electrolyte.Following corrosion reaction happens immediately after immersing an Mg substrate into the treatment electrolyte.
Due to the corrosion reaction,the pH on the surface of Mg rises.The pH-increase shows the quick nucleation of HAp with simultaneous production of Mg(OH)2on the surface of Mg.Generally,the coating incorporation with positive ions was proceeded with a cascade of the following partially overlapping occurrences:hydrolysis,negative charging,and electrophoresis.So,Ca2+could be present in the solution for a long time.Within the PEO procedure,the applied high voltage produced an electric field between the cathode and the anode making the driving force for the P and Ca to migrate to the anode of Mg before participating in the production of the coatings.It must be considered that the OH−ionic radius is smaller than that of theand particles of calcium.Consequently,the movement speed of OH−is higher than that of theand particles of calcium[113].Within the PEO procedure,the Mg anodic surface is first encapsulated with OH−,followed by theand particles of calcium due to the ions different distribution on the surface of the Mg anode,OH−is priority to enter into the microdischarge and after that isand particles of calcium.This leads to an agreement with the elemental distribution in the PEO coating.The ceramic coating growth is due to the applied dielectric breakdown voltage within the PEO procedure that is accompanied with the micro-discharge events feasible on the surface.Indeed,the micro-discharge lifetime is less than 1ms,but the instantaneous temperature in the micro-discharge channels can vary from 800K to 3000K[114]that is adequate to cause the substrate to be molten and produce ions.So,Mg2+was produced via the Mg alloy the dissolution driven by existence of the electric field.Mg2+reacted with OH−leading to the production of MgO.
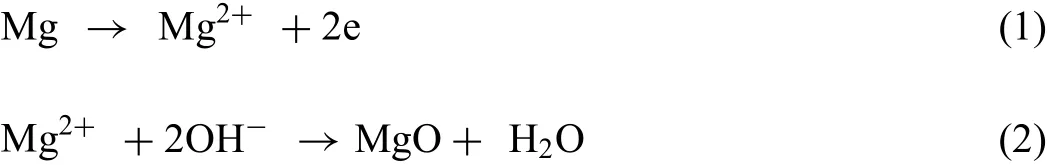
The micro-discharge intensity augments by rising voltage.Thus,the zone for every micro-discharge bets bigger by rising voltage.Therefore,more ions in the solution could be absorbed via the micro-discharge channels.Under the high temperature effect,calcium particles,and OH−can react and form HA based on the following equation.

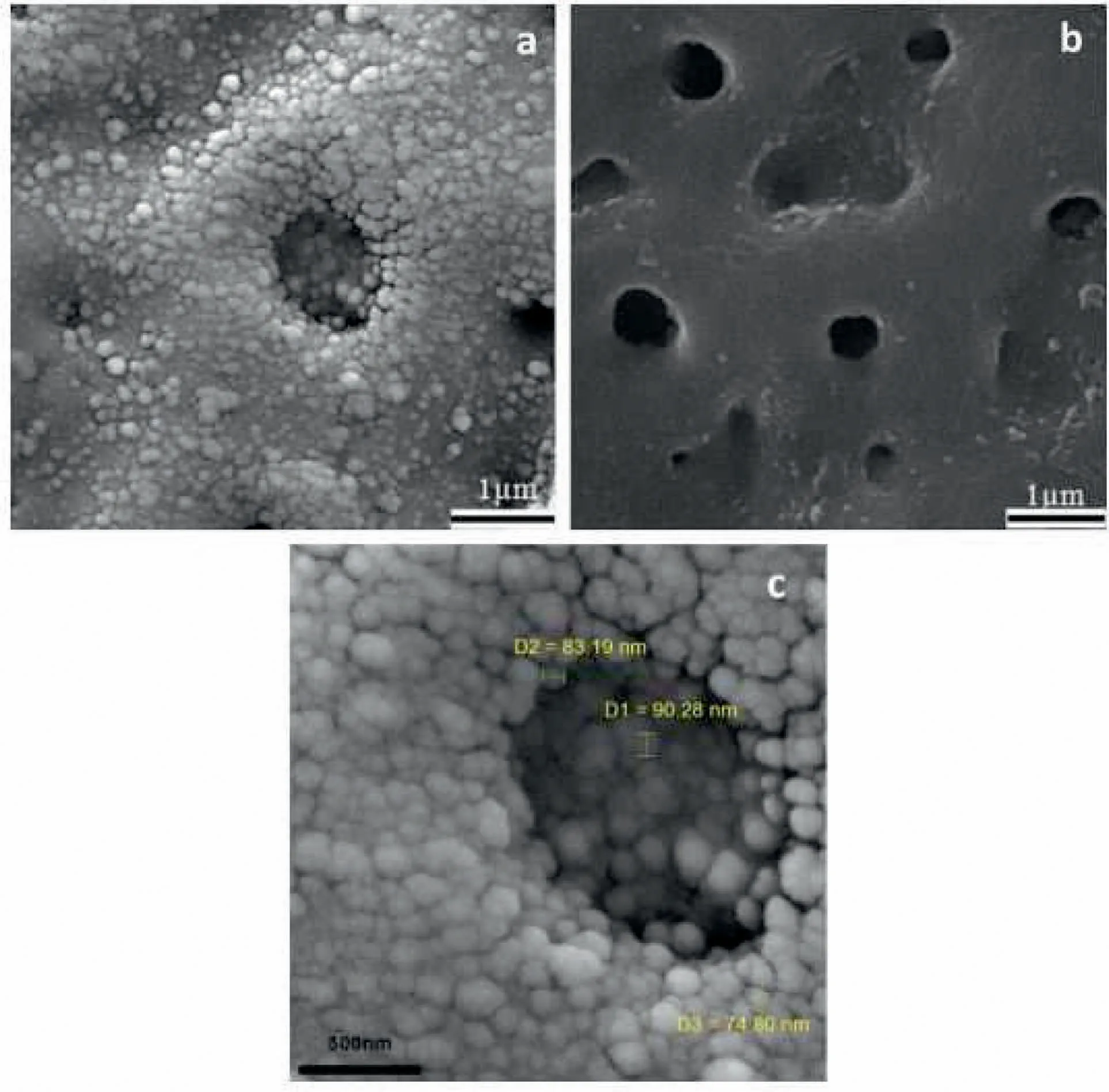
Fig.7.(a)SEM images of nanocomposite film,(b)pure PEO film and(c)higher magnification of(a)[117].(With permission from[117];License Number:4794660713493,Mar 23,2020,Elsevier).
The temperature of micro-discharge channels can be augmented via employing a higher voltage that could accelerate the amorphous phases transformation into crystallines.
4.1.Microstructure and morphology
In situ-grown PEO coatings on Mg-Ca alloys show great interfacial adhesion and proper biocompatibility[115]and they have porous microstructures and low levels of porosity that are useful for growth of tissue.Basically,PEO coatings have two kinds of pores:through-pores and non-through pores.The next one has open pores and pores of blind or isolated.The open pores,settled in the outermost film are straightly subjected to electrolyte.The single pores in the center of the coating,straightly get in touch with the electrolyte within the immersion primary step[116].Nevertheless,the through-pores being located all over the coating prepare routes for the electrolyte to penetrate into the substrate and so causes the substrate to have a direct touch with the electrolyte.
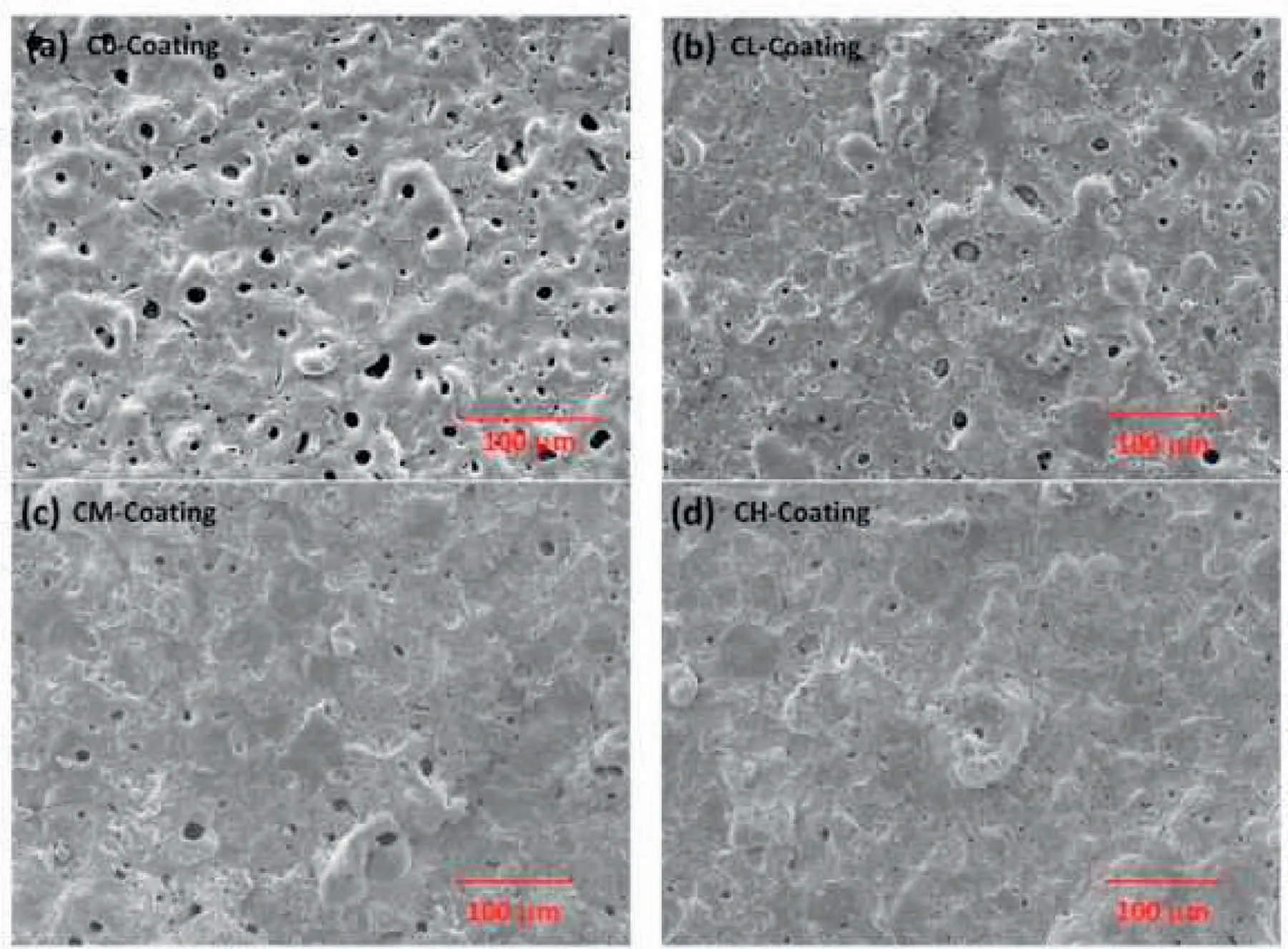
Fig.8.Surface morphology of PEO coatings with and without HAp(a)C0-,(b)CM-,(c)CL-and(d)CH-coating[121].(With permission from[121];License Number:4794660975736,Mar 23,2020,Elsevier).
Seyfoori et al.[117]proved that,the Ca-P concentration influenced the morphology of biofilm after the PEO procedure.The scanning electron microscopy(SEM)images of the surface morphology of coatings that were illustrated in Fig.7 indicate the nanoparticles(NPs)incorporation into the grown layer in comparison to pure PEO specimen[117].NPs entrapment in the growing ceramic layer can be done efficiently via eruption and perturbation of molten oxide in every spark that happens at the samples surface within the PEO process.Considering prior publications[91,118],for particles incorporation in these coatings,their ongoing existence next to the anode is required too.One of the most important parameters that can bring the NPs in the affinity of the anode,is electrophoretic force[119].While it is observed in Fig.7(a and c),the repletion of NPs in the affinity and in the pores is more than other areas.According to Fig.7(c),near the single NPs,some of the agglomerated NPs can be observed near and inside the porosities.This occurrence may be because of the molten oxide fluctuation at the Mg alloy surface.Molten oxide Fluctuation and its quick solidification within every spark can result in the reposition of the absorbed NPs.Xu et al.[120]presented DCPD in alloy of Magnesium that offered the Ca-P coating is an efficient technique to amend the bioactivity of surface.Yang et al.[121]investigated the consequence of adding HAp NPs(0,5,10 and 20g/L)on characteristics of pure Mg.Fig.8 illustrates the morphology of surface for PEO coatings produced in presence and absence of different HAp particles concentrations[121].As can be seen,all the produced coatings show the common morphology of surface for PEO films,characterized using micro-cracks,micropores and molten oxide deposition.This customary type of volcano-like morphology is produced because of the molten oxide ejection out of the discharge channels and the latter solidification via the solution as it gets to the surface of coating[122,123].Both size and number of the pores on the surface feel like declining and the surfaces get denser by rising the amount of HAp particles.Ma et al.[124]published that the surface of PEO coating indicated a glossy microgram and higher ratio of void,while the PEO HAp coating showed a rougher surface having white specks and fairly lower ratio of void.It can be observed that several little pores on the PEO–HAp surface were coated,leading to the lower void ratio than PEO coating and this could ascribe to HAp additives that were absorbed on the surface and sealed little pores produced on the coating of PEO–HAp.
Tang et al.[125]studied coatings having HAp on surface of Mg alloy at various voltages.Five used voltages were opted to produce the PEO coatings.The consequences show that the number of micro pores in the layers rises but their dimensions decline after applying a higher voltage.Surface morphologies of the PEO coatings prepared distinct voltages as indicated in Fig.9[125].Significant value of micro pores and several micro cracks can be observed on the coatings surface.Micro pores were produced as the gas bubbles and molten oxides were ejected out of the micro-arc channels of discharge(MAD)and micro cracks were created from the thermal stress within the rapid solidification of the molten oxides in cool solutions[126].Also,it can be seen that the number of micro pores on the PEO coating declined but their size augmented at a higher voltage.The size increase is ascribed to the high-energy sparks occurrence as the anode is at a stronger electric field.Meanwhile,more mass of product was ejected to produce discharge channels that later deposited on the alloys surface leading to a coarser and thicker coating.Concentrations of Ca and P and their ratios(Ca/P)within the PEO layers produced at different voltages from 250 to 500V are indicated in Fig.10[125].Concentration of Ca and the ratio of Ca/P are discovered to be augmented with the applying voltage.The tendency of P concentration considering the rising voltage,nevertheless are less clear.At 500V,the Ca/P ratio is about 1.68,that is alike the amount of HAp(1.67).Also,the consequences show that the primary concentration and the ratio of Ca/P in the PEO coatings can be simply controlled via changing the voltage of anodic[125].
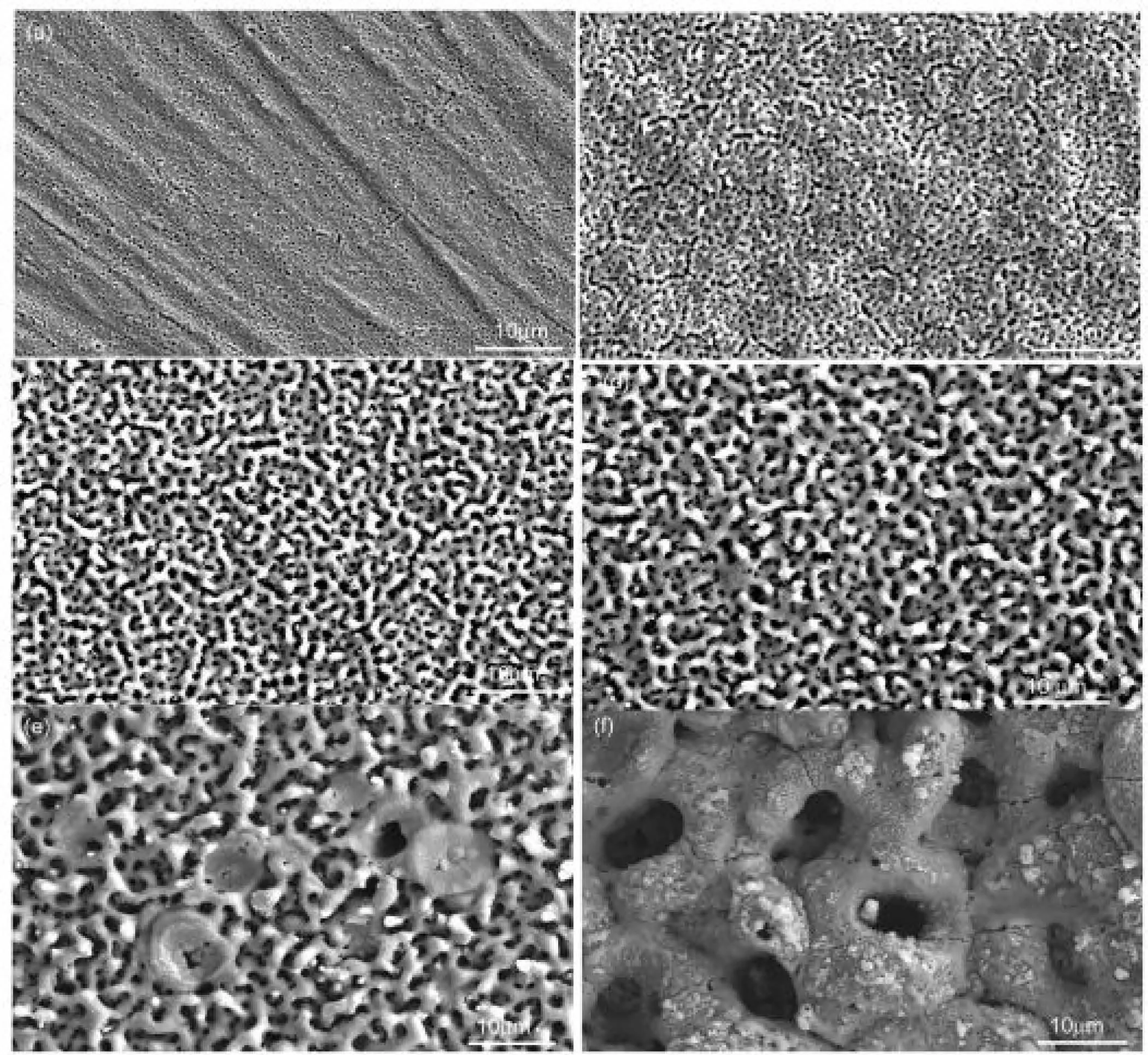
Fig.9.Surface morphologies of the PEO coatings formed with different applied voltages:(a)PEO 25,(b)PEO 30,(c)PEO 35,(d)PEO 40,(e)PEO 45,and(f)PEO 50[125].(With permission from[125];License Number:4794661232637,Mar 23,2020,Elsevier).
4.2.Coating thickness
Based on the growth process,PEO coatings grow to the contrary directions concurrently,containing inwards growth towards the substrate and outwards growth towards the interface of coating/solution[127].The inwards growth can be ascribed to the oxygen diffusion to the substrate of Mg,whereas the outwards growth of thickness is due to the ongoing ejection of molten material on the surface of coating and its following solidification.The outer film is much thicker than the other two parts of the coating,having more than half of coating thickness.So,it is possible that the outer film cannot prepare enough protection of substrate against corrosion.Ma et al.[124]proved that the present defects in the outer film are inclined to be sealed by adding particles of HAp.In Fig.11(a and c),morphology of the cross-sectional for the PEO and PEO–HAp coatings were completely clear and both of them indicated a contiguous and rough feature[124].Nevertheless,the PEO coating was more uneven in which the ratio of surface void was higher than that of PEO–HAp coating.The other major characteristics of the PEO specimen were numerous inlaid pores within the coating,but every pore was independent instead of intercommunicating.Contrarily,micropores in the PEO–HAp coating that the rough feature of the cross-section was only shown on the superficial film instead of inlaid inside the coating.The coating of PEO–HAp was compactly combined with the substrate of Mg alloy as no cracks or pores were discovered between them,showing that HAp additives could fill in the pores of discharging within the PEO procedure.
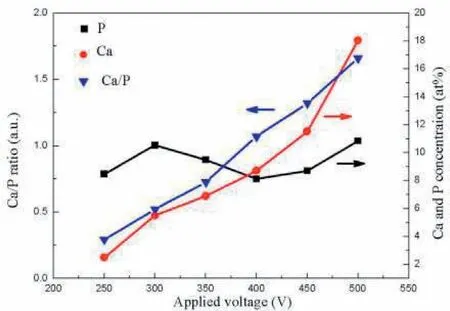
Fig.10.Effects of the applied voltages on the Ca,P contents and Ca/P ratios of the PEO coatings[125].(With permission from[125];License Number:4794661232637,Mar 23,2020,Elsevier).
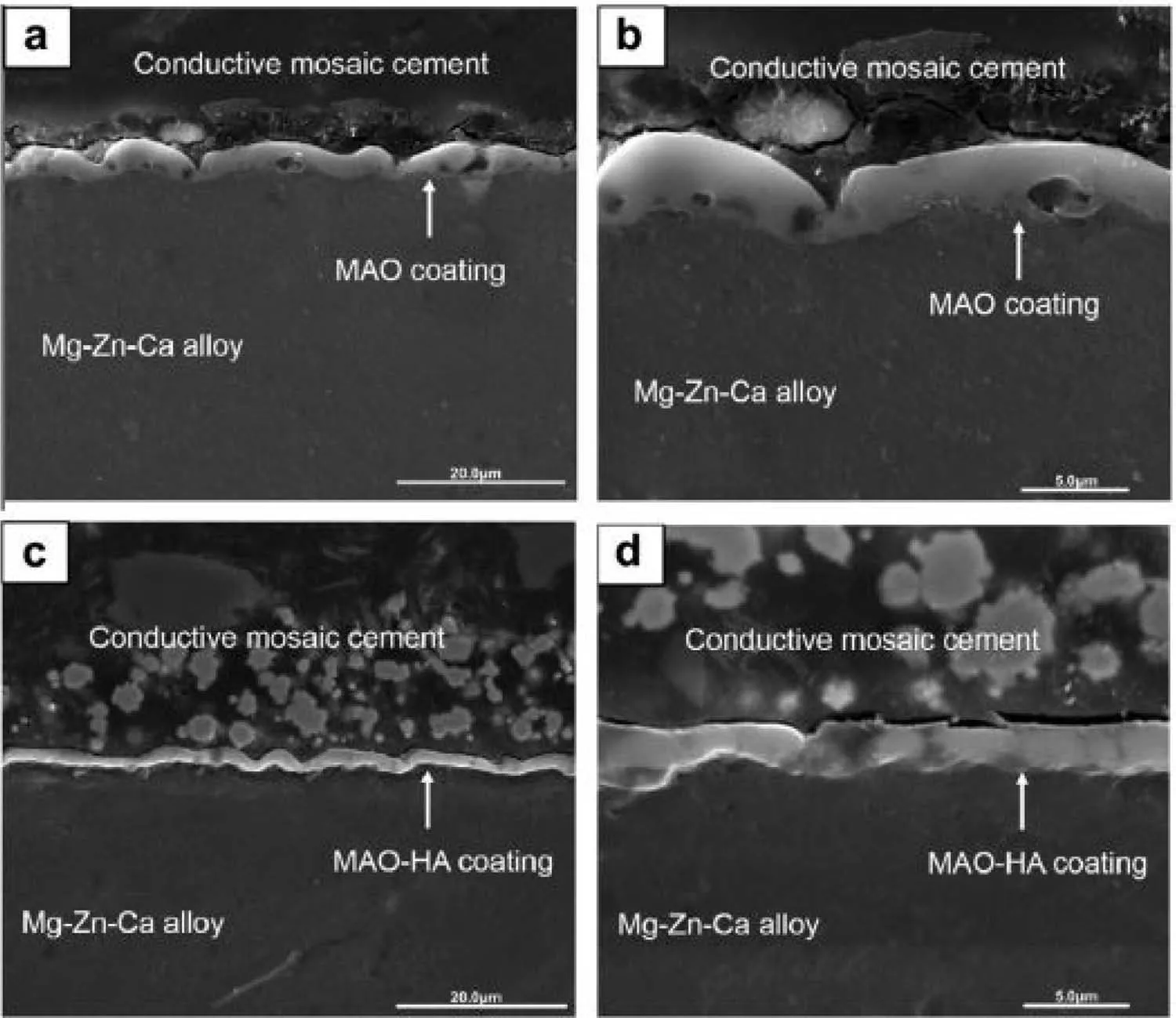
Fig.11.Cross-sectional morphologies of PEO coating(a and b)and PEO–HAp coating(c and d)under SEM[124].(With permission from[124];License Number:4794670007170,Mar 23,2020,Elsevier).
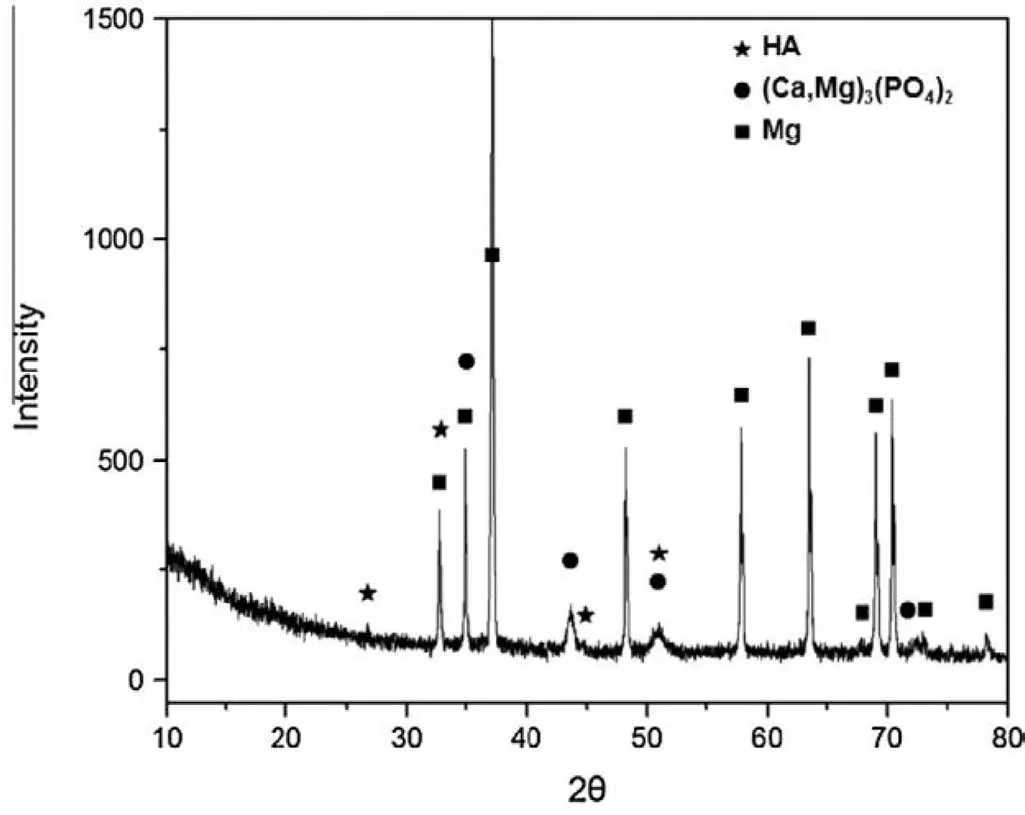
Fig.12.XRD patterns of the PEO–HAp coating synthesized on the substrate of Mg–Zn–Ca alloy[124].(With permission from[124];License Number:4794670007170,Mar 23,2020,Elsevier).
Chaharmahali et al.[4]investigated the influence of calcium hydroxide concentration on the PEO coating and corrosion resistance and surface microstructure of these coatings on AZ31B alloy.The results obtained from coated specimens showed that the thickness of the specimens augmented by rising concentration of calcium hydroxide.Also,bigger drain channels cause particles to penetrate much more easily into them.So,the rate of the oxide film formation rises that leads more molten material to be deposited on the surface.Yang et al.[121]showed that the present defects in the outer film incline to be sealed by adding and rising the HAp particles concentration.This optimization in morphology of cross-section is in a good agreement with corresponding microstructure of surface that shows the HAp particles could influence the behavior of discharge and alter the composition of coating.
4.3.Phase and chemical composition of the coatings
The particles incorporation into PEO coating is highly dependent on the size of particle and their melting temperature[91,128].In comparison to the pores sizes on the surface of coating,most of the HAp particles are little enough to penetrate into the coating via the channels of discharge and being deposited in the pore band of the coating.The channels of discharge are also filled up with solution as well as the entrapped particles.As the breakdown of the oxide coating through the spark discharge happens,maximum high pressure and temperature are demonstrated[129].Ma et al.[124]discussed the addition of HAp particles.The XRD pattern of the PEO–HA coating was shown in Fig.12.The result of analysis showed that in addition to the diffraction peaks from the substrate of magnesium alloy,the diffraction peaks of(Mg,Ca)3(PO4)2and HA were eliminated,showing that(Mg,Ca)3(PO4)2and HA were formed as the principal components of the coating on Mg–Zn–Ca alloy after PEO–HA.
The coating TCP phase was mostly from the decomposition of HAp and the similar conclusion was achieved[117].As HAp andmigrated to the Mg anode surface under the electric field within the procedure of PEO.Meanwhile,the temperature next to the anode that was produced by the intense MAD due to the high voltage can change from 525°C to 2725°C[130].As the temperature surpassed 1325°C,HAp would produce decomposition and bring forth TCP:
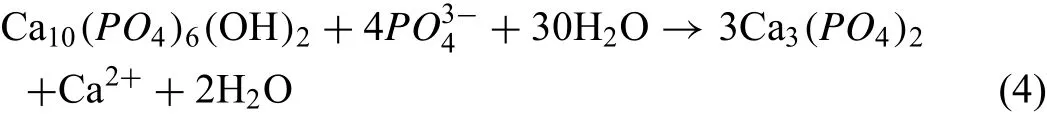
In the meantime,the high temperature may make Mg2+dissolve out from the substrate within the intense MAD and so Mg2+reacted with PO43-in the high temperature media and produced the phase of magnesium phosphate:
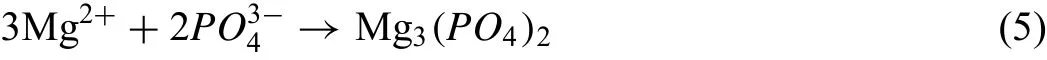
Tang et al.[131]investigated coating with HAp are produced via PEO treatment on alloy of AZ31 Mg at different times of treatment.XRD patterns of PEO of the surface of coatings being formed at various durations are presented in Fig.13[131].Mg,MgO and HAp phases are obtained in the PEO coatings via test of XRD.Same wide range of patterns is produced for all the specimens.The spectra indicated just reflections related to HAp.The composition of coating is composed of MgO as a main phase and HAp as a minor phase.The Mg intensity considerably declines by rising the duration time.The intensity of MgO and HAp rises by augmenting the duration time.The coating crystallinity within PEO is also related to the discharge intensity.Higher energy needed for breakdown of the coating and higher intensity discharge can be seen on the Mg surface within PEO procedure for longer time of growth.This makes a higher temperature in the PEO procedure that favors a better HAp crystal.
Uddin et al.[132]investigated the effect of HAp NPs addition in various values of current density(3,6 and 13mA/cm2)on properties of AZ31 magnesium alloy.Fig.14 indicates SEM micrographs and EDS spectra of HAp coating produced at three various current densities[132].Regular plate-like HAp crystal structures were seen(Fig.14(a,c and e))for all current densities.Platelets may be correlated to the orientation of plane in pseudo-hexagonal crystal system[133].As the current density is low(up to 6mA/cm2),the crystals sound more oriented and regular and lapped near each other,making a structure of microporous.As the current density rises to 13mA/cm2,the size of crystal augments and their distribution seems to be more randomized.This might produce a more macroporous structure.Fig.14(b,d,and f)reveals EDS spectrums showing primary composition of a selected region of HAp coating.As predicted,like O,mainly the basic elements–Mg,P,Ca exist in HAp coating.As the current density rises from 3 to 6mA/cm2,the percentage weight of Ca+P augments quickly.Table 2 indicates the information of substrate,thickness,electrolyte composition,phase composition of coatings and condition of operation in Ca-P coatings[4,39,121,131,134–139].
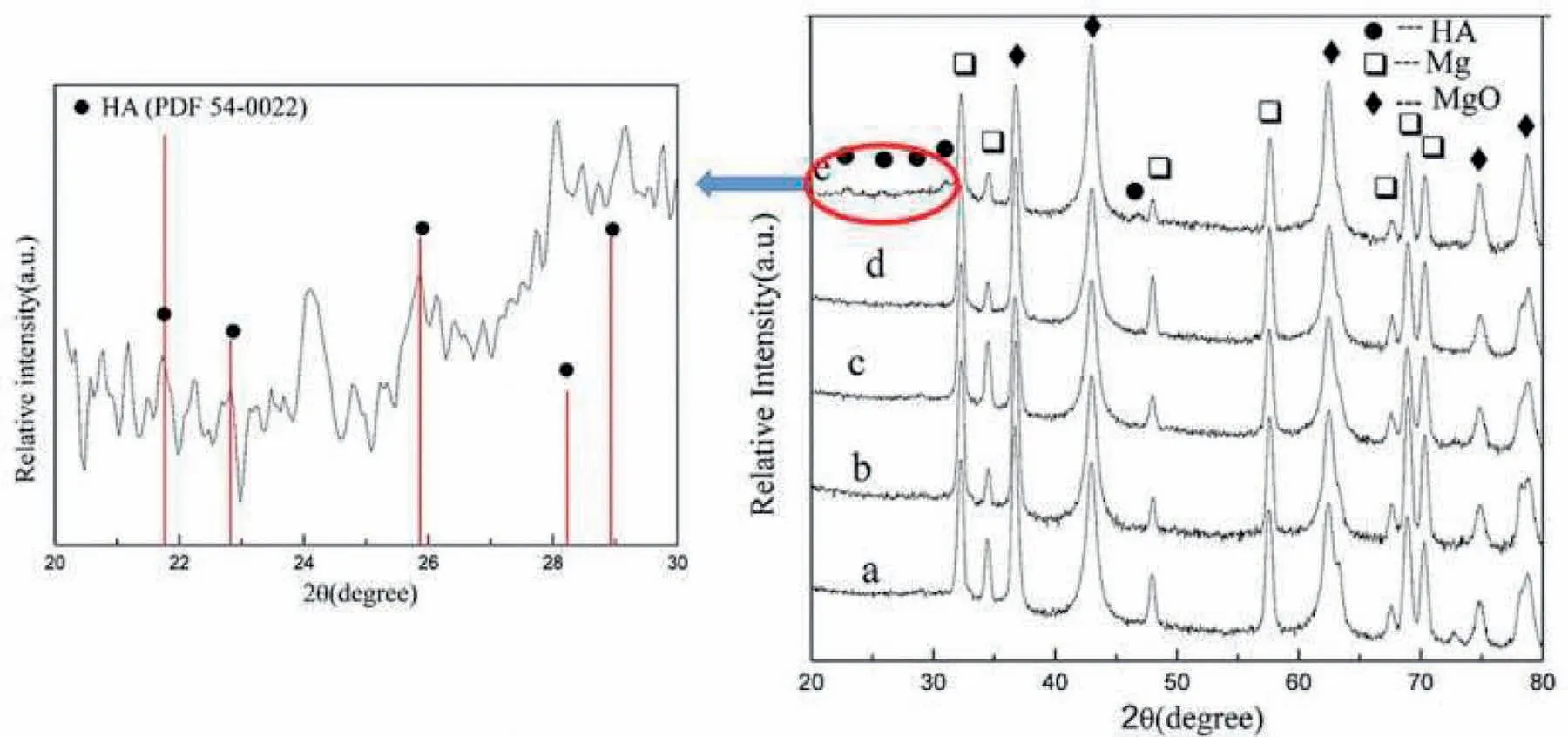
Fig.13.XRD patterns of the PEO coatings:(a)5,(b)10,(c)20,(d)40,and(e)60min[131].(With permission from[131];License Number:4794670261721,Mar 23,2020,Elsevier).
4.4.Corrosion behavior
The PEO coating protective behavior has been investigated by many investigators[140].It has been proved that corrosion resistance is augmented by applying PEO coating and so the function of corrosion protection is enhanced[141–143].There are a couple of kinds of models subjecting the corrosion behavior of PEO coatings formed on alloys of Mg which that are according to much research about the PEO coatings microstructure.The first kind is the model of chemical dissolution.Guo et al.[144]suggested that the PEO coating degradation on an alloy of AZ91D had two steps:the incorporation and the growth of metastable pits.Gu et al.[145]suggested a model for the corrosion behavior of the MgO element in two-layered PEO coatings applied on an alloy of AZ31 in SBF.Nevertheless,Zhang et al.[57]suggested a model for a PEO coating on an alloy of AZ31 with three films:an outer porous film having big and deep pores,a compact inner film and a thin interlayer between the substrate of AZ31 alloy and the dense film.Significantly,the models prohibit the effect of through-pores on degradation.Electrochemical corrosion created by the electrolyte penetration into the substrate by through-pores within the coating has been overlooked in the primary step of immersion.The electrochemical corrosion model is the second kind of model is.Mostly,the substrate electrochemical corrosion happens because of the solution transport into the substrate through micro-cracks and through-pores in PEO coatings on alloys of Mg.Shi et al.[146]depicted the great function of the PEO coating to the substrate alloy higher corrosion resistance and the lower density of through-pores that corrosion started at the interface of the substrate and PEO coating.
In general,biodegradable implants are supposed to present enough mechanical integrity and corrosion resistance[147].Nevertheless,attention has slightly was attracted towards bioactive coatings that can develop the healing procedure with minimum adverse effects as preparing enough corrosion protection by declining the rate of degradation is still the initial strategy.According to this demand,considerable investigations have been carried out on producing PEO coatings with biologically friendly compositions[10,93].It is realized that the ratio of Ca/P in PEO coatings depend on the parameters of procedure,i.e.a longer processing time leads to a higher ratio of Ca/P in the PEO coating.Except for the research activities about the procedure optimization parameters to prepare stable Ca and P phases in PEO coatings,other efforts are made on the development of coating bioactivity using post treatments[148].Not only can the bioactivity be amended,but also the corrosion resistance can be also boosted because:(a)the pores formed in the PEO coating can be sealed via the top film and(b)the bioactive film itself makes an extra barrier film protecting the substrate against corrosion attack[149].
The existence of micro pores and micro cracks in the form of ceramic-like PEO structure is unavoidable for two purposes.On the one hand,ordinarily electrical discharges,the intense and the formation of gas bubbles on the surface result in many microchannel which some of them do not get solidified within the PEO procedure[150].Furthermore,a considerably lower Pilling-Bedworth(P-B)for Mg oxide than the substrate of metal results in a loose Mg oxide layer produced on the Mg surface[151].Also,the micro-cracks in structure of oxide coating are produced because of the thermal stresses made by the quick solidification of molten oxide inside solution within the cooling.According to Fig.15,the pores produced by electrical microdischarges make the penetration of corrosive ions and as a result the protective coating failure[68].PEO coating corrosion resistance in corrosive media and for long durations depends on different parameters like thickness,composition and the present defects inside the coating.Irregular cracks and pores in the oxide film result in the production of penetration directions for corrosive ions and so decrease in protective behavior of PEO coatings to corrosive agents[60,152].
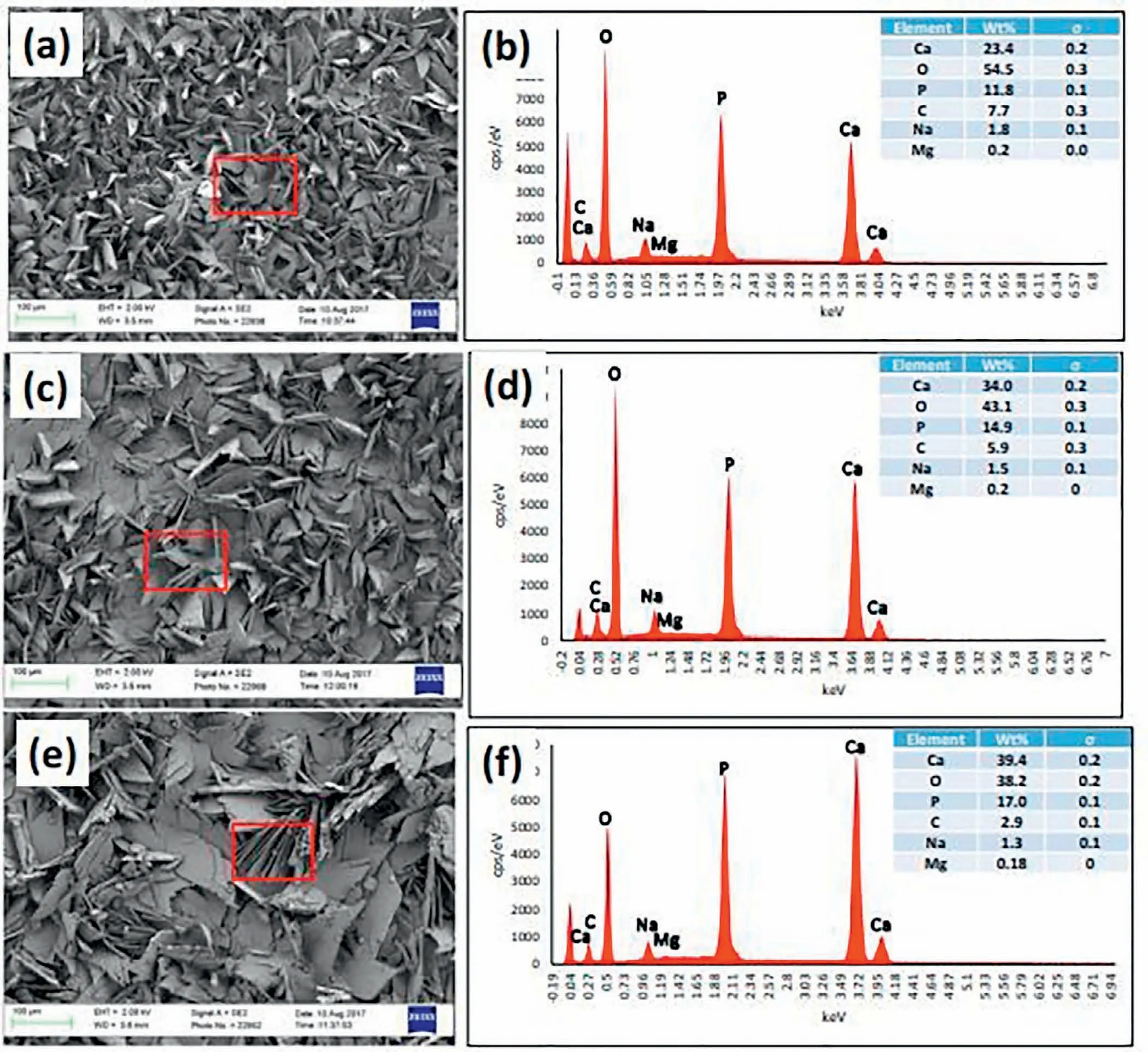
Fig.14.SEM image and EDS spectra of HAp coated AZ31B surface topography at a current density of(a,b)3,(c,d)6,and(e,f)13mA/cm2.Rectangular box selection indicates the area for EDS analysis[132].(With permission from[132];License Number:4794670529466,Mar 23,2020,Elsevier).
Chaharmahali et al.[153]investigated the effect of HAp NPs addition on corrosion resistance of Mg alloy.Fig.16 shows the curves of potentiodynamic polarization(PDP)for coated and uncoated samples at different concentrations of HAp after half an hour immersion inside SBF electrolyte[153].As the AZ31B alloy was coated by ceramic coatings all plots of the PDP for coatings have moved to a bigger negative potential and lower corrosion current density than that of for the substrate.This indicates a thermodynamic intention for corrosion happens by using a ceramic coating,while the kinetics of corrosion is declined.
Chun and co-workers[154]provided Ca-P coatings on alloys of Mg via electrochemical deposition and the coated substrates corrosion resistances in Hank’s electrolytes were considerably amended.Lin et al.[155]proved that the coating with HAp was denser.The HAp coating had better formation of apatite ability and long-term corrosion resistance compared with the HAp-free coating that may come from the amended structure and composition of coating.Fig.17 illustrates the plots of Nyquist and Bode for thecoatings with and without addition of particle[117].The outer film resistance augmented by 3 times owing to particle incorporation.The effect of Ca-P addition on the corrosion behavior of PEO coatings is conveyed below in addition to Table 3[4,117,131,134,137–139,153,156–158].
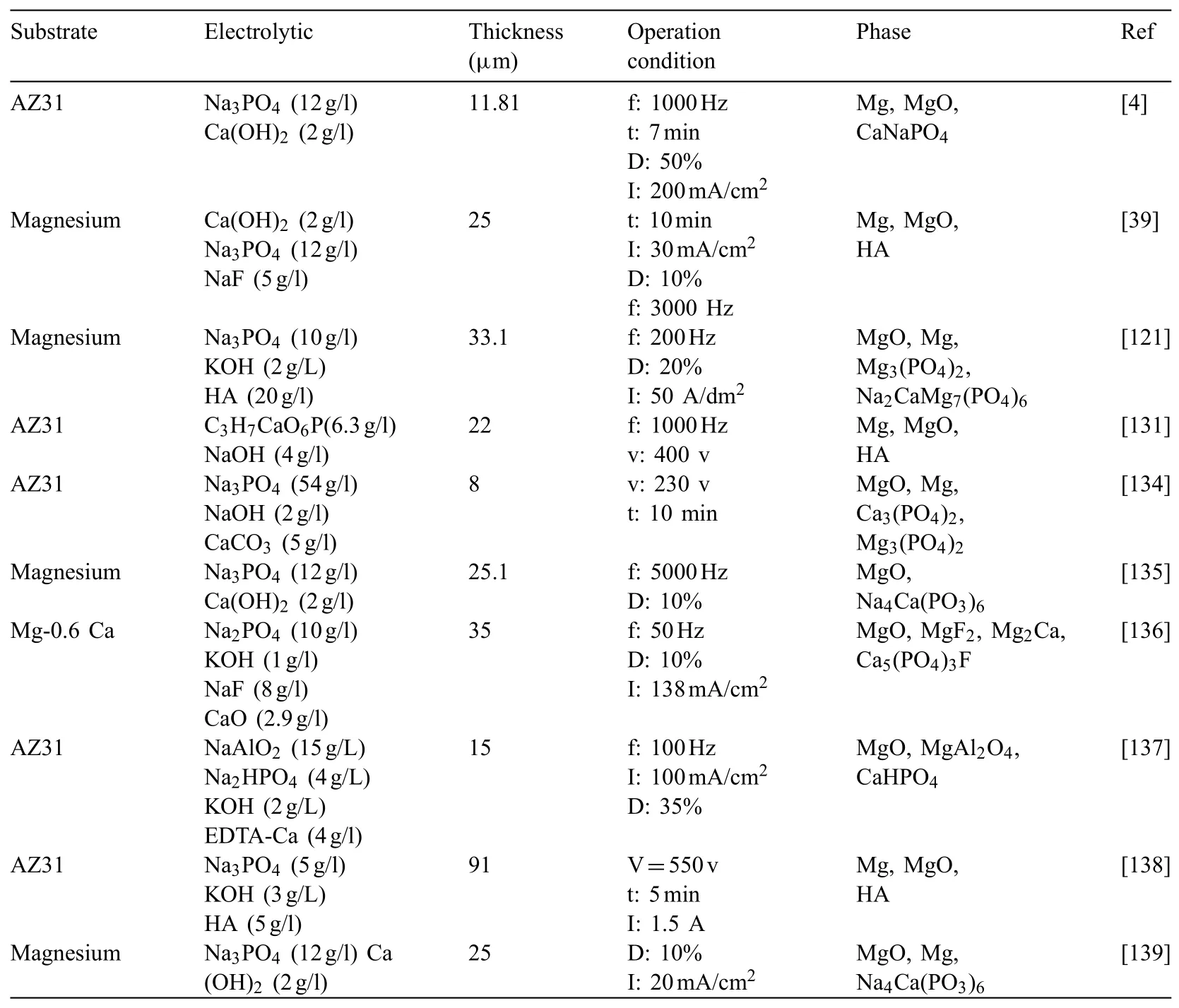
Table 2Detailed information of the substrate,electrolyte,coatings phase composition,thickness and Operation condition in Ca-P coatings.
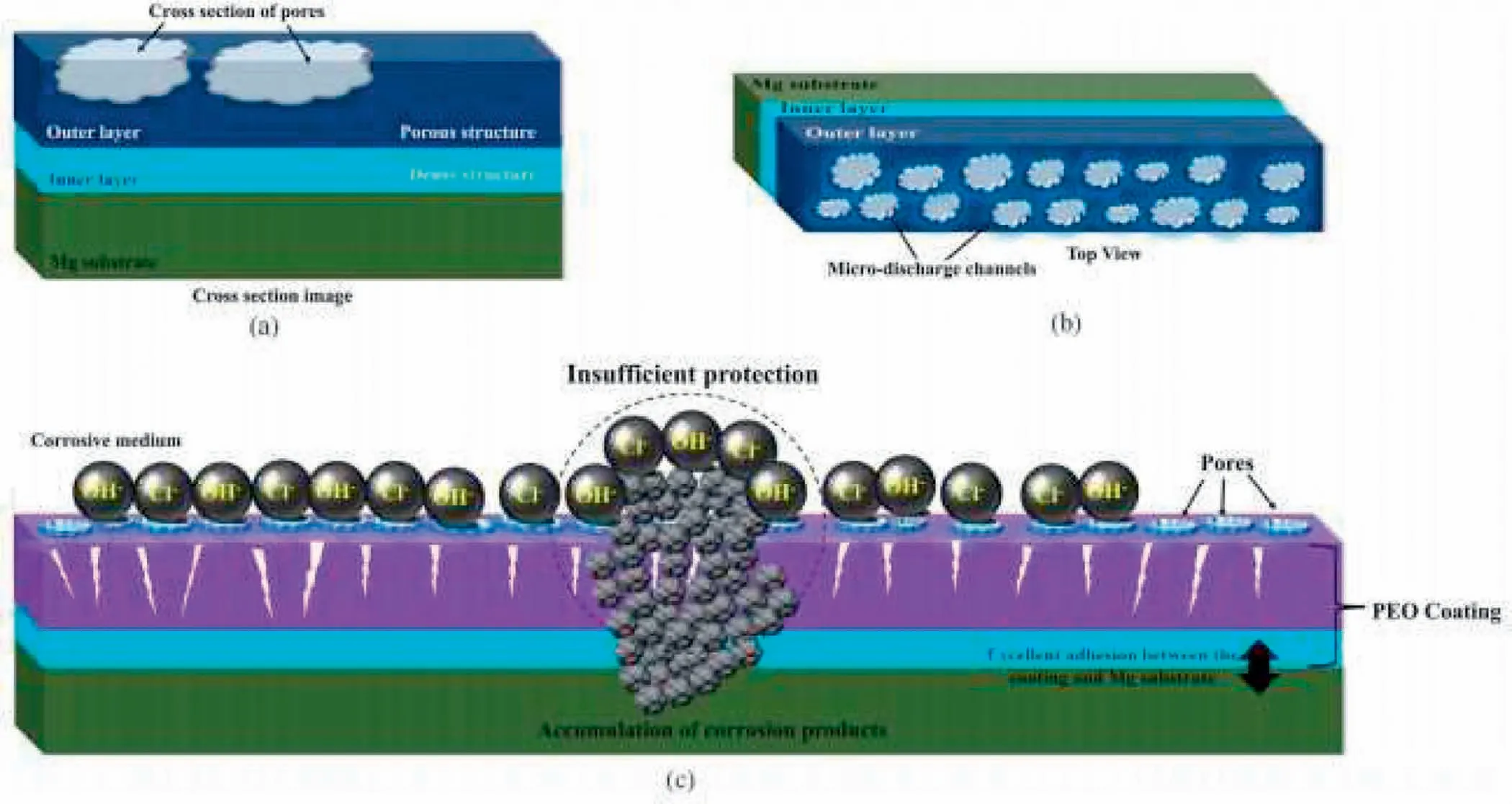
Fig.15.(a)Cross-sectional view;(b)top view of the PEO coating structure;(c)penetration of corrosive agents through pores and coating degradation[68].(With permission from[68];License Number:4794670726533,Mar 23,2020,Elsevier).
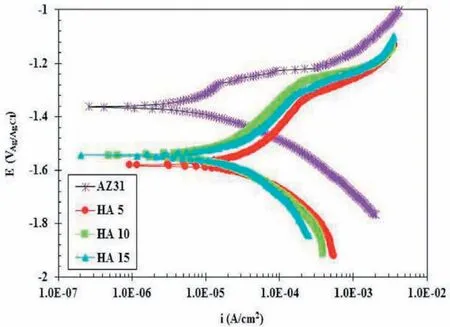
Fig.16.PDP curves at distinct concentrations of 5,10 and 15g/L of HAp in SBF solution for 30 min[153].
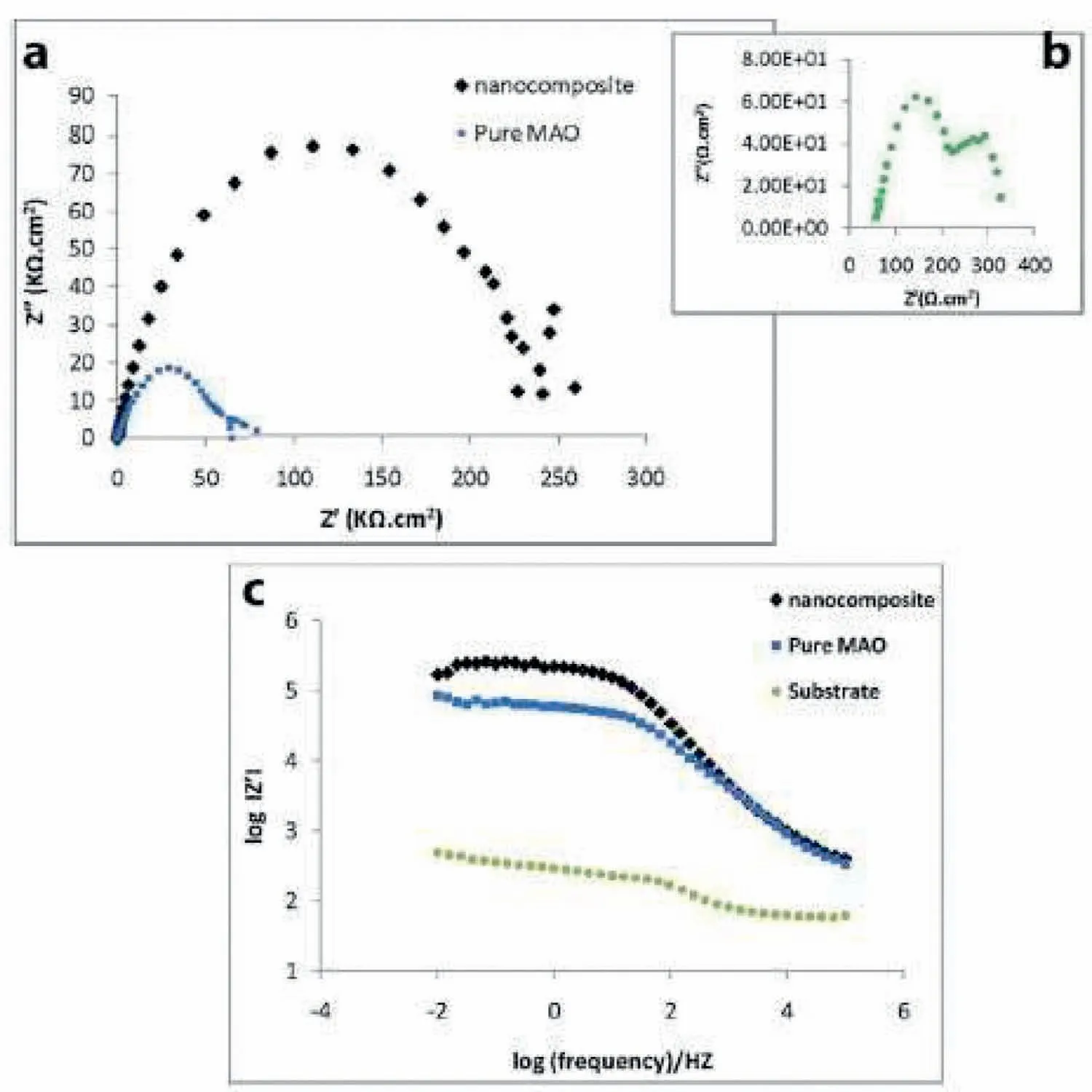
Fig.17.(a,b)Nyquist and(c)bode plots of pure PEO,nanocomposite films and bare Mg alloy[117].(With permission from[117];License Number:4794660713493,Mar 23,2020,Elsevier).
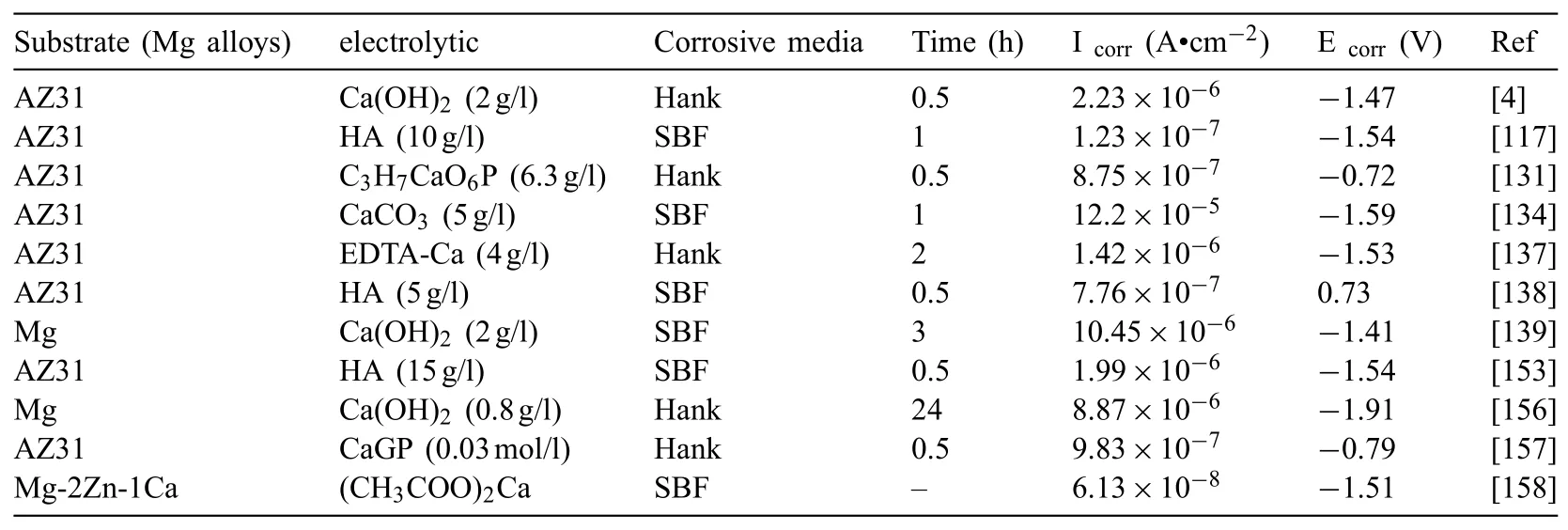
Table 3Influence of particle addition on corrosion properties.
4.5.Biocompatibility and bioactivity properties
Bioactivity is feature of the implant that induces biological integration of living hard and soft tissues.The bioactivity core mechanism is surface biomineralization of calcium phosphate crystallites coated on implant.The biomineralization is initiated using surface functional groups electrostatic interaction with the phosphate and calcium ions in the solutions.SBF is a calcium phosphate electrolyte supersaturated with respect to apatite.A chemical stimulus of surface functional group can trigger the apatite nucleation.Forming ability of Apatite can be correlated with two distinct factors.The first one can be related to the HAp bioactive nature in the structure of oxide layer so that it can develop bone like apatite nucleation via the hydroxyl and other charged groups including Ca2+andon its surface[159].The second factor that can affect the value of apatite deposition is the roughness of surface for the nanocomposite coating.The amount of efficient existing areas on the surface of the samples in contact with the SBF solution by rising the surface roughness of the nanocomposite layer in comparison to pure PEO layer and so the rate of biomimetic heterogonous apatite nucleation is augmented.So,HAp NPs addition to the oxide layer can efficiently develop bone like formation of apatite and as a result can result in stronger chemically bonding between the host tissue and implant through a rich Ca-P film.Also,existence of minor values of bone like apatite on the surface of pure PEO layer can exhibit its bioactivity.One part of the deposited apatite is owned by the corrosion nature of Mg that can rise the pH of SBF near the surface and as a result,lead to apatite nucleation[117].Osseointegration of implant with the neighboring tissues is a major parameter for successful fixation and implantation.Thus,the apatite-forming ability of the treated specimens in SBF is considered as a sign of osseointegration ability.
Fig.18 indicates images of SEM for the PEO-ST coating after soaking in SBF for 24 h and 72 h[142].After being immersed in SBF for 24 h,several sphere-like particles were deposited on the micro-flowers and attached on the microrods and aggregated simultaneously.But,the original microrods and microflowers can be still obviously differentiated without remarkable coating on the surface.The original 3D flowerlike architecture is vanished by rising the immersion time to 72 h.A coralloid surface can be seen under SEM.As the layer is entirely coated by a precipitate film.The purposes for the changing the morphology of surface after immersing for 72 h are not that clear.Nevertheless,this might ascribe to the apatite growth in preferred orientation.
Yang et al.[121]investigated the biocompatibility behavior of coatings formed at different concentrations of hydroxyapatite in a simulated body fluid.They found that as the concentration of hydroxyapatite increased,the ability to form biological apatite on the surface increased so that the porosity was completely sealed and therefore increased the corrosion resistance of the coating after immersion.Zhu et al.[160]demonstrated the bioactive and biodegradable properties of hydroxyapatite coatings.Immersion tests were carried out at different times.The results showed that in the primary times of immersion,the surface remained with uniform depositions.Cracks and cavities were seen on the surface by increasing immersion time and at the same time many depositions accumulated.At the end of immersion,deep cracks were created on the surface compared to other samples.EDX analysis of calcium and phosphorus elements after immersion showed that depositions containing more calcium and phosphorus content first decreased and then increased.ions were released in the primary times of immersion and so the amount of phosphorus in the coating decreased and cracks were observed on the surface.Then the supersaturation degree of the simulated biologic fluid was augmented,leading to precipitation of biological apatite crystals having simultaneous incorporating of different ions exhibited in the SBF.
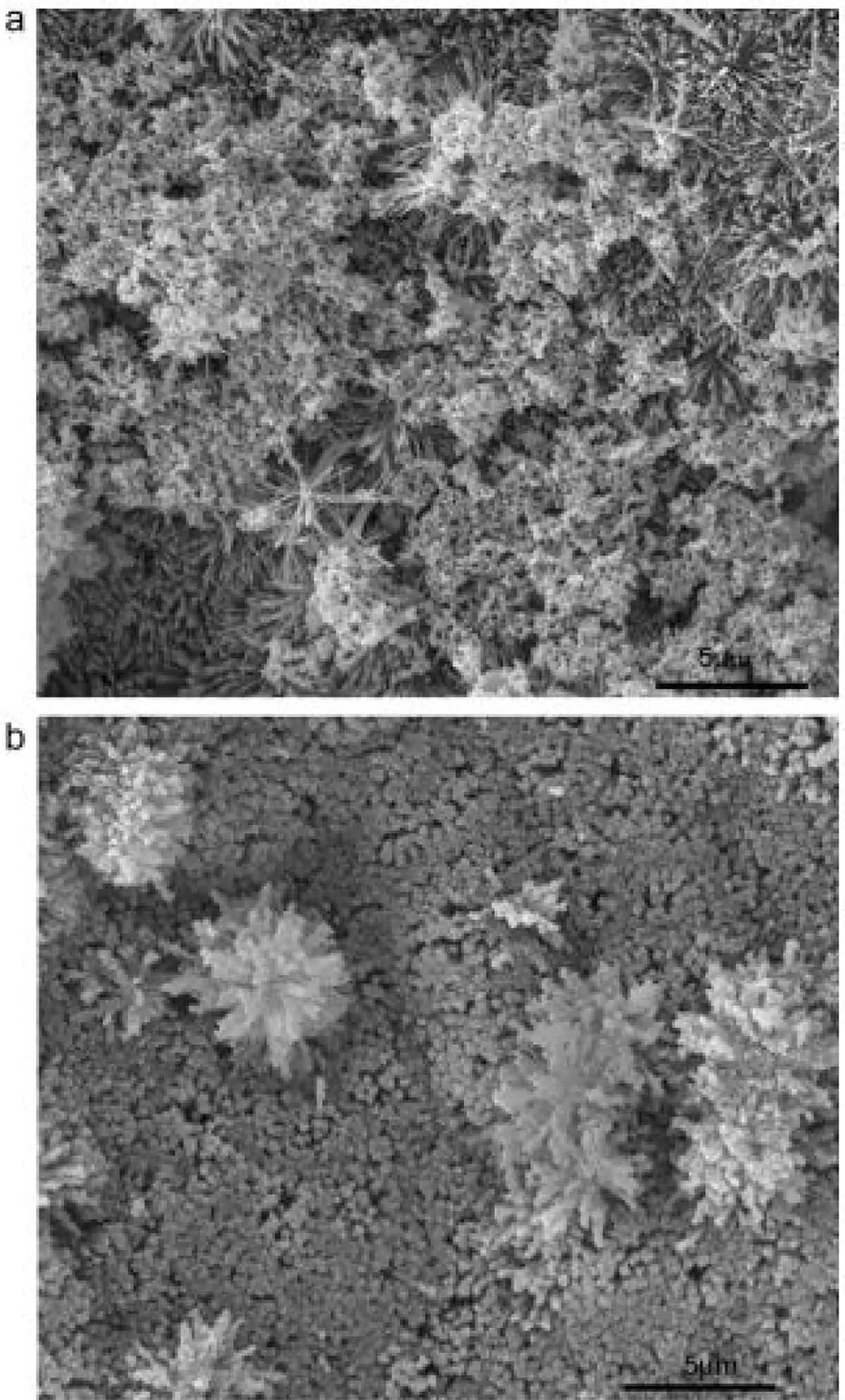
Fig.18.Surface morphologies of PEO-ST coating immersed in SBF solution for(a)1 day and(b)3 days[142].(With permission from[142];License Number:4794671115441,Mar 23,2020,Elsevier).
Hiromoto et al.[161]studied the effect of adding Octacalcium phosphate(OCP)and hydroxyapatite(HAp)on biocompatibility of AZ31 magnesium alloy.The HAp coating indicated a higher protection efficiency than the OCP coating on pure Magnesium to the corrosion in a NaCl electrolyte,likely as the HAp inner film was microscopically more compact than the OCP inner film.Thus,the OCP and HAp coatings are also expected to indicate distinct protection effects within physiological media that is a helpful property to monitor the rate of corrosion.For orthopedic usage,long-term degradation(corrosion)treatment must be found,because the corrosion morphology affects the mechanical properties of the substrate of magnesium alloy.In addition,the biocompatibility of both OCP and HAp coatings produced on magnesium must be analyzed as OCP and HAp crystals typically indicate distinct degradation treatment in vivo.
They studied the biocompatibility behavior of HA and OCP coatings in the living media for 16 weeks.The reaction of the tissue in the vicinity of implantation in the living environment was like no change was seen at the beginning of implantation.Nevertheless,after 4 weeks,the tissue around the magnesium alloy having no coating was swollen and a lot of hydrogen gas was released but the tissue around the coating containing HA And OCP was not swollen.There is no significant difference between HA and OCP coatings and both are directly bidden to the soft tissue.OCP and HA coatings can be damaged inside the body.OCP coating can be destroyed and replaced with bone tissue inside the body and HA coating remains for a longer time than OCP coating.
5.Conclusions
Many investigations have been done on the coating of PEO of light metals particularly Mg and its alloys.Metallic biomaterials are biodegradable and cannot react with the host tissue.They will have limitations in making bones due to inappropriate relation between the metal implant and the host tissue.So,the metal surface is coated by calcium phosphates to increase their osteoporosis in orthopedic usages.The PEO treatment can produce a bioactive oxide film on the surface of Mg and its alloys.As demonstrated in this research,using various solutions having calcium phosphates and various electrical parameters can modify the surface produced by the PEO procedure.This modified level is very efficient on biological activity.Consequently,various microstructures and thicknesses are created.This technique showed that the corrosion behavior of Mg and its alloys was improved considerably.Suggestions for the Ca-P base composite layers are listed including the mechanical properties study of coatings such as wear behavior,hardness and adhesion of coatings in addition to the other different nanoparticles addition such as copper,silver and zirconia to coating electrolytes to amend the biological behavior.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Recent developments and applications on high-performance cast magnesium rare-earth alloys
- Towards developing Mg alloys with simultaneously improved strength and corrosion resistance via RE alloying
- Magnesium matrix composite reinforced by nanoparticles–A review
- The design of Co3S4@MXene heterostructure as sulfur host to promote the electrochemical kinetics for reversible magnesium-sulfur batteries
- A new die-cast magnesium alloy for applications at higher elevated temperatures of 200–300°C
- Exploring the concept of castability in magnesium die-casting alloys
