The design of Co3S4@MXene heterostructure as sulfur host to promote the electrochemical kinetics for reversible magnesium-sulfur batteries
2021-03-10QinnnZhoRonghuWngYuxinZhngGungshengHungBinJingChoheXuFushengPn
Qinnn Zho,Ronghu Wng,Yuxin Zhng,Gungsheng Hung,c,Bin Jing,c,Chohe Xu,c,∗,Fusheng Pn,c,∗
a College of Aerospace Engineering,Chongqing University,Chongqing 400044,China
b College of Materials Science and Engineering,Chongqing University,Chongqing 400044,China
c National Engineering Research Center for Magnesium Alloys,Chongqing University,Chongqing 400044,China
d State Key Laboratory of High Performance Ceramics and Superfine Microstructures,Shanghai 200050,China
Received 10 November 2020;received in revised form 8 December 2020;accepted 8 December 2020
Available online 19 January 2021
Abstract The rechargeable Mg-S batteries are attractive because of their resource abundances of Mg and S,high volumetric energy density,and less dendrite property of Mg anodes.However,the development is barred by the intrinsic low electronic conductivity of S and the discharge products as well as the lack of understanding the hidden electrochemical kinetics.Here,a Co3S4@MXene heterostructure is proposed as effective sulfur host for reversible Mg-S batteries.XPS results and density functional theory(DFT)calculation confirm that the chemical interaction between the decorated Co3S4 nanocrystals host and polysulfide intermediates could well absorb and catalyze the polysulfides conversion,thus improve the electrochemical redox kinetics.Meanwhile,the MXene matrix could promote Mg ion diffusion dynamics greatly.As a result,the developed Mg-S batteries using the Co3S4@MXene-S as the cathode material could demonstrate high sulfur utilization with specific capacity of 1220 mAh g−1 and retain a capacity of 528 mAh g−1 after 100 cycles,together with a satisfactory rate performance even at 2 C.This work shed light on the advanced cathode design for reversible high energy Mg-S batteries.
Keywords:Magnesium sulfur batteries;MXene;Co3S4;Electrochemical sulfur conversion;Mg ion diffusion.
1.Introduction
The advanced energy storage systems(AESSs)have highly attracted the research community’s interests due to the everincreasing demand of power source and environmental concern like carbon emissions[1,2].Among diverse AESSs,metal-sulfur batteries,thanks to their high charge-storage capacities and energy densities,are generally considered as the most promising candidates in the post Li-ion batteries era[3,4].Firstly,the sulfur cathode(S8)can yield 1675 mAh g−1specific capacity by storing two electron per sulfur atom to generate metal sulfide,which is 4–5 times higher than the metal-ion intercalation material(typically<300 mAh g−1)[5].Moreover,compared with nowadays commercial lithiumion battery cathodes,the nontoxic sulfur is natural abundant making it cost-effective and environmental friendly(sulfur,$150 ton−1vs.LiCoO2,$10 000 ton−1)[6].Then talking about the metal anodes,nowadays,most research about metalsulfur batteries were focused on lithium anode.However,direct use of the metallic Li may cause some other concerns,such as its limited natural reservation,the expected price increases[7],as well as dendrite formation which will further lead to safety problem.Noted that underlying the application of the energy storage devices is their reliability for grid application,thus the cost of raw materials is vital for the commercial development[8].Hence,coupling the sustainable metal anode with sulfur cathode will be of more practical significance,taking the most advantage of the low price of the meta-sulfur batteries.Magnesium(−2.36V vs.NHE)is regarded as one of the most auspicious metal anodes due to its natural abundance in the earth crust and high specific volumetric capacity of 3833 mAh cm−3(vs.Li,2046 mAh cm−3)[9–11].Furthermore,Mg metal shows easier processing features,less reactivity toward moisture/air and less dendrites growth than Li benefitting from the strongly bonded Mg atoms as well as good ions mobility[12,13].All these merits of low cost,high energy density and good safety make metallic Mg a potential anode in practical batteries[14].
According to the above concerns,the development of rechargeable Mg-S batteries becomes a more attractive option than current Li-S batteries.Nevertheless,developing highenergy-density Mg-S batteries also faces several challenges.First of all,the intrinsic electronic and ionic insulation properties of sulfur always require the addition of a large amount of conductive additives during electrode preparation process.During cycling,the electrochemical processes involve magnesium polysulfide(MgPS)intermediates and solid MgS deposition and decomposition,which is a more complex multielectron solid-liquid-solid conversion than that in the Li-S batteries[15].When soluble polysulfides generated,the shuttling phenomenon inevitably occurs resulting in capacity loss,Coulombic efficiency decay and anode corrosion.On the other hand,large ionic size and slow diffusion capability of the bivalent Mg ion will further slow the reaction kinetics[16].Therefore,how to promote the cathode reaction kinetics and achieve fast Mg ion conduction and diffusion plays a vital role in reversible Mg-S batteries[9,17].Recent studies found that polar transition metal oxide/chalcogenides/phosphides could chemically adsorb lithium polysulfides and catalyze the electrochemical conversion efficiently in Li-S batteries[5,18,19].Moreover,enhancing conductivity of the cathode host will effectively lower the energy barriers and accelerate the Li-ion diffusion kinetics[4,20].These empirical conclusions in Li-S batteries provide strong references in fabricating advance cathode host material for reversible Mg-S chemistry.
Herein,we construct a Co3S4@MXene heterostructure as sulfur host to promote the electrochemical sulfur conversion kinetics for reversible Mg-S batteries,compatible with the classic APC electrolyte.XPS results and density functional theory(DFT)calculations verified the existence of strong chemical interaction between Co3S4clusters and polysulfide intermediates,which would be greatly helpful for catalyzing the sulfur species conversion;while,the MXene matrix could greatly promote Mg ion diffusion dynamics.As a result,the designed Mg-S batteries can deliver a high capacity of 1220 mAh g−1and show a satisfactory rate performance.
2.Experimental section
2.1.Preparation of Ti3C2Tx nanosheets
The Ti3C2Txnanosheets were prepared by etching the Ti3AlC2powders with a mixed solution of hydrochloric acid(HCl)and lithium fluoride(LiF).Typically,1.6g of LiF was added in to 30ml of 9M HCl solution,followed by adding 2.0g of Ti3AlC2powders under ice bath condition.After 24h etching at 40°C,the precipitates were washed with DI water for several times until the pH of the supernatant reached 6.Then,80ml deionized water was poured into the precipitates and sonicated for 5h under the protection of N2atmosphere.At last,the mixture was centrifuged at 3500 r.p.m.,and the resultant dark supernatant is the few-layer Ti3C2Txnanosheets colloid.
2.2.Preparation of CoOx quantum dots
CoOxquantum dots were used as the precursor for preparation of Co3S4and simply synthesized according to our previous work[21].Briefly,1.0g of cobalt acetate and 5ml of ammonium hydroxide were sequentially added into 50ml of ethanol under continuous stirring.The resulting mixed solution was refluxed at 100°C for 3h to produce the CoOxquantum dots dispersion.
2.3.Preparation of the Co3S4@MXene composite
5ml of the as-prepared CoOxquantum dots dispersion was uniformly mixed with 10ml Ti3C2Txcolloid under stirring,named as solution A.350mg thioacetamide(TAA)was added into 15ml DI water under continuous stirring until the mixture turned clear,named as solution B.Then,these two solutions were mixed together and kept stirring for 0.5h,next transferred the mixture into a Teflon-lined autoclave and heated at 200°C for 16h in an oven.After that,the Co3S4@Mxenes precipitates were washed with DI water for several times and dried at 80°C overnight.
2.4.Materials characterizations
SEM was carried out on a JEOL7800 field emission SEM instrument.TEM was performed on a JEOL 2010F operating at 200keV.Powder X-ray diffraction(PXRD)was recorded on a Rigaku D/max 2550PC(Cu Ka).X-ray photoelectron spectra(XPS)were used to reveal the chemical environment on a KRATOS AXIS DLD spectrometer.
2.5.Cell assembly and electrochemical tests
The active material was synthesized by heating 75wt.%sulfur and 25wt.% Co3S4@MXene at 155°C for 12h with the help of CS2for an ideal sulfur impregnation.The cathode slurry composed of Co3S4@MXene-S composite,super P and PVDF(weight ratio of 8:1:1)was balling milled for 6h in a NMP solution.The as-collected slurry was coated on a Cu foil by a doctor-blading method and dried at 50°C for 24h in a vacuum oven.After drying,the cathode film can easily peel off from Cu foil,and was further cut into disks for full cells assembling.Mg foils were polished and used directly as an anode.The electrolyte was 0.4M(MgPhCl)-AlCl3in the solution of THF.For symmetric batteries,two pieces of Co3S4@MXene disks immersed with the magnesium polysulfides(MgPSs)solution were used as the cathode and anode;a glass fiber membrane containing the electrolyte was employed as the separator.All batteries were assembled in an Ar-filled glovebox(<0.5ppm of O2and H2O).The galvanostatic charge/discharge performance was measured on a program-controlled battery test system(Shenzhen Neware Battery Co.China)with a voltage range of 0.4–2.1V.CV results were recorded on a CHI 760E electrochemical workstation.
2.6.Computational methods
All the calculations were performed within the framework of the density functional theory(DFT)as implemented in the Vienna Ab initio Software Package(VASP 5.3.5)code within the Perdew–Burke–Ernzerhof(PBE)generalized gradient approximation and the projected augmented wave(PAW)method[22–25].The cutoff energy for the plane-wave basis set was set to 400eV.The Brillouin zone of the surface unit cell was sampled by Monkhorst–Pack(MP)grids,with a k-point mesh for Co3S4and MXene structure optimizations[26].The Co3S4surface was determined by 3×3×1 Monkhorst−Pack grid and Co3S4surface was determined by 4×4×1 Monkhorst−Pack grid.The convergence criterion for the electronic self-consistent iteration and force was set to 10−5eV and 0.01eV/°A,respectively.A 2×2 supercell of the Co3S4surface including 5 layers was constructed to model the Co3S4catalyst in this work.The PBE+U approach was applied to calculations of the electronic structure of Co3S4and MXene which can partly reduce the underestimation of the electronic band gap and the excessive tendency to delocalize the electron density.In this work,we set the Hubbard parameter to U−J=3.4 for Co and 3 for Ti,which ensures a good qualitative description of structure and electronic properties of Co3S4and MXene.A vacuum layer of 15 °A was introduced to avoid interactions between periodic images.
3.Results and discussion
3.1.Design and preparation of the heterostructural Co3S4@MXene
Delaminated MXene phases,as a new category of 2D early-transition-metal cabide/carbonitrides materials,are exciting energy storage material with inherently high electronic conductivity and active Lewis acidic surfaces[27–29].MXene nanosheets are usually produced by selective etching of A-site element from MAX phase’s ceramics,where M is an early transition metal,A is IIIA or IVA group elements and X is C and/or N elements[30].According to previous literature,sufficient electronic conductivity of the MXene backbone and abundant oxygen species on its surface could promote the reaction kinetics in Li-S batteries by fast electron/ion transport and chemisorption through Lewis acid-base interaction.However,the strong electronegativity of the F-terminal group on the functional surface greatly restricts the full chemical capturing and chemical catalytic conversion for the polysulfides to some extent[31].On the other hand,transition metal chalcogenides with good conductivities were reported as efficient sulfur host materials,served as chemical bonding/absorption sites for accelerating the flood polysulfides redox reactions and regulating uniform nucleation of the solid discharge precipitates of Li2S[5].
Considering the respective merits,we hereby designed a Co3S4@MXene heterostructure as sulfur host by a simple solvothermal sulfurization method.The preparation process of the Co3S4@MXene-S composite is illustrated in Fig.1a.By a controlled etching,the Ti2C3TxMXene was synthesized by using the MAX ceramic powders as the raw material.After intensive ultrasonication for 10h under the protection of N2atmosphere,the loose packed MXene was fully delaminated into nanosheets.Then,MXene nanosheets,CoOxquantum dots and TAA were mixed together to form a homogeneous solution and sulfurized by solvothermal reaction to produce the final Co3S4@MXene host[18,21].Afterwards,sulfur was incorporated into the host by the typically heat-melting method.In addition to inheriting the intrinsic advantages of MXene and Co3S4,the Co3S4@MXene composite also shows significant synergetic effects in enhancing the Mg-S batteries’ electrochemistry.MXene nanosheets could build a continuous conductive network for accelerating the electron transport.The negative F-terminal on the surface could protect the sulfur species from being attacking by the nucleophile electrolyte reagent.At the same time,the Co3S4nanodots tightly anchored on MXene could act as the sulfophilic sites and further catalyze the magnetism polysulfides conversion efficiently.As well,the dual-phase active interface of MXene and Co3S4could realize fast Mg ions transport for uniform MgS deposition,ensuring the good electrochemical performance of the Mg-S batteries.Moreover,benefitting from the 2D sheetlike structure,the Co3S4@MXene-S cathode exhibits good flexible property which could be further used as the flexible electrode of high performance Mg-S battery(as described in Fig.1b).
3.2.Characterization of the Co3S4@MXene heterostructure
XRD was firstly conducted to identify the characteristic phase of the as-prepared materials(Fig.2a).After chemical etching,the resultant Ti3C2TxMXene shows a typical peak shift compared with the original MAX phase.SEM image in Fig.2b clearly presents smooth 2D nanosheet morphology,which could work as the backbone to hybrid with Co3S4nanodots.The corresponding elemental mapping images depict uniform C,O,Ti and F distributions,with no residual Al observed,which can confirm the successful etching and fully delamination process(Fig.2c–f).After solvothermal sulfurization,the Co3S4@MXene composites retain the nanosheet morphology with uniform Co3S4nanodots decorated on its surface,which is confirmed by SEM image and EDS mappings(Fig.3a–g).XRD results of the Co3S4@MXene composites collected at different sulfurization time were also recorded in Fig.3h for comparison.The samples after 4 and 8 h sulfurization exhibits relative weak diffraction peaks,which is attributed to the low crystallinity of Co3S4in the composites.When extending to 12h,the Co3S4peaks become more obvious.Further prolonging the sulfurization time to 24h,the as-prepared Co3S4@MXene-24 heterostructure showed no obvious change compared with Co3S4@MXene-12(Fig.3h).So,the Co3S4@MXene-12 sample with appropriate sulfurization degree is mainly discussed in this work when the energy consumption during the material synthetic process is considered.TEM images further reveal the inner structure of the Co3S4@MXene composites as shown in Fig.3i–l.Obviously,Co3S4nanoparticles with size of about 20nm are tightly anchored on the MXene matrix even after intense ultrasonication for preparation of TEM sample(Fig.3i and j).HRTEM image in Fig.3j shows typically(220)and(440)planes of the cubic Co3S4(JCPDS No.47–1738),while MXene showing an amorphous state without visible lattice fringes were founded[5,32].The EDS mapping results in Fig.3k and l represent uniform spatial distribution of Ti,S and Co elements,confirming the uniform distribution of Co3S4nanoparticles on the Ti3C2Txsheets.
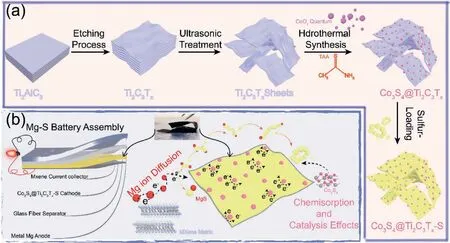
Fig.1.Schematic illustration of(a)the fabricate process of the Co3S4@MXene heterostructure and the Co3S4@MXene-S cathode material,(b)scheme of function of the Co3S4@MXene heterostructure in a Mg-S battery.
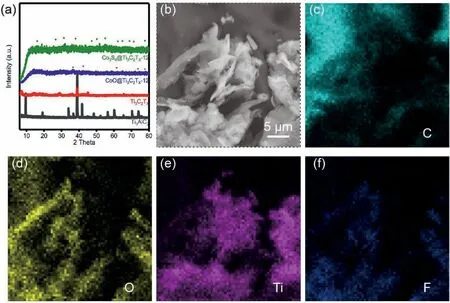
Fig.2.(a)XRD patterns of the Ti3AlC2 MAX powders,Ti3C2Tx,CoOx@Ti3C2Tx and Co3S4@Ti3C2Tx.(b)SEM images and the corresponding elemental mappings of the MXene nanosheets.
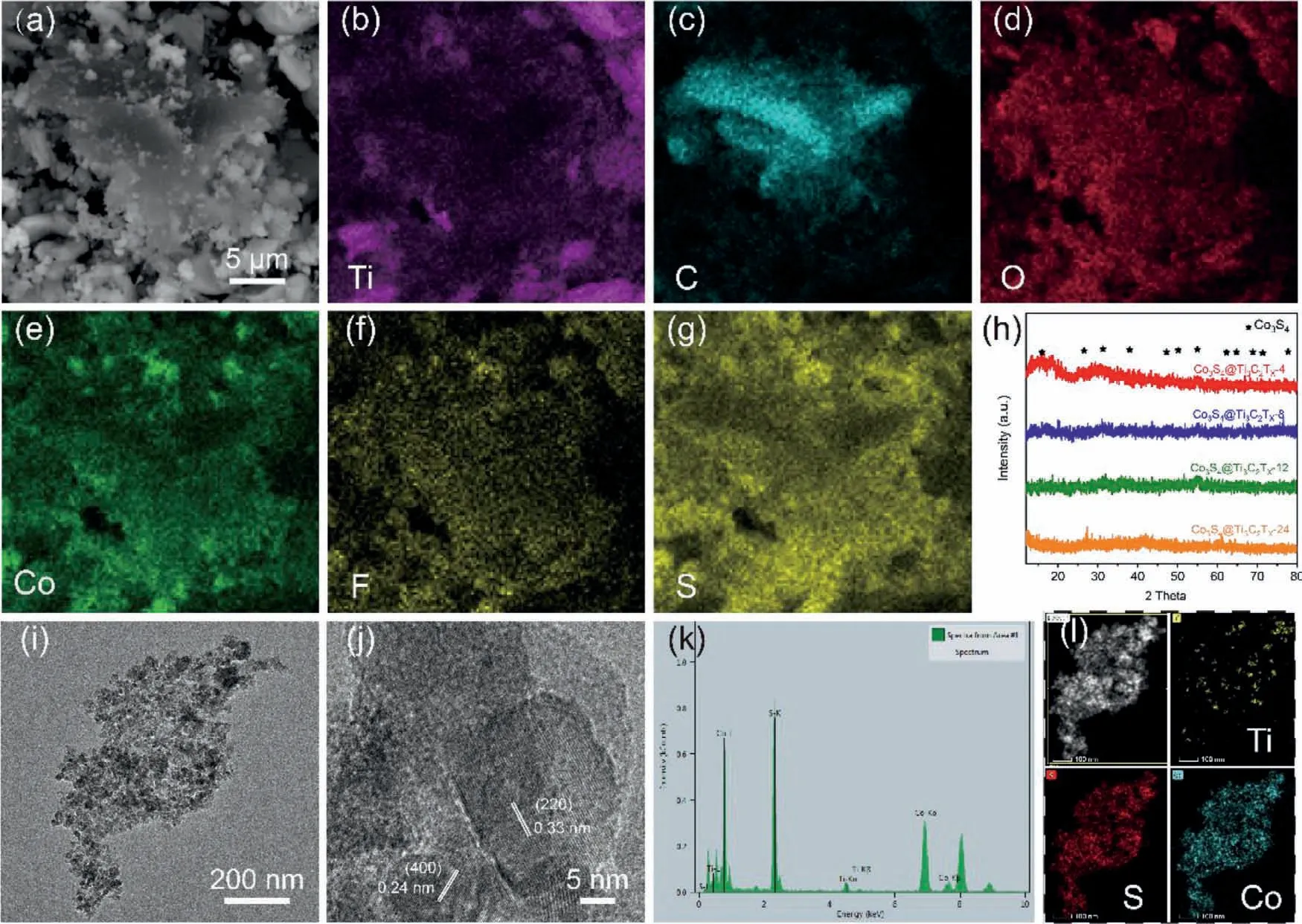
Fig.3.(a-g)SEM images and the corresponding elemental mappings of the MXene nanosheets.(h)XRD patterns of the Co3S4@Ti3C2Tx with 4,8,12 and 24h hydrothermal treatments.TEM and EDS results of the Co3S4@MXene heterostructures:(i)TEM and(j)representative HRTEM images,(k-l)EDS curve and elemental mapping images.
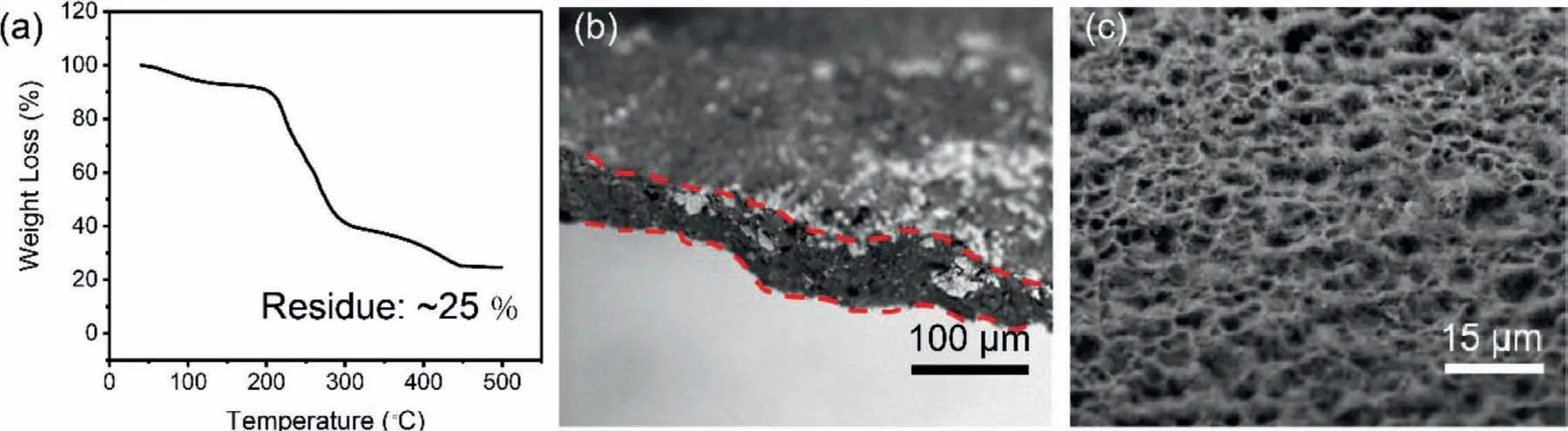
Fig.4.(a)TGA result of the Co3S4@MXene-S cathode material.SEM images of the flexible Co3S4@MXene-S cathode(b)cross section and(c)top view images.
TGA tests were applied to detect the accurate sulfur loading in the Co3S4@MXene-S composite,as seen in Fig.4a.According to TGA plot,the sulfur content was calculated to be about 75wt% which is consistent with the sulfur feed ratio.For the characterization of the flexible Co3S4@MXene-S cathode,SEM images of the cross section and top view were showed in Fig.4b and c.The cross section image showed the thickness of freestanding cathode is about 50μm while the surface of cathode is porous without any bulk sulfur aggregation which is beneficial for the mass transfer and boosting the sulfur conversion.
3.3.Electrochemical performance of the Co3S4@MXene-S cathode material
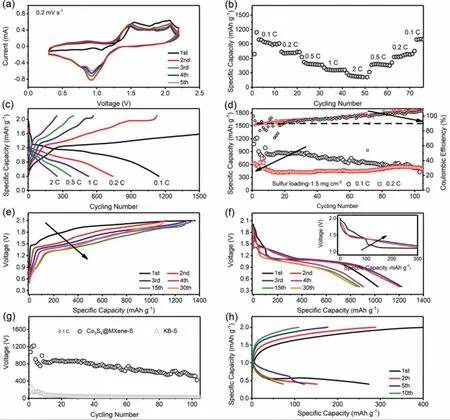
Fig.5.(a-f)Electrochemical performance the specific designed Co3S4@Mxene-S cathode,(a)CV profiles,(b)rate performance,(c)the discharge-charge profiles at different rates,(d)cycling performance at 0.1 and 0.2 C,(e)charge and(f)discharge profiles at different cycles.(g)Comparison of the electrochemical cycling performance of Mg-S batteries with Co3S4@MXene-S cathode and KB-S cathode at 0.1 C.(h)Voltage profiles of different cycles of Mg-S battery with the KB-S cathode.
A prototype Mg-S battery with APC electrolyte was assembled for electrochemical performance evaluation.As seen in the CV profiles(Fig.5a),one obvious redox peak at about 0.9V and two oxidation peaks at about 1.5 and 2.1V are observed,which can be attributed to the multi-stage phase conversion during discharge and charge process.The CV curves overlap well for five cycles scanning,indicating the good electrochemical reversibility of the Mg-S batteries.The rate and cycling performance were further tested.The Co3S4@MXene-S cathode material shows reversible capacities of 1144,739,632,410,and 297 mAh g−1at varied rates of 0.1,0.2,0.5,1 and 2 C(Fig.5b and c);the reversible specific capacities of 319,515,755,and 993 mAh g−1could be immediately recovered after the current densities turned back to 1,0.5,0.2 and 0.1 C.Furthermore,the Co3S4@MXene-S electrode delivers good cycling performance as revealed in Fig.5d.At 0.1 C,the specific capacity is as high as 1220 mAh g−1,which almost approaches to the peak capacity,indicating sufficient sulfur utilization.The fluctuation of the cyclic specific capacities for the first several cycles is probably caused by the activation of the active material.Specifically,as seen in Fig.5e and f,during the first three cycles,the charge and discharge profiles display decreasing and increasing trends of the voltage plateaus.After activation,the as-constructed Mg-S battery with this unique Co3S4@MXene-S cathode shows stable cycling capacities of 521 and 527 mAh g−1even after 100 cycles.However,Mg-S batteries with the common KB/S cathode material deliver much lower specific capacities,and could hardly reversibly cycle for long cycling(light gray cycling performance in Fig.5g).Voltage profiles with much lower discharge platform and almost no charge platform were further observed,revealing the sluggish reaction kinetics in the KB/S cathode,as shown in Fig.5h.

Fig.6.Digital photos of(a)the as-prepared magnesium polysulfides(MgPSs),(b)solubility of MgPSs in the electrolyte solvent,(c)comparison of the original the MgPSs solution and the blank electrolyte,(d-f)process record of the interaction between sulfur host and MgPSs by adding CoOX@MXene and Co3S4@MXene composites in No.1 and 2 sample in(c),respectively.
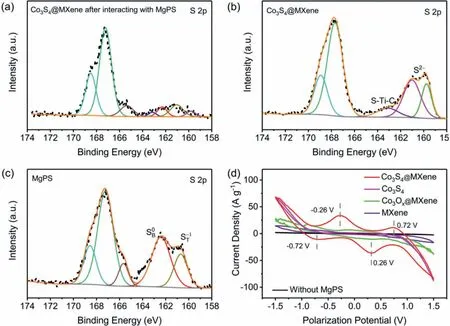
Fig.7.XPS results of S 2p spectrums,the Co3S4@MXene host(a)after and(b)before MgPS adsorption test(c)MgPS.(d)Comparisons of the CV profiles of Co3S4@MXene,Co3S4,CoOX@MXene and MXene as host materials for the magnesium polysulfides conversion.
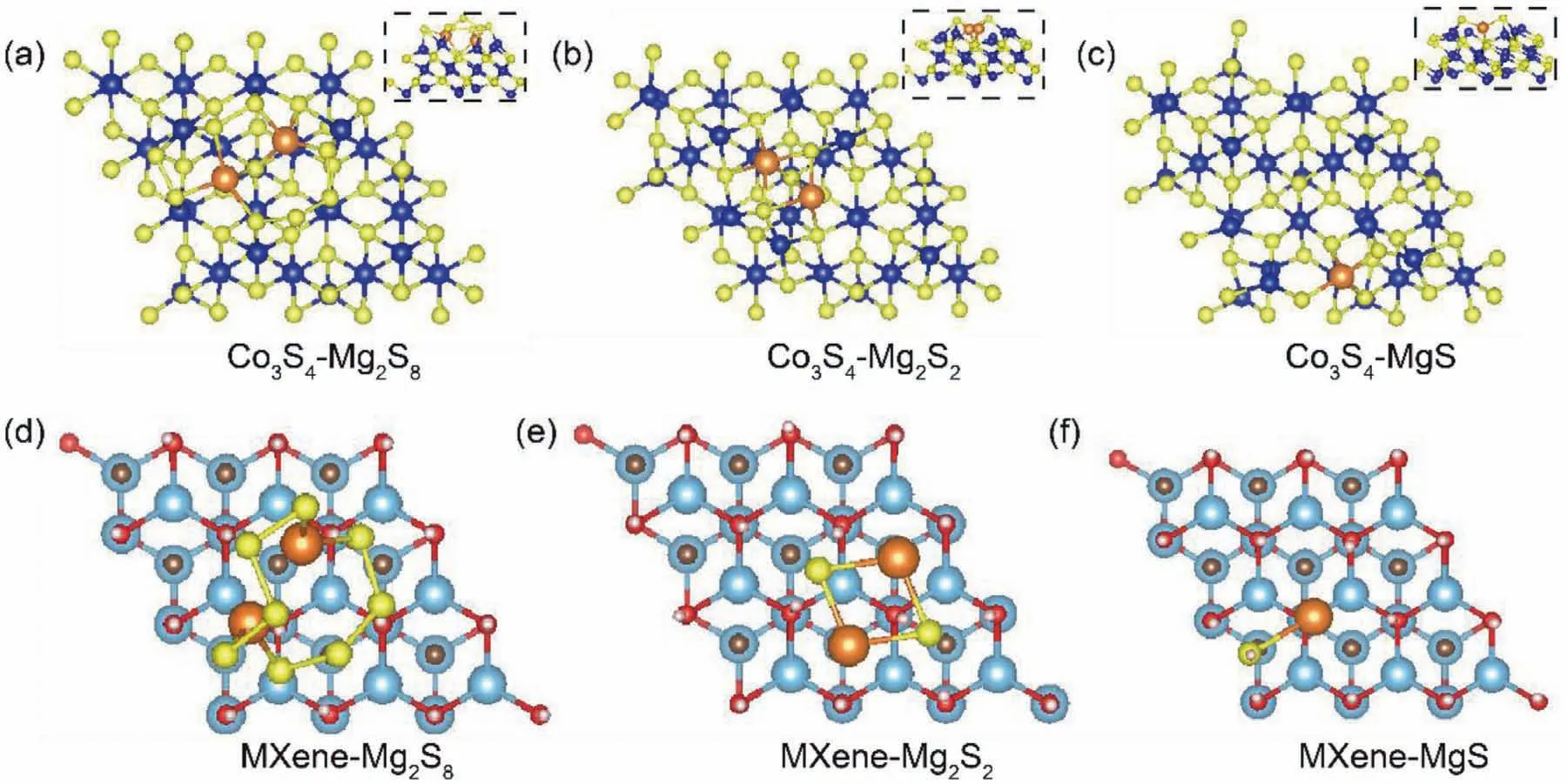
Fig.8.Optimized configurations of the magnesium polysulfides adsorption on the surface of Co3S4(a-c)and MXene(d-e).Insets in(1-c)shows the side view of the magnesium polysulfides adsorption on the surface of Co3S4.The royal blue,yellow,brown,blue,dark gray,red and pink balls represent the cobalt,sulfur,magnesium,titanium,carbon,oxygen and hydrogen atoms.
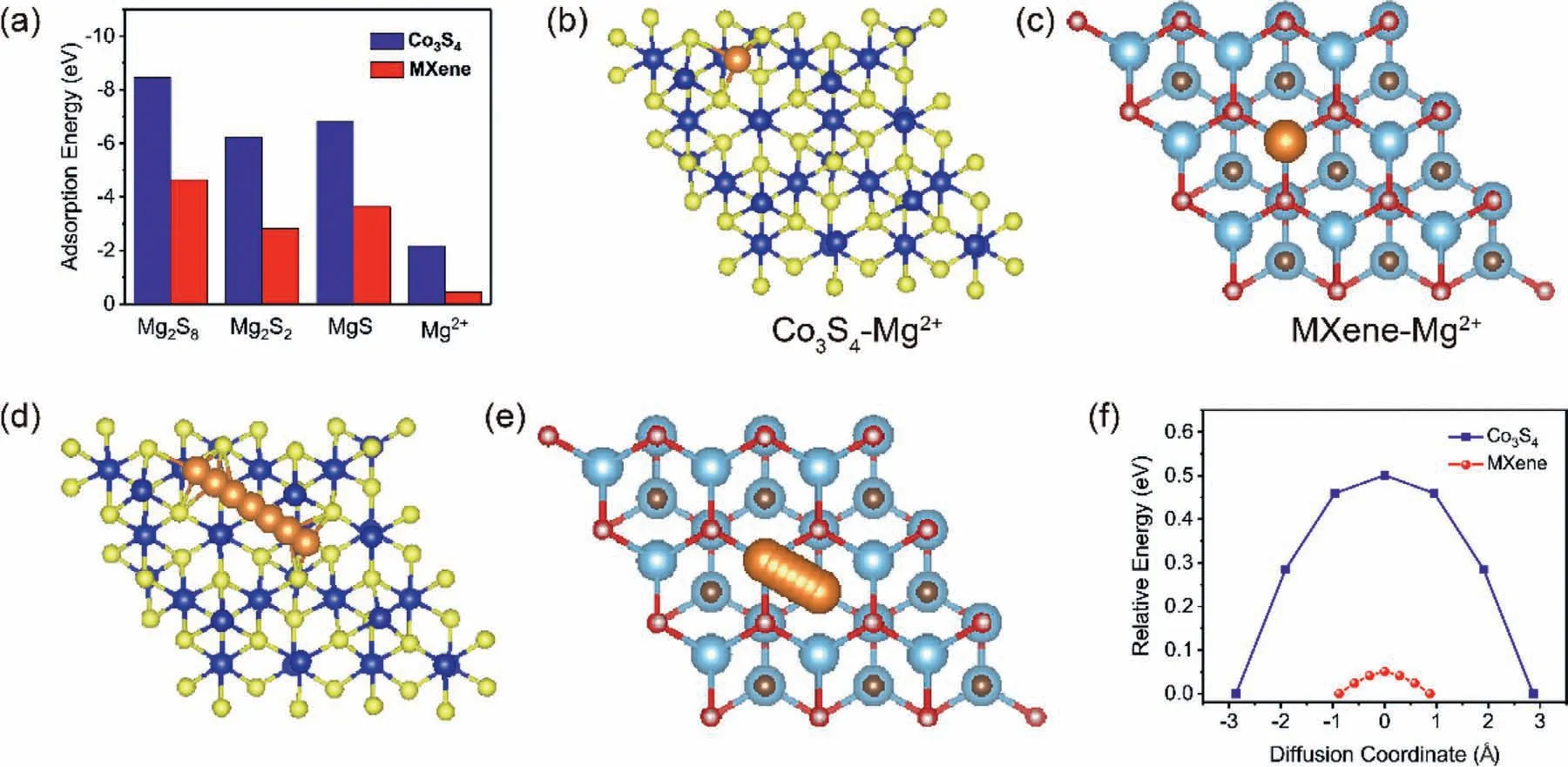
Fig.9.(a)Adsorption energies of the magnesium polysulfides and Mg ion,(b-c)the optimized structures of the Mg ion adsorption,(d-e)top view of the Mg ion diffusion pathways,(f)energy profiles for diffusion processes of Mg ion on the Co3S4 and MXene,respectively.
In order to clarify the specific mechanism of the asprepared Co3S4@MXene host in the electrochemical conversion,optical adsorption tests combined with XPS analysis,CV tests of the symmetric battery with and without MgPSs,and DFT calculation were further conducted.The chemical interaction was firstly investigated by optical adsorption experiments and XPS tests.The red MgPSs solution was synthesized according to the previous literature[15].Similar to the lithium polysulfides shuttling in Li-S batteries,the solubility of MgPSs also means that the shuttling effects will happen in the Mg-S batteries(Fig.6a–c).So as to restrain the active materials inside the cathode,strong chemisorption ability towards MgPSs is indispensable in the host materials.However,it is surprising that the optical MgPSs adsorption effect is different.After adding the Co3S4@MXene host in MgPSs solution,the mixture turned dark red color and kept stable even after 10h(Fig.6d–f).Besides,the changes of sulfur species in the cathode material before and after optical adsorption were discriminated by XPS tests.Noted that the peak at 163.3eV in the Co3S4@MXene corresponds to the S-Ti-C bonds which confirmed the interaction between the Co3S4and MXene matrix in the heterostructure(Fig.7b)[33].By comparing the XPS results of the Co3S4@MXene host,the Co3S4@MXene interacted with MgPS as well as the original MgPS(collected after vaporizing the solvents in vacuum oven),S2p spectrum of the Co3S4@MXene after adsorption presented much lower peak intensity of the terminal sulfur(ST–1)and bridging sulfur(SB0)which come from the MgPS molecules,which further confirmed the existing chemical interaction(Fig.7a–c)[34].The polarization profiles of the corresponding MXene,CoOX@MXene,Co3S4and Co3S4@MXene symmetric batteries filled with magnesium polysulfides show different redox currents(Fig.7d).Obviously,the symmetric battery coupled by two pieces of Co3S4@MXene electrodes exhibits two pairs of highly visible reduction and oxidation peaks rather than drawn-out peaks in single MXene and Co3S4electrodes.Larger contribution of capacitive current in the Co3S4@MXene electrodes than the CoOx@MXene electrodes confirms that Co3S4nanoparticles possess much more effective catalysis effects than the raw CoOx,further highlighting the necessity of sulfurization in this work[35,36].Briefly,the Co3S4@MXene host exhibits a synergetic enhancement in catalyzing the redox conversion in the electrochemical reactions of Mg-S battery.
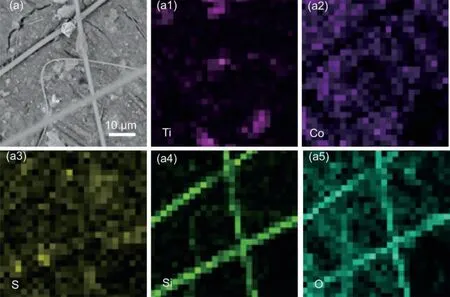
Fig.10.SEM images and corresponding EDS mappings of the Co3S4@MXene-S cathode in the Mg-S battery after cycling.
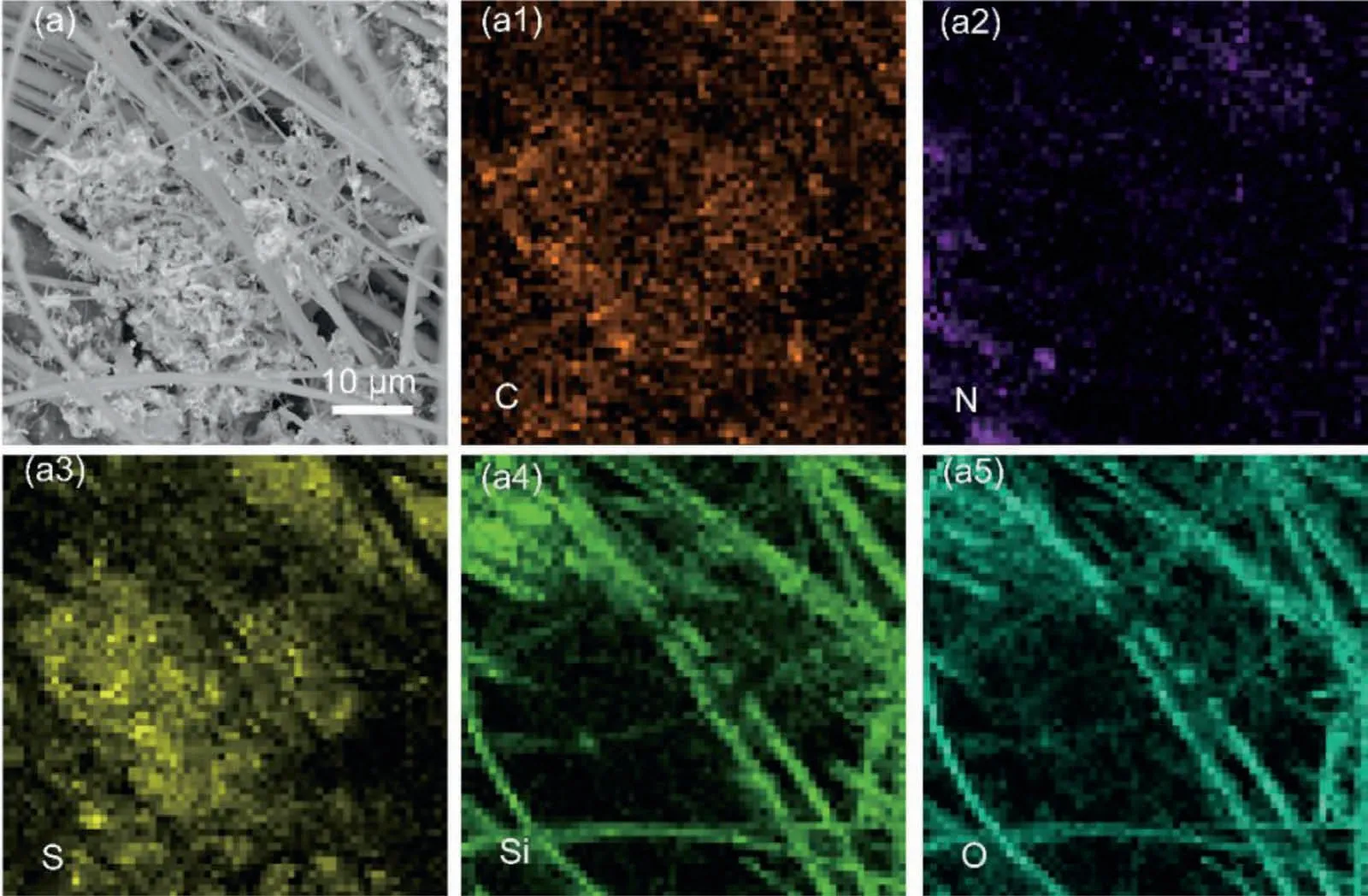
Fig.11.SEM images and corresponding EDS mappings of the KB-S cathode in the Mg-S battery after cycling.
DFT calculations of the MgPS chemisorption ability and the migration of Mg ion diffusion on Co3S4and MXene surface were further conducted and analyzed to discover their synergetic mechanism of tripping and conversion.Fig.8 shows the adsorption conformation for soluble Mg2S8and solid product of Mg2S2and Mg2S while the corresponding adsorption energies were summarized in Fig.9a.According to the calculation results,Co3S4shows much higher MgPS adsorption abilities than MXene matrix indicating Co3S4works as efficient MgPS anchoring sites on the conductive MXene matrix.This strong chemical adsorption was achieved through the formation of S-Mg bonds between S atom of Co3S4and Mg ion in MgPS,as shown in the insets in Fig.8.Afterwards,MXene matrix exhibits lower Mg ion adsorption capability than the Co3S4,as shown in Fig.9a–c.Furthermore,the top views of acting that MXene could effectively accelerate the Mg ion diffusion kinetics.Summarily,Mg ion diffusion pathway on Co3S4and MXene matrix are also inscribed in Fig.9d and e.The diffusion energy barriers were calculated to be 0.5 and 0.05eV for Co3S4and MXene,respectively,further illustrated on the above discussion,we can conclude that Co3S4works as a chemical anchor with catalysis abilities for the MgPS conversion;the MXene supply smooth Mg ion diffusion and transfer for fast reaction kinetics in Mg-S batteries.These synergetic effects ensured the highly reversible electrochemical reaction and high sulfur utilization in Mg-S batteries.
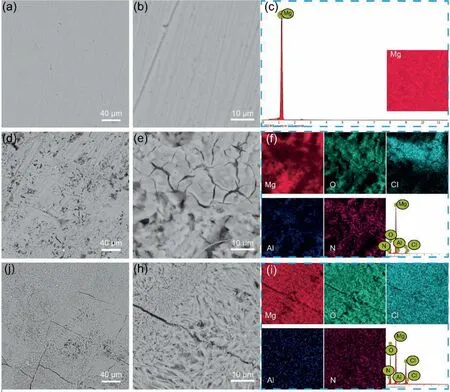
Fig.12.SEM images and corresponding EDS mappings of the Mg anode(a-c)before and after cycling(d-f)with Co3S4@MXene-S cathode and(j-i)KB-S cathode.
To better understand the Mg-S batteries,series of postmortem examinations were further carried out to reveal the specific electrochemical behaviors.We firstly characterize the morphologies of the Co3S4@MXene-S cathode and the KB-S cathodes after cycling.As seen in Figs.10 and 11,although thoroughly rinsed several times using blank electrolyte to remove the floating glass fiber of the separator,there are still some glass fiber attached on the surface of the cycled cathode.The corresponding EDS mapping results well demonstrate that sulfur and the other contained elements are uniformly distributed in the cycled Co3S4@MXene-S cathode(Fig.10).However,as a control,the cycled KB-S cathode shows obvious aggregation of sulfur species(Fig.11),which will block the channels of the glass fiber separator.This dead sulfur will cause fast capacity decay and severely affect the mass and electron transfer,making the battery crash quickly.
On the other side,comparing with the smooth surface of the original Mg foils(Fig.12a–c),the cycled Mg anode coupled with the Co3S4@MXene-S cathode exhibits relative rougher surface,and no large cracks are observed in Fig.12d.Higher magnification SEM images and corresponding EDS mapping in Fig.12e–f confirmed porous SEI layer formed on the anode surface.To some extent,SEI layer with this kind morphology is generally fragile.This may be the main reason for the capacity decay as shown in Fig.5d.Further optimization of the APC electrolyte for the Mg anode stabilization is still need to investigate in the further.When compared with the situation of cycled Mg anode with KBS cathode,the above results were still encouraging.The cycled Mg anode with KB-S cathode shows several large cracks(Fig.12j)and a great many of Mg dendrites(Fig.12k).Additionally,the SEI layer covered on the dendrites is rather thick as seen in Fig.12i.All these negative results will induce the electrolyte exhausted,dead Mg formation,electrochemical reversibility decrease of the batteries and even cause some safety disasters in the KB-S cathode battery.To sum up,our designed Co3S4@MXene-S cathode material can improve the reversibility of Mg-S batteries and the cycled Mg anode morphology to some extent.However,it is still necessary to optimize the electrolyte or adopt other strategies to further stabilize the Mg anode in the future study for high performance Mg-S batteries.
4.Conclusions
In summary,a heterostructural Co3S4@MXene sulfur host was successfully prepared and applied in Mg-S batteries.Benefitting from the excellent conductivity of the MXene matrix and the catalysis effects of the Co3S4,the composite host efficiently improved the electrochemical reaction kinetics in Mg-S batteries.XPS results and DFT calculations confirm that the designed Co3S4@MXene host could chemically adsorb the polysulfides through chemical bonding and supply fast Mg ion transportation.As a result,our designed Co3S4@MXene sulfur cathode ensure a high sulfur utilization in the Mg-S batteries with specific capacity of 1220 mAh g−1,satisfactory cycling performances with 528 mAh g−1capacity delivery after 100 cycles and rate performance even at 2 C.
Declaration of Competing Interest
There are no conflicts to declare.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China(No.21603019),the Opening Project of State Key Laboratory of High Performance Ceramics and Superfine Microstructure(SKL201807SIC),and program for the Hundred Talents Program of Chongqing University.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Recent developments and applications on high-performance cast magnesium rare-earth alloys
- Surface characterization and corrosion behavior of calcium phosphate(Ca-P)base composite layer on Mg and its alloys using plasma electrolytic oxidation(PEO):A review
- Towards developing Mg alloys with simultaneously improved strength and corrosion resistance via RE alloying
- Magnesium matrix composite reinforced by nanoparticles–A review
- A new die-cast magnesium alloy for applications at higher elevated temperatures of 200–300°C
- Exploring the concept of castability in magnesium die-casting alloys
