Bonding effect of liquid magnesium with open-celled carbon foam in interpenetrating phase composite
2021-03-10MrinGodzierzAnitOlszwkMylskNtliSobzkRfNowkPtrykWrzesniowski
Mrin Godzierz,Anit Olszówk-Mylsk,∗,Ntli Sobzk,RfłNowk,Ptryk Wrze´sniowski
aSilesian University of Technology,Faculty of Materials Engineering,Krasi´nskiego 8 street,40-019 Katowice,Poland
b Institute of Metallurgy and Materials Science,Polish Academy of Sciences,Raymonta 25 street,Kraków,Poland
cŁukasiewicz Foundry Research Institute,Zakopia´nska 73 street,30-418 Kraków,Poland
Received 3 April 2020;accepted 15 June 2020
Available online 25 August 2020
Abstract The issue of bonding formation in liquid metal/open-celled carbon foam(Cof)systems was examined,taking into account the practical aspects of the synthesis of a new type of Mg-C metal material composite.The problem is complex due to the strong oxidation and intense evaporation of liquid magnesium,as well as the 3D geometry of the carbon component,where metal transport occurred through the foam cells’windows.Laboratory experiments performed at 700°C in ceramic crucibles showed that spontaneous carbon foam infiltration by liquid metal is impossible under the applied conditions,either in an air atmosphere coupled with flux protection or under argon protection.
Keywords:Magnesium matrix composite;Open-celled carbon foam;Interpenetrating phase composites;Wetting;Interface.
1.Introduction
In metal matrix composites(MMCs),the microstructure and properties of interfaces are determined by the physicochemical interactions occurring between the components during the processing of MMCs,and such interactions establish the properties of the final product.Only after the verification of various methods and the characterization of the obtained composite microstructure and properties,can the most useful conditions for a new material’s processing be chosen.In the case of liquid-assisted processes,knowledge of the interactions between the MMC components(liquid metal matrix and solid reinforcement),particularly their wetting and infiltration behavior,is the fundamental issue in the consolidation of the parameters’design[1-4].Therefore,measurements of the contact angle(θ)at different temperatures and times are conducted for flat and compacted samples of the reinforcing material and the liquid metal drop[2-3].Since a value of 90° or less for the contact angle is usually desirable,an enrichment of the base metal matrix with alloying elements,the surface modification of the reinforcing phases,or an increase in the liquid metal temperature can be applied in order to decrease the contact angle.It must be mentioned that the data reported in the literature for experiments conducted using the same systems and temperature can be different and even inconclusive,since the measurement conditions are dependent on the applied method and equipment used[2-3].In the case of wettability tests on metal/ceramic systems,the main problem is related to metal oxidation,which may cause the formation of a native oxide film at the drop surface even under a protecting atmosphere.This layer,sometimes only a few nanometers thick,plays the role of a barrier affecting the reliability of contact angle measurements by the sessile drop method.The effects of primary and secondary oxidation of a metal sample can be efficiently reduced by the application of capillary purification[3].
In composite technologies,the wetting of a reinforcement by a molten metal matrix is more complex than that found during sessile drop measurements.The reinforcing particles are usually irregular in shape,and their surface at a microor nano-scale is rough.Moreover,they can be enriched with different elements or phases in the milling processes or during storage.The agglomeration of ceramic particles is another effect that impedes their wetting.In the case of fiber rovings or textiles,the space between single fibers forms irregular capillaries that affect the liquid metal/solid bonding formation.Such cases,involving not very strong compacted powder or fabricated fibrous preforms,can be examined by the sessile drop method,and the obtained qualitative results are usable in technological practice for the comparison of different methods.
The problem analyzed here concerns metal matrix composites reinforced with open-celled foam,which have been the subject of very intensive research conducted in the last decade[5-19].In the case of this matrix/reinforcement system,the liquid metallic component is supplied to the foam cells through the windows located in the cells’walls.The foam cells’filling process should be gradual and can be supported by an increased or reduced pressure of a value that prevents damage to the soft porous structure.
In the research described here,a procedure not previously reported in the literature was applied,where in the sessile drop test,a dense ceramic substrate was replaced by a carbon foam sample.The advantage of the proposed method is the in situ observation of liquid metal behavior in contact with ceramic foam.Such knowledge of the consolidation kinetics can also be useful in explaining the processes occurring in the fabrication of composites with the same phase composition by different technologies.Moreover,this experimental procedure seems to be helpful and complementary in the examination of cross-sectioned and fractured composite products,where the interface processes are characterized.
In the presented study,glassy carbon open-celled foam(Cof)and magnesium of technical purity were chosen.Magnesium matrix composites with carbon reinforcement are known in literature,and the carbon component has been applied as fibers,particles,nanotubes,and graphene[20-36].For the components’consolidation,many different technological techniques have been exploited,e.g.infiltration[22-26],gravity and pressure casting of magnesium suspensions[27-29,35],powder mixture sintering[20-21],and the extrusion of sintered or cast composites[36].The main reason for the application of carbon components in magnesium base matrices is improvement in the mechanical properties,friction coefficient,and wear resistance.The application of open-celled carbon foam in a magnesium matrix is a new materials engineering concept,proposed by the authors[37-39]to avoid reinforcing phase segregation and to achieve an increase in stiffness and wear resistance.Due to the selective corrosion of the components[40]and the biocompatibility of them both,potential applications of Mg-Cofcomposites as biomaterials can also be taken into account.
The aim of the presented experiments was to exhibit the effects of the pressureless interaction between the liquid magnesium and the open-celled carbon foam together with the kinetics of their bonding,since this problem is not described in the literature and seems to be important with regard to the application of foams in metal matrix composites.Therefore,two types of experiments were conducted:firstly,the interactions in the Mg-Cofsystem were examined by applying high temperature tests in ceramic crucibles either in an air atmosphere combined with flux protection or under argon protection.In equipment dedicated to contact angle measurements by the sessile drop method coupled with the capillary technique[3-4],the contact angleθwas then determined at a temperature of 700°C.Finally,during the sessile drop test,a special procedure,which allowed external pressure on the liquid metal drop to be applied in order to mechanically push it inside the open-celled foam,was used.The solidified Mg/Cofcouple was then examined using macroscopic and microscopic observations.The results of contact angle measurements and the structural characterization of interfaces were compared with those reported in the literature.Our analysis,made taking into account recent findings reported in[35]for magnesium matrix composites with carbon fibers and glassy carbon particles fabricated by metal casting processes,allowed us to propose a general structural scheme for bonding formation between liquid magnesium and carbon foam.
2.Materials and experimental procedure

Fig.1.SEM micrograph of Cof with indicated single cell(1),windows(2),and walls(3).
In this study,the open-celled carbon foam(Cof)produced at the Silesian University of Technology[41-42]by pyrolysis of organic material was applied as the porous component of the Mg-C composite.The Cofsamples had 97 vol.% porosity and pore sizes of 20 ppi(pores per inch).SEM characterization(Fig.1)showed that it was built of cells with walls of 0.1 mm thickness and different-sized circular windows.To confirm the tendency of carbon materials to absorb oxygen,a cross section of the Cofsample was examined by X-ray spectroscopy using the WDS technique(Fig.2).As the metallic component of the Mg-C composite,magnesium of technical purity was used,which allowed us to exclude the influence of alloying elements on the wetting behavior and interfaces in the Mg/C system(e.g.Al4C3formation in the case of Mg-Al alloys).For all experiments,a temperature of 700°C was chosen since it is the maximal value allowed in consolidation processes involving magnesium with carbon reinforcements,due to the strong evaporation and self-ignition of Mg at high temperatures.
For the examination of the interaction between the liquid metal and Cof,three different procedures were applied:
(1)Flux-assisted processing(Fig.3a),where the Mg disc,(ϕ=25 mm and h=8 mm)was stacked on the Cofdisc(ϕ=28 mm and h=8 mm),placed in ceramic crucible ofϕ=30 mm,and covered with Melrasal TE Flux to protect against magnesium self-ignition.The Mg/Cofassembly of two discs was placed in a laboratory furnace preheated up to 700°C.After isothermal heating for 30 minutes,the Mg/Cofassembly was cooled to room temperature in air.Finally,the ceramic crucible and the sintered flux were removed;the sample was incorporated in thermosetting resin and then cross-sectioned for structural characterization(Fig.4).
(2)Argon-assisted processing,where the same Mg/Cofassembly was placed in a furnace with flowing argon and then heated up to 700°C at a rate of 12°C/min,held at this temperature for 30 minutes,and cooled along with the furnace.Finally,the Mg/Cofsample was incorporated in transparent resin and cross-sectioned for structural observations(Fig.5).
(3)Processing under high purity static argon in a UHV chamber accompanied by non-contact heating of two different materials.For this method,a sessile drop wettability test was adopted,in which a 17×17×5 mm Cofsample was placed in a UHV chamber equipped with a special manipulator for non-contact heating and capillary purification of a metal,allowing its native oxide film to be removed directly in the UHV chamber and producing an oxide-free Mg drop,as described in detail in paper[43].In this procedure,the Mg sample was placed in a graphite capillary located above the Cofsubstrate and heated separately up to 700°C in a static argon atmosphere(p=850-900 mbar).Images of the Cof/Mg were recorded at a rate of 100 fps using a high-speed high-resolution CDD camera.The values of the contact angles were estimated using ASTRA software[44-45].Firstly,the metal droplet was deposited on the Cofsubstrate over a time of 300 s.Secondly,in order to constrain the liquid metal’s infiltration,the graphite capillary was moved down and pushed onto the drop.Finally,after about 30 s,the capillary was raised,and the system was cooled down to room temperature.The solidified samples were removed from the vacuum chamber and subjected to structural evaluations.

Fig.2.SEM micrograph of cross-section of Cof(in fabricated state)(a),and WDS line scan of oxygen concentration(b).

Fig.3.View of Mg/Cof couple applied in experiment[46](a),scheme of components(Cof and Mg)and flux configuration in ceramic crucible before metal melting(b).
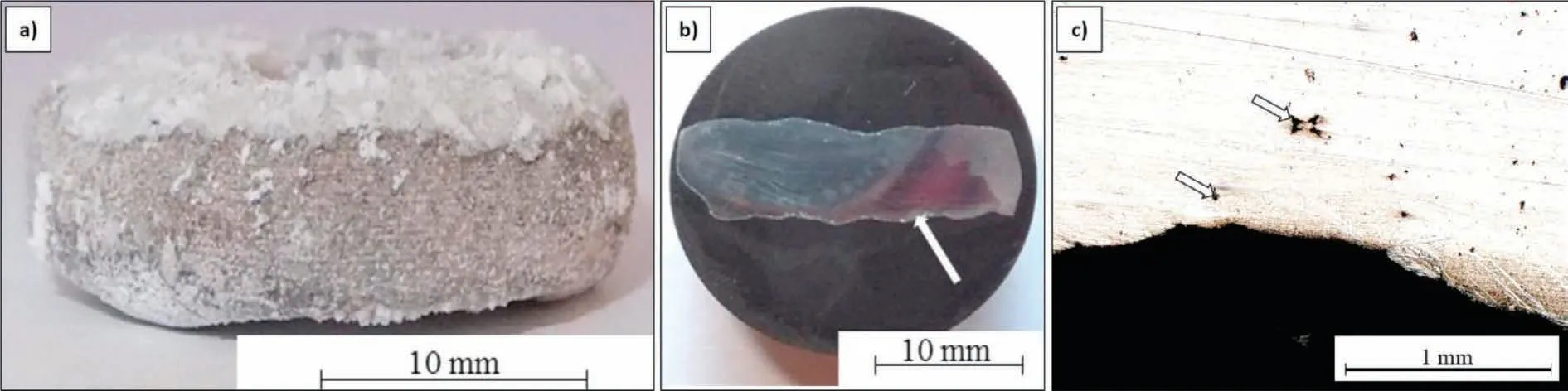
Fig.4.Mg/Cof sample produced by flux-assisted process(a),cross-section sample(b),LM micrograph taken from the region where Cof was connected with magnesium(c).Arrow in Fig.4b shows the region analyzed by LM in Fig.4c.Arrows in Fig.4c correspond to the locations of carbon-rich phase.

Fig.5.Mg/Cof sample after trial under argon(a),cross-section sample showing good bonding between solidified magnesium and Cof(b),LM micrograph of composite Mg-Cof zone formed between the two contacting materials(c).
The structural examinations of the Mg/Cofsamples were conducted with an optical microscope(Nikon MA200,Tokyo,Japan)and a scanning electron microscope(Hitachi 3400N SEM,Tokyo,Japan)equipped with a wavelength-dispersive X-Ray spectrometer(WDS MagnaRay,Thermo Scientific,Waltham,Massachusetts,USA).By applying the WDS method,elemental mapping and scan lines for magnesium(TAP crystal),carbon(NiC80 crystal),and oxygen(NiC80 crystal)were obtained.
3.Results and Discussion
Fig.4 shows the results of optical microscopy characterization of the Mg/Cofsample obtained by the flux-assisted process in the conventional furnace.It was found that during the processing of the sample,the foam was partially degraded at the macroscale.However,the analysis of the solidified metal at the metal/Cofregion of contact showed a continuous zone of approx.1 mm thickness that was reinforced with carbon foam.These observations suggest an absence of spontaneous infiltration and indicate that the Mg-Cofcomposite zone was formed due to the slight external pressure generated by the slope of the liquid metal due the gravitational effect;this pressure acted on the carbon foam located under the metal.It should be mentioned that the applied flux successfully secured the system against self-ignition.However,under the conditions of this trial,the influence of the surrounding air on Mg oxidation could not be efficiently eliminated.
The second trial to produce an Mg/Cofsample was conducted under an argon atmosphere at 700°C,but with longer heating and cooling times.It showed similar effects as the first trial,i.e.a composite zone between the metal and Cofwas formed,which was no thicker than 1 mm.However in this case,the foam was well bonded to the metal,while foam degradation was not observed due to the effective argon protection.

Fig.6.Magnesium drop on carbon open-celled foam at 700°C at the moment of drop deposition(a),after 60 s(b),and after 300 s contact time(c).

Fig.7.Effect of graphite capillary push on the magnesium drop at 700°C:(a)metal connected with carbon open-celled foam when Cof substrate was freestanding on alumina support and connected with the Mg drop squeezed through the graphite capillary,(b,c)the Mg/Cof couple after capillary pick up.
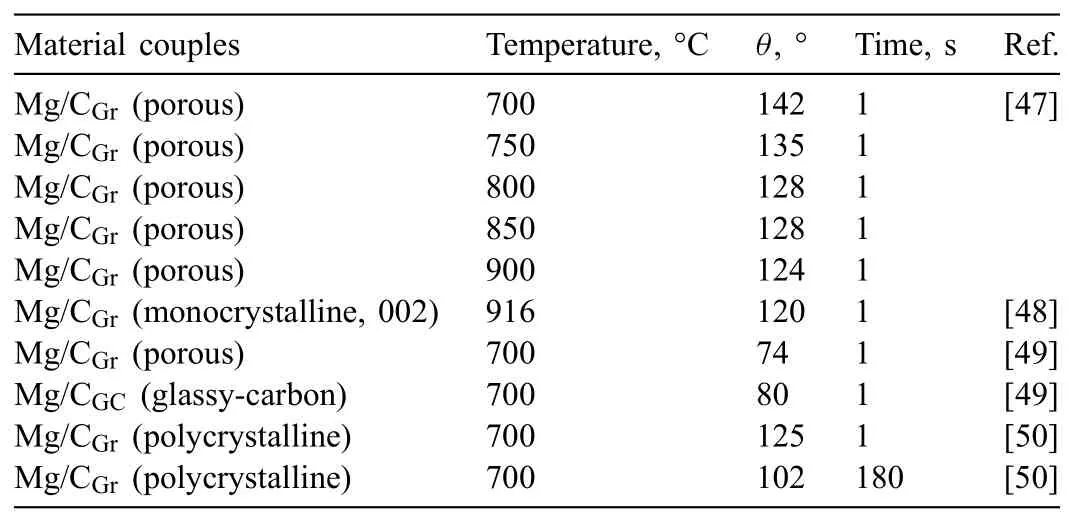
Table 1Results of contact angle measurements(θ)in Mg/C couples reported in the literature.
The most representative moments of the sessile drop test are shown in Fig.6.No evident changes in the contact angle values were noted for the 300 s test,which showed a final value of about 135°,assuming that the foam sample surface was planar.These observations are in good agreement with literature data showing the non-wetting character of the Mg/C system,since the contact angle values reported for different Mg/C couples at the same temperature of 700°C(Table 1)were in the range of 102-142°.In the case of paper[47],where the experiment was conducted at temperatures higher than 700°C,a decrease inθvalue was noted from 142° at 700°C to 124° at 900°C.
After recording the liquid drop geometry,the experiment was continued,and the graphite capillary was gently pushed onto the drop,kept there for about 30 s,and then raised up(Fig.7).The reasons for this procedure were to improve the contact of the liquid metal with the carbon foam and to force the melt to flow inside the channels of the foam.However,infiltration of the foam was still not observed,while some mechanical destruction of Coftook place.After cooling and metal crystallization,the Mg/Cofcouple was split in two parts,i.e.one part presented Cofwithout clear evidence of metal infiltration,but it had a trace amount of shiny metal(Fig.8a),while the second part consisted of the metallic drop connected with the graphite capillary and some Cofat the opposite surface of the solidified drop(Fig.8b).The first part was examined by SEM+EDS to analyze the effects that had taken place at the Cofsurface after its short contact with liquid magnesium.For this purpose,no special sample preparation was needed(Fig.9).In case of the solidified metal drop,SEM+WDS examinations were focused on Mg/C bonding(Fig.10),while the sample was mounted in resin,ground,and polished in a typical manner for a metallographic sample.
Fig.9 displays the most representative results of SEM observations of the Cofsurface with metal traces shown in Fig.8a.It reveals a few regular crystals deposited at the uniform and brittle cracked layer.The corresponding X-ray patterns revealed that regular crystals were formed by Mg(Fig.9b),while the mapping of a brittle layer(Figs.9 c-e)indicated MgO formation.These structural observations,being a result of the short time contact of liquid Mg with Cof,suggest that during the sessile drop test a thin oxide film formed at the interface.It means that in fact the measured contact angle concerns a transition from an initial couple of Mg/Cofto a new one,i.e.liquid Mg/MgO-film/Cof.Examination of the composite region formed at the drop-side interface of the cross-section of the drop(Fig.10)detected a thick(1-2μm)transition zone,where an evident oxygen content increase was measured by WDS at the X-ray characteristic line scans(Fig.10b).
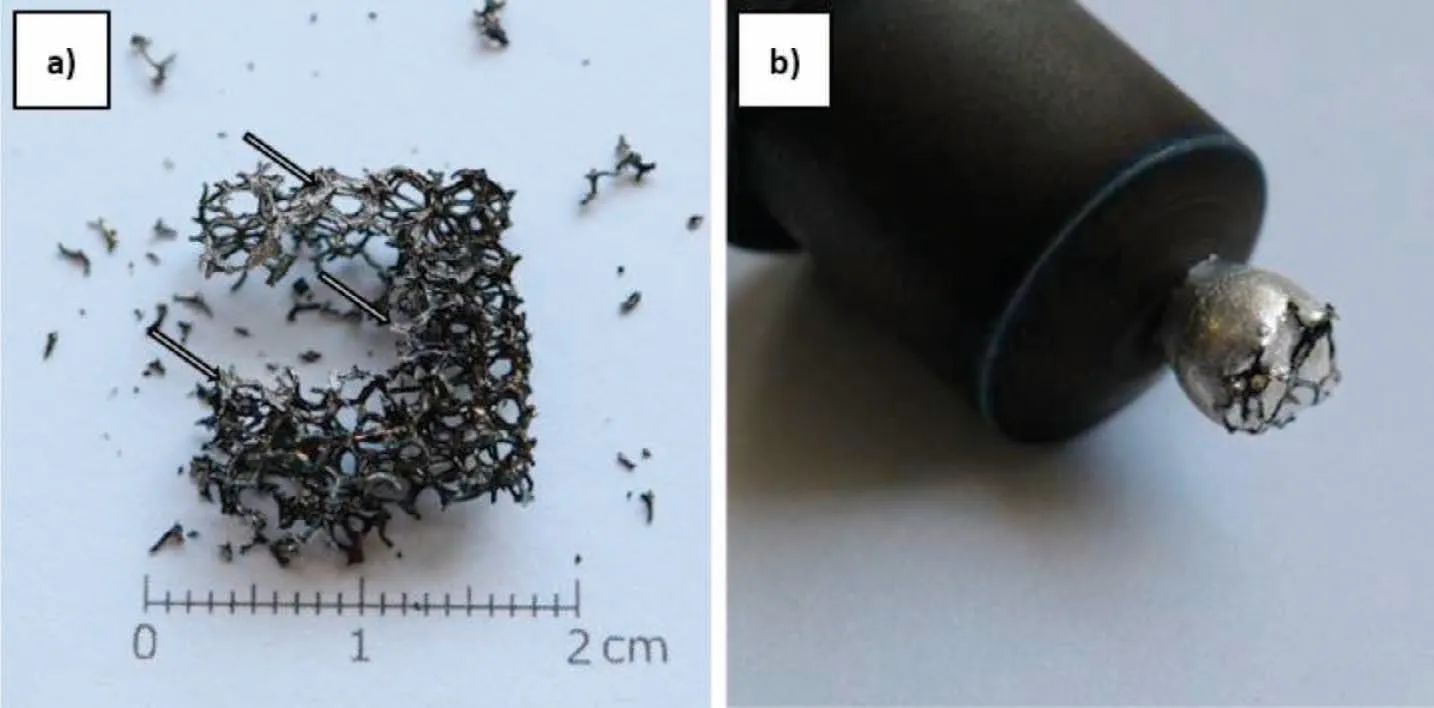
Fig.8.Macrograph of Mg/Cof couple divided after conducted experiment into two parts,(a)destroyed Cof with trace content of shiny Mg and(b)Mg drop connected with graphite capillary and Cof.
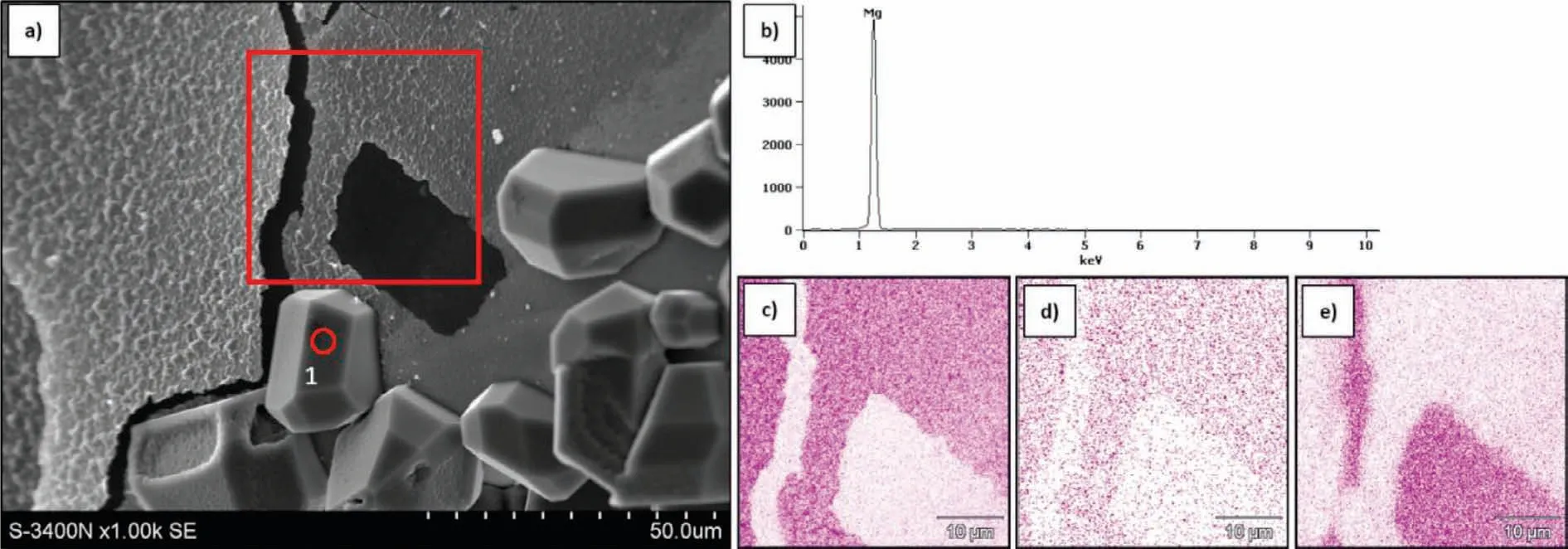
Fig.9.SEM micrograph(a)of Cof surface after short-term contact with pure liquid magnesium in sessile drop test(macro in Fig.8a),regular Mg crystals and cracked oxide layer visible,and WDS spectrum(b)of magnesium crystal(1)with mapping of Mg(c),O(d),and C(e).
These results of the SEM+WDS analysis confirm the presence of an oxide layer between the components,and they are in a good agreement with that obtained at the Cofsurface after the sessile drop test and demonstrated in Fig.9.Additionally,the profile of the oxygen line showed an enrichment of Cofin oxygen,in comparison to the magnesium matrix.This directly indicates a source of the oxygen in the process of oxide interlayer formation under a protective atmosphere,as well as being compatible with the WDS result for the oxygen measurement in applied Cof(Fig.2).Moreover,an effect of oxygen absorption by carbon components is known,and the mechanism has been described in detail,e.g.in[66].Despite the very short contact of the metal with the reinforcing component in the conducted wetting test,our experimental results are in a good agreement with those focused on the microstructural characterization of Mg-C composites fabricated by such techniques as stir casting,liquid infiltration,and powder mixture sintering,where the processing time and intense diffusion are a matter of hours long[29,35].The oxide type of interface is characteristic and reported in the literature independently of the applied type of carbon reinforcement and the magnesium base matrix composition,as shown in Table 2.
An additional achievement of the capillary purification technique applied in this study is related to the metal drop pushing action applied on the carbon foam allowing realtime observation of liquid Mg-Cofbonding formation.From now on,independently of the carbon reinforcement shape and size(particles,fibers,or foam)and magnesium alloying elements,this problem may be considered simply by examination of the final composite sample or product microstructure.Moreover,previous tests and trials reported in the literature were conducted with different techniques and focused on the microstructure of the interfacial zone as well as the changes induced in the components by the consolidation procedure.

Fig.10.SEM micrograph(a)of Mg/Cof interface formed in sessile drop test at 700°C with corresponding WDS scan lines of C,O,and Mg(b).
In this study,it was shown that the fabrication of a magnesium matrix composite with open-celled carbon foam by spontaneous infiltration is impossible,and confirmed the results of our earlier laboratory trials concerned pressureless infiltration.Nevertheless,we believe that this problem is not an important technological issue because the trials with the pressure infiltration of Cofby pure magnesium and some magnesium alloys under vacuum presented in other studies[38,40]gave favorable results.They revealed the possibility of consolidation without indivisible metal temperature increase due to applying an appropriate external pressure,despite the unfavorable value of the contact angle in the pressureless measurements in this study.They also revealed the formation of oxide-type bonding,thus indicating reactive diffusion as the main mechanism of the components’bonding and final consolidation.
Based on the real-time observations presented here and the previous experience with glassy carbon-magnesium systems possessed in our laboratory and industrial practice[29,35],a structural model of bonding formation between the opencelled carbon foam and pure liquid magnesium was proposed(Figs.11 and 12).According to this model,on a macroscale(Fig.11)a low additional pressure applied to the liquid metal enables its transport through the windows of the carbon foam cells.It should be highlighted that due to the non-wetting character of the Mg-Cofsystem,liquid metal penetration inside porous Cofpreforms,and thus the components’bonding,is not possible without such a pressure.

Table 2Summary of literature data on interface characterization of different types of Mg-C composites.
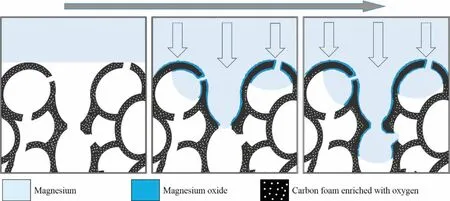
Fig.11.Schematic presentation of interaction and bonding between the open-celled carbon foam and liquid magnesium.
On a microscale(Figs.11 and 12),the contact of liquid metal with the Cofsurface induces the formation of the MgO film at the interface,which occurs due to magnesium’s reaction with the residual oxygen absorbed by the raw carbon component.This reactive diffusion induces a decrease in the oxygen concentration in infiltrated foam in comparison with its metal-free part.
Moreover,the proposed model includes two additional structural effects that were not detected in this study due to the applied conditions of the experiment.The first one is the presence of the nanozone enriched with some oxygen and formed at the front of liquid metal that is moving through the foam.This phenomenon is the consequence of the extremely high tendency of magnesium to oxidize(ΔG=-991,9 kJ for the reaction 2Mg+O2→2 MgO at 700°C)and the imperfections of the protective gasses or vacuum atmosphere inside the foam cells,thus resulting in the secondary oxidation of the oxide-free Mg.The presence of this zone can be confirmed only by high resolution methods.The second phenomenon is an enrichment of the carbon material in magnesium as a result of high temperature diffusion.This enrichment was easily experimentally demonstrated by the EDS method in the Mg-C composites,the processing of which was accompanied by a few hours’interaction between the Mg matrix and carbon reinforcement(e.g.in material processing by sintering,stir casting,composite ingots’remelting,and pressure casting[29,31,33,35]).
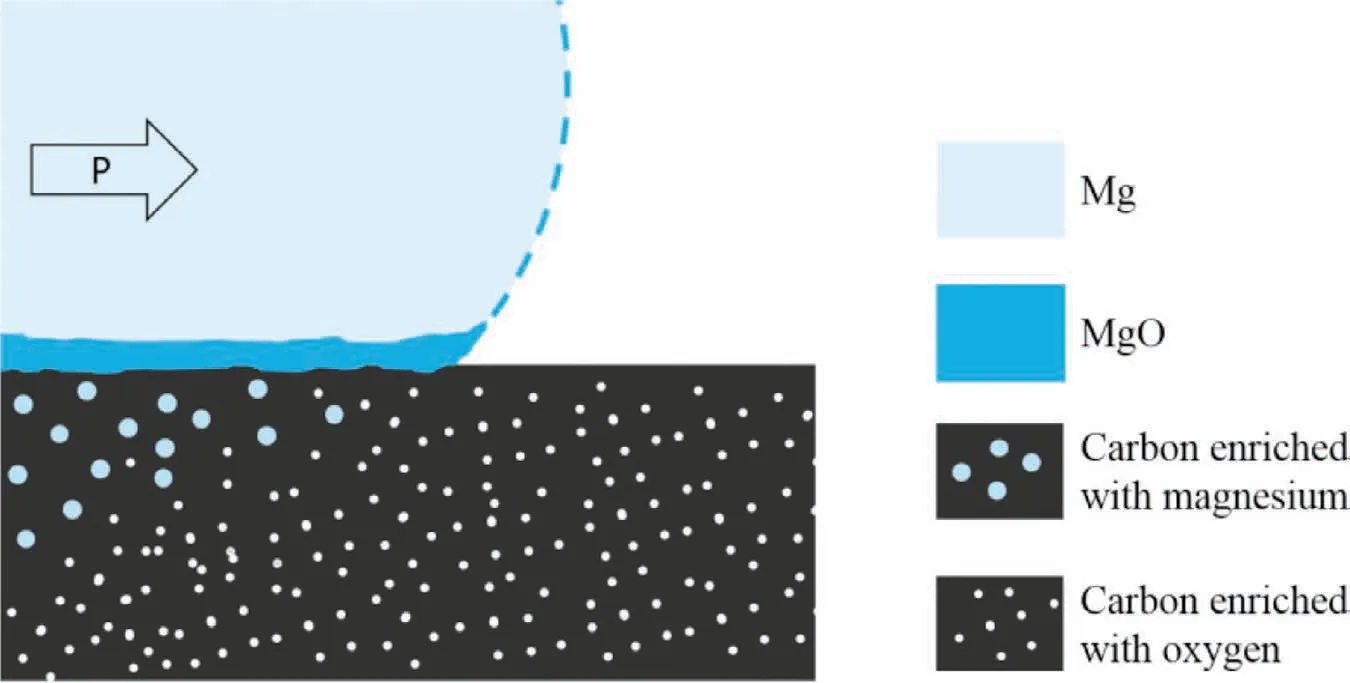
Fig.12.Scheme of microstructural effects occurring at the interface formed between the liquid magnesium and glassy carbon induced by reactive diffusion
4.Conclusions
This study was focused on the analysis of the factors affecting bonding formation between open-celled carbon foam and liquid magnesium.High temperature interaction between these materials was examined at a temperature of 700°C using different processing conditions accompanied by different times and atmospheres,in order to evaluate the possibility of the spontaneous infiltration of molten magnesium into porous carbon foam.The results of this study revealed the following:
(1)Our trials showed no possibility of infiltrating a thick Cofpreform of 97% porosity with molten pure magnesium without external pressure applied on the liquid metal due to the non-wetting character of the Mg-Cofsystem.
(2)The value of the contact angle of the Mg/Cofcouple measured by the sessile drop method combined with non-contact heating and the capillary purification procedure was about 135°,and it did not change over the measurement time of 300 s.These results are in good agreement with those reported in the literature for measurements with graphite substrates and support the results of trials showing the problems with effective pressureless infiltration of a Cofpreform with molten Mg.
(3)Under the conditions of this study,the contact of liquid magnesium and Cofcreated a thin MgO film situated between the components,and this phenomenon was induced by reactive diffusion of the oxygen absorbed by the Cof.The formation of the oxide film plays a fundamental role in the infiltration progress and bonding process.This effect also determines the oxide nature of the bonding,which is characteristic for different types of Mg-C composites.
(4)The experimentally revealed poor wettability of the carbon material by the liquid magnesium should be treated as a technological disadvantage only.This problem can be overcome when an appropriate additional external pressure is applied to the metal during the consolidation process,ensuring a direct contact between the components and inducing MgO interlayer formation.
CRediT authorship contribution statement
Marcin Godzierz:Conceptualization,Validation,Formal analysis,Investigation,Visualization,Writing-original draft.Anita Olszówka-Myalska:Conceptualization,Resources,Formal analysis,Writing-original draft,Writingreview & editing,Supervision.Natalia Sobczak:Methodology,Writing-original draft.RafałNowak:Methodology,Investigation.Patryk Wrze´sniowski:Investigation.
Acknowledgements
This research was funded by the Silesian University of Technology,Faculty of Materials Engineering as a part of statutory research for 2020.
The authors would like to thank theŁukasiewicz Foundry Research Institute in Krakow for the possibility to carry out the sessile drop tests by the capillary purification technique,completed as a part of the academic training of PhD students Marcin Godzierz and Patryk Wrze´sniowski.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Recent developments and applications on high-performance cast magnesium rare-earth alloys
- Surface characterization and corrosion behavior of calcium phosphate(Ca-P)base composite layer on Mg and its alloys using plasma electrolytic oxidation(PEO):A review
- Towards developing Mg alloys with simultaneously improved strength and corrosion resistance via RE alloying
- Magnesium matrix composite reinforced by nanoparticles–A review
- The design of Co3S4@MXene heterostructure as sulfur host to promote the electrochemical kinetics for reversible magnesium-sulfur batteries
- A new die-cast magnesium alloy for applications at higher elevated temperatures of 200–300°C
