Novel technique of sutureless glueless intrascleral fixation of single-piece intraocular lens
2021-03-10
Abstract
•KEYWORDS:scleral fixation; single-piece intraocular lens; Scheimpflug imaging
INTRODUCTION
Till now,the endocapsular placement of the intraocular lens (IOL) is undoubtedly the most preferred site after cataract surgery. There are various options to offer optimum visual rehabilitation in cases with poor or no capsular supporti.e. anterior chamber IOLs (ACIOLs),iris fixated IOLs and scleral fixated IOLs (SFIOL)[1]. Placement of the IOL in the anterior chamber increases the risk of damage to the anterior chamber angle structures and corneal endothelium[2-3].
Use of non-absorbable sutures to suture the IOL to sclera has been the traditionally accepted technique of IOL placements,but associated with various complications like suture-induced inflammation,suture degradation and delayed IOL subluxation or dislocation due to broken suture[4-5]. Later,Schariothetal[6]developed a technique of sutureless scleral fixation of a multipiece IOL using intrascleral placement of the haptics in a scleral tunnel parallel to the limbus. Agarwaletal[7-8]popularized the similar surgical technique using fibrin glue for adherence of scleral flaps. Both of these techniques used three-piece IOL. We aim to assess clinical efficacy,safety,and complexity of sutureless glueless scleral fixation of single-piece IOL in aphakia.
SUBJECTS AND METHODS
The ethical committee of the hospital approved the study and followed the tenents of the Declaration of Helsinki. Well written informed consent was obtained from all patients before surgery. Patients were not given any stipend. It was a hospital-based descriptive type of observational study that included 50 eyes of 50 patients. All aphakic patients above 12y who were ready to give consent were included in study. Exclusion criteria included patients with corneal opacity,retinal disorder,high myopes,optic atrophy,bleeding disorder,pregnancy and those who were unwilling to give consent. Preoperative and postoperative visual acuity,slit lamp and fundus examination,applanation tonometry,keratometry,biometry (CARL ZEISS MEDITEC IOL MASTER),optical coherence tomography (OCT) (TOPCON 3D OCT-2000) were done for an extensive evaluation of anterior and posterior segment.
StatisticalAnalysisWith the use of Software-IBM SPSS 19.0,qualitative data was summarized in the form of proportion. Quantitative data were summarized in the form of mean and SD. The significance of the difference in proportion measured by the Chi-square test. The significance of the difference in mean measured by unpairedt-test or ANOVA whichever is appropriate.P<0.05 was considered as significant.
SurgicalTechniqueAs per the American Academy of Ophthalmology (AAO) recommendations for sulcus implanted IOL power adjustment[9],in patients with a predicted IOL power of less than 18 D,power reduced by 0.5 D; in those with a power of 18 to 25 D,power reduced by at least 1 D; and for lenses greater than 25 D,power was reduced by 1.5 D. Under peribulbar anesthesia,a 5.0 mm conjunctival peritomy was done at 2 o’clock and 8 o’clock positions. Then,2 T-shaped incisions were made 2.0 mm from the limbus exactly 180 degrees apart diagonally. An infusion cannula or anterior chamber maintainer was inserted. To prevent interference with the creation of the T-shaped incision,the infusion cannula should be positioned at 4 o’clock. Anterior vitrectomy (deep core) was performed,if necessary. Sclerotomy was done parallel to the iris at the T-shaped incision with a 23-gauge angled microvitreoretinal (MVR) knife and a scleral tunnel was made parallel to the limbus at the branching point of the T-shaped incision. 2.8 mm keratome was used to make a corneal incision at 10 o’clock through which single-piece foldable IOL was implanted with an injector,the trailing haptic was left outside the incision. The tip of the haptic was then grasped with 24-gauge IOL haptic gripping forceps,pulled through the Sclerotomy,and externalized on the left side. After the trailing haptic was inserted into the anterior chamber and the haptic tip was grasped with a 24-gauge forceps,pulled through the second sclerotomy,and externalized on the right side. The haptic insertion into the anterior chamber may be difficult depending on the material or shape of the haptics,which can cause the IOL to rotate clockwise and the leading haptic to slip back into the eye. To prevent such risks,the IOL optic was pushed to the back of the iris and moved to the 2 o’clock position with a push-and-pull hook inserted through the side port at the 1 o’clock position. The tip of the haptic was subsequently inserted into the limbus-parallel scleral tunnel. Conjunctival peritomy closed. Scheimpflug imaging (OCULUS PENTACAM) was done to evaluate the proper centration of IOL. Follow up was done on 1st,7th,28thpostoperative day,at 3mo and 6mo.

Figure 1 Uncorrected visual acuity on various follow up.
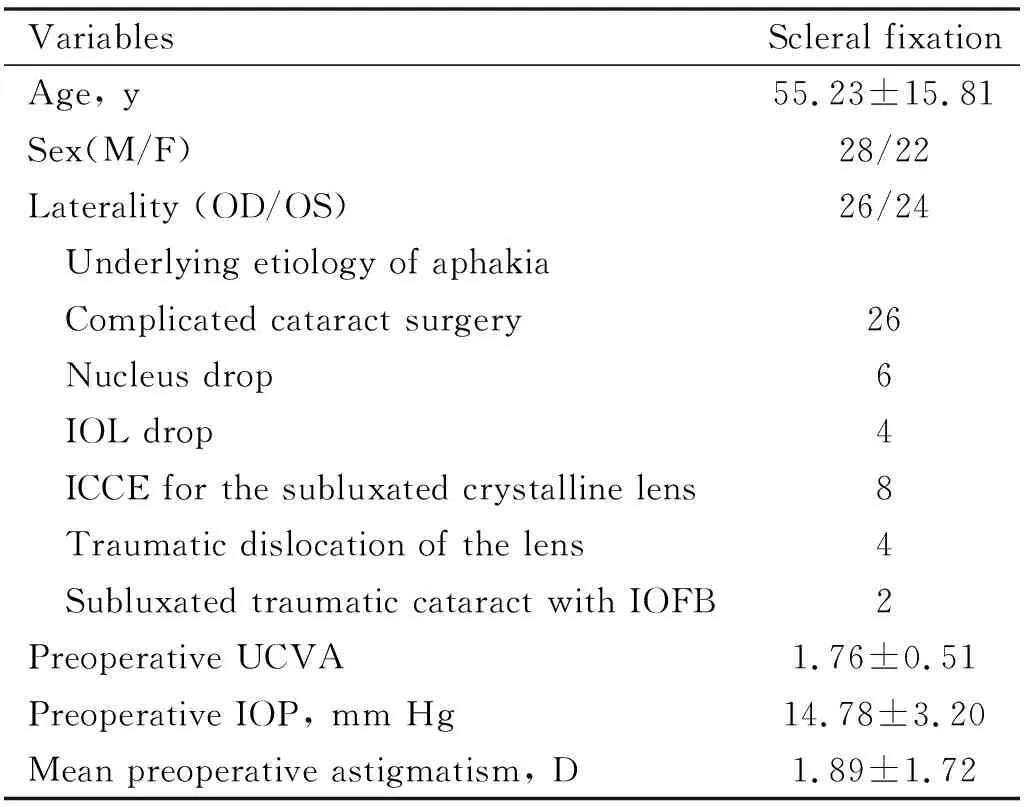
Table 1 Patients demography and preoperative characteristics
RESULTS
The study population consisted of 50 patients (22 females and 28 males). Baseline demography and preoperative ocular characteristics of patients are shown in Table 1.
Cases of aphakia due to complicated cataract surgery underwent deep core anterior vitrectomy. Anterior chamber maintainer was used and a single 23-gauge pars plana incision was made 3.5 mm behind the limbus to allow the unidirectional flow of vitreous (anterior to posterior chamber). Thereafter secondary IOL was implanted. In the rest of the cases,23-gauge primary pars plana vitrectomy with a 360° endolaser was done. Secondary IOL was implanted after 4wk so that the corneal edema or anterior chamber reaction because of the underline etiology or primary surgery get resolved.
Change in uncorrected visual acuity (UCVA) in LogMAR from preoperative (1.76±0.51) value to 6mo postoperatively (0.47±0.29) was highly significant (P<0.01) (Figure 1).
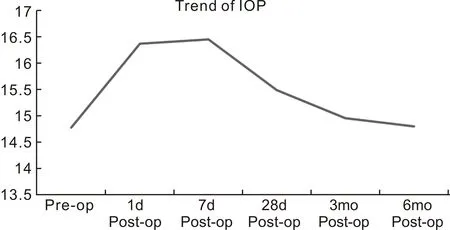
Figure 2 Intraocular pressure on various follow up.

Figure 3 Well-centered intraocular lens on slit lamp undilated pupil (A) and dilated pupil(B).
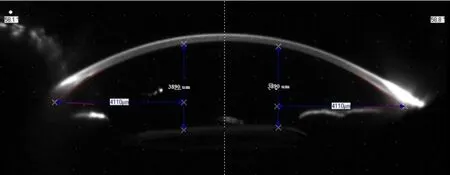
Figure 4 Well-centered intraocular lens on Scheimpflug imaging.
Changes in best-corrected visual acuity (BCVA) in LogMAR from preoperative (0.55±0.19) to 1d follow up (0.59±0.29) was not significant (P=0.8707) but at the end of 6mo (0.34±0.30) change was highly significant (P=0.01). Changes in astigmatism (preoperative: 1.89±1.72 D,after 6mo: 1.72±1.48 D) as measured by Scheimpflug imaging was found insignificant (P=0.6028),showing that scleral tunnel made in this technique does not affect corneal astigmatism.
Mean intraocular pressure (IOP) was 14.78±3.20 mmHg preoperatively while it was 14.80±1.66 mmHg postoperatively at 6mo (Figure 2). Mean IOP change from the preoperative period to 6mo postoperative period was not statistically significant (P=0.9688). Mean change in IOP from preoperative period to postoperative 1d (16.24±2.23) (P=0.0096) and 7d (16.42±4.66) (P=0.0429) was found significant as there were some cases of raised IOP due to inflammation or pigment release due to maneuvering. These cases were treated medically and on the next follow up their IOP came out normal.
On 1d,there were 2 (4%) cases of corneal edema due to surgical manipulations which got resolved on next follow up,on 7d there were 3 (6%) cases of raised IOP which were managed medically and 1 (2%) case of decentration which was recentered surgically,at 3mo,there was 1 (2%) case of cystoid macular edema (CME) which was managed medically and 1 (2%) case of decentration which was recentered surgically. In the end,all cases have well-centered IOL with good visual acuity (Figures 3 and 4).
DISCUSSION
The crystalline lens-zonule barrier provides a stabilizing effect on the eye. When our eye moves,kinetic energy is acquired from its muscles and attachments and this energy is dissipated to the internal fluids as it stops. So placing the IOL closer to the rotational center of the eyei.e. just anterior to the vitreous face,reduces the centrifugal forces on the lens and stabilizes the ocular contents,thereby decreasing the probability of complications such as iritis,CME,and retinal detachment. And also,there is the superior optical quality when the lens is positioned closer to the nodal point and center of rotation of the eye[10-11]. Sulcus fixation of single-piece IOLs is not popular because bulky single-piece IOL haptics are large and thick enough to contact the posterior iris when placed in the sulcus. Also,the haptics are planar rather than angulated and therefore do not vault the optic posteriorly from the iris,results in complications like pigment dispersion,iris transillumination defects,secondary glaucoma and dysphotopsia[12-14].
Single-piece Tecnis®1 foldable lenses have an offset design and in our technique,the greater part of haptic is externalized. So neither the optic nor haptics come in contact with the iris,thereby minimizing the above-mentioned complications[15-16]. Single-piece IOL haptics are sturdy,this allows easy exteriorization. Sclerotomies are blocked completely by the thick stubby haptics and provides stable fixation; mitigating the need for suturing. However,sclerotomies can be sutured with absorbable suture when needed,preventing the haptics from early slippage. To avoid IOL tilt,sclerotomies should be placed exactly 180° apart and 1.5 mm behind the limbus on both sides. An adequate and equal amount of haptic should be inserted in the respective scleral pockets to ensure exact horizontal alignment. The two most important requirements for ciliary sulcus placement of IOL are sufficient posterior iris clearance and secure fixation. The stable fixation ensures long-term centration and avoids lens movement or tilting that can cause uveal irritation,microhyphema,and pigment dispersion syndrome. The haptics should be angulated posteriorly to maximize iris clearance,otherwise,posterior iris rubbing can cause chronic inflammation and pigment dispersion[17-18]. Ideally,the anterior optic surface should be smooth and have rounded edges to minimize iris chafing when any posterior iris contact occurs. It has been shown that the overall length (12.5-14.0 mm) of the IOL helps ensure firm,stable fixation at the posterior chamber behind the iris,where the average diameter in emmetropic eyes is approximately 13.0 mm. Also,the large optics lowers the risk of clinically significant postoperative decentration[19-20]. Externalization of the greater part of the haptic along its curvature and fixation in a scleral tunnel (as in our technique) stabilizes the axial positioning of the IOL and thereby prevents IOL tilt.
In this study,we have seen favorable and safe results with stable and well-centered single-piece IOL. However,like any other technique,there is a learning curve involved. This technique is evolving and long term safety concerns,especially regarding lens centration,haptic erosion,dislocation,need to be addressed.
