Transjugular portosystemic shunt for early-onset refractory ascites after liver transplantation
2021-03-05GiuseppeBiancoMarcoMariaPascaleFrancescoFrongilloEridaNureSalvatoreAgnesGabrieleSpoletini
Giuseppe Bianco, MarcoMaria Pascale , Francesco Frongillo, Erida Nure, Salvatore Agnes,Gabriele Spoletini
General Surgery and Liver Transplant Unit, Fondazione IRCCS - Policlinico Universitario “A. Gemelli”, Rome 00168, Italy
Liver transplantation (LT) is the most effective treatment for end-stage liver disease and complications of portal hypertension(PHT). However, PHT can relapse as a consequence of viral, alcoholic or metabolic chronic liver disease (CLD) recurrence, rejection, vascular abnormalities, small-for-size syndrome and technical complications (e.g., portal or hepatic veins stenosis and/or thrombosis) [1].
LT recipients with PHT may suffer from com plications similarly to non-transplanted cirrhotic patients (e.g., refractory ascites,variceal hemorrhages and hydrothorax) although with higher morbidity and mortality due to their postoperative status and concomitant immunosuppressive therapy [2].
Refractory ascites (RA) has been reported in 5%–7% of LT recipients. Most cases develop after recurrent CLD and PHT while a minority of recipients develop early after LT [3]. Treatment options include medical, surgical and endovascular treatments. Medical treatment is based on the use of diuretics, although this often may increase the risk of nephrotoxicity, especially in patients with previous hepatorenal syndrome and on calcineurin inhibitors [4]. Surgical treatment is based on perito-venous derivation and peritoneum-to-bladder pumps but has limited role in the LT recipient setting [5]. Endovascular treatment is utilized when medical management fails or is contraindicated. Transjugular portosystemic shunt (TIPS) is largely utilized in the management of complicated PHT. Instead, splenic artery embolization was regarded as a valid alternative to TIPS in these patients, especially in the past, with a relevant risk of splenic infarction [5]. However, the use of TIPS in LT recipients is still sparse and based on case reports and series.
The aim of our work was to describe the use of TIPS in LT patients from our center who developed early-onset RA, in the absence of recurrent CLD, and to concisely review the existing literature.
After interrogating our prospectively maintained database of 593 consecutive LTs performed between 1991 and 2018, we identified three patients who developed early-onset RA (i.e., within 90 days from LT) due to PHT without recurrent CLD and treated with TIPS. Since 2004, our standard implant technique consists of sideto-side piggyback caval anastomosis. RA was defined as ascites resistant to diuretic therapy (spironolactone up to 400 mg/day and furosemide up to 160 mg/day), salt restriction and requiring repeated paracentesis. Patients who developed RA after LT in our center were managed according to the decisional pathway depicted in Fig. 1 . Ascites was analyzed routinely to rule out infections, malignancies and other extra-hepatic etiologies of ascites. Each case was discussed within our multidisciplinary team (MDT) before finalizing the treatment. The characteristics of the three cases are summarized in Table 1 .
Case #1: A 42-year-old male patient underwent LT in 2017 for cryptogenic liver cirrhosis complicated by partial portal vein thrombosis (grade 1 according to Yerdel’s classification), on anticoagulant treatment. Model for end-stage liver disease (MELD) score was 13. During LT, three liters of clear ascites were drained. At post-LT day 22, the patient developed RA which required paracentesis due to respiratory distress, after a failed trial of high-dose diuretics. Ascitic analysis ruled out infections.
Recurrent PHT was suspected and hepatic venous pressure gradient (HVPG) was measured, revealing a 20 mmHg porto-systemic gradient, thus confirming the diagnosis. Contrast-enhanced computed tomography (CT) and phlebography did not reveal venous outflow obstruction through the caval anastomosis. A liver biopsy showed no intrahepatic cause for PHT. Sixty-four days after LT, TIPS was performed. Access was obtained through the right subclavian vein. The cavo-caval anastomosis was crossed without technical difficulties; a communication between the right hepatic vein and the right portal branch was obtained. Using a double stent graft,an 8 mm caliber porto-systemic shunt was created.
Over the next few days after TIPS positioning there was a progressive reduction in ascites accumulation and diuretic treatment was gradually weaned off. Neither encephalopathy nor hemodynamic dysfunction was developed. The patient had recurrent pneumonia, which required antibiotics again. During the course of the hospitalization, TIPS function was monitored with serial ultrasound scans. Body weight and abdominal circumference decreased until stabilizing to satisfactory levels. After 28-month post-TIPS followup, the patient has not developed RAs and the TIPS maintains good function, without shunt-related side effects.
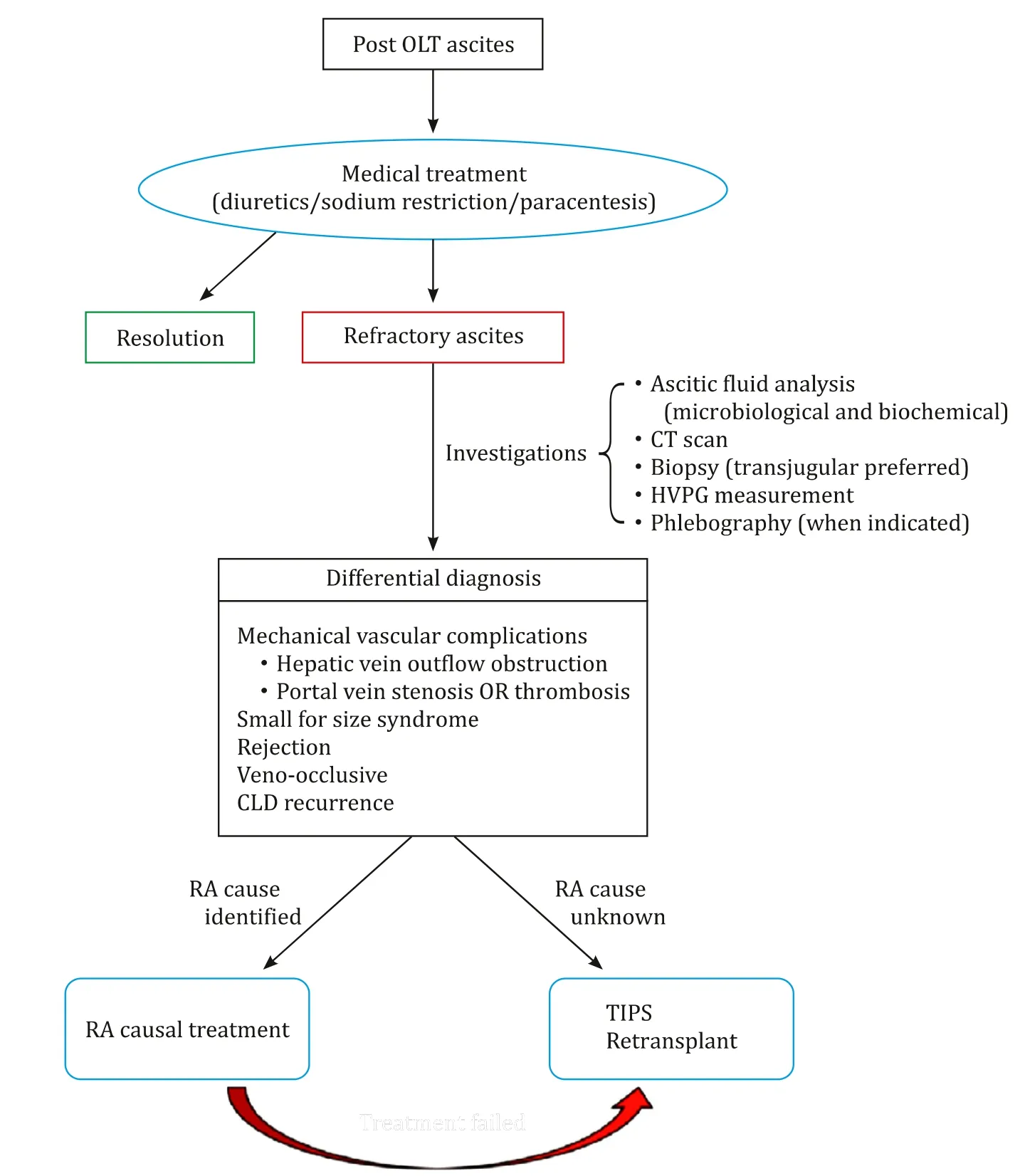
Fig. 1. Flow chart describing the decisional pathway for TIPS in patients with refractory ascites after LT. OLT: orthotopic liver transplantation; CT: computed tomography;HVPG: hepatic venous pressure gradient; CLD: chronic liver disease; RA: refractory ascites; TIPS: transjugular portosystemic shunt.
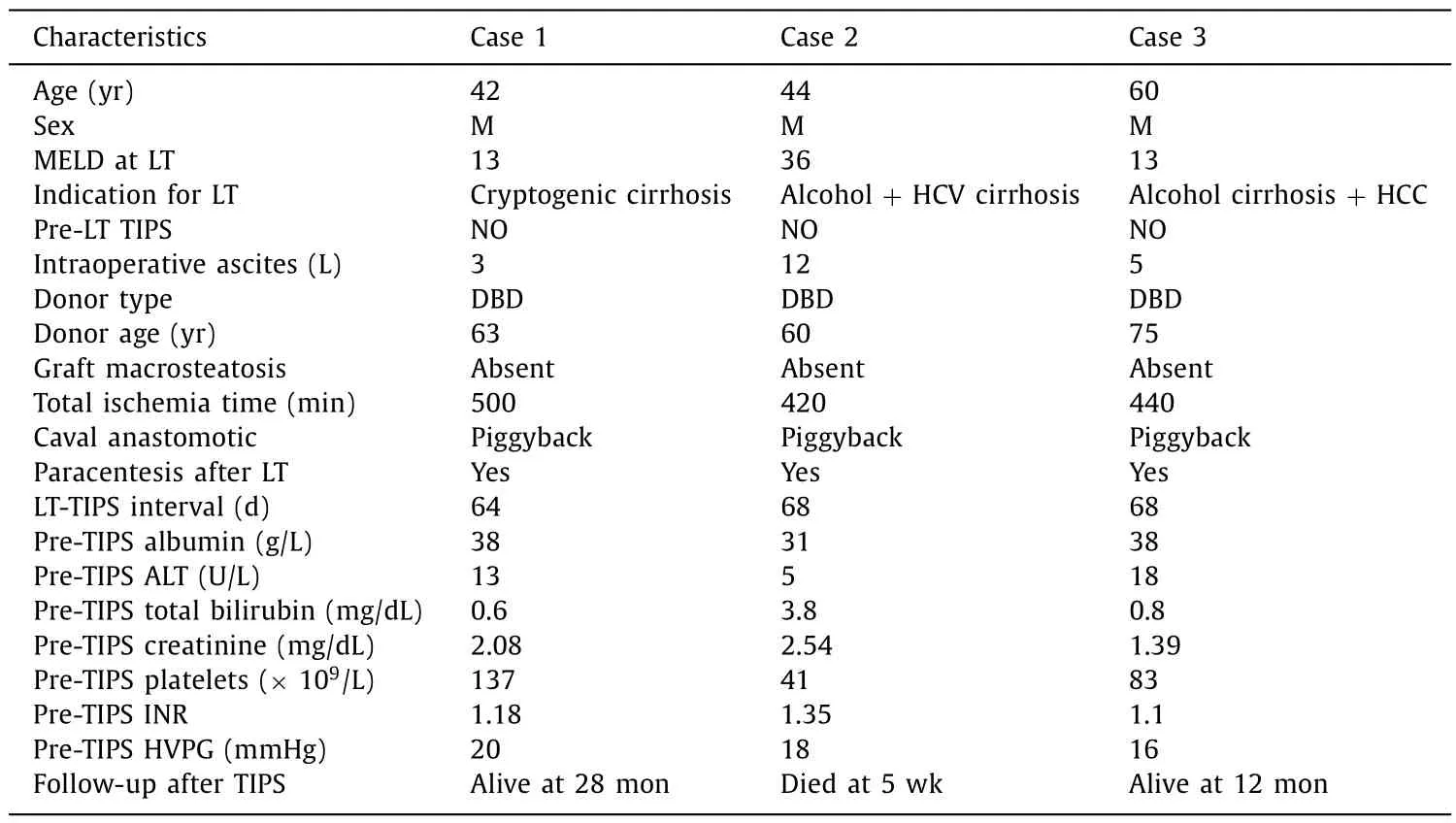
Table 1 Summary of patients treated with TIPS for post-liver transplant refractory ascites.
Case #2: A 43-year-old male patient underwent LT in 2017 for hepatitis C virus (HCV) and alcohol-related decompensated liver cirrhosis, with a MELD score of 36. The patient suffered from RA but did not undergo TIPS prior to LT due to decompensated cirrhosis. During LT, about 12 liters of clear ascites were drained.
The postoperative course was characterized by a mild transaminase peak [highest alanine transaminase (ALT) value: 303 U/L; on post-LT day 4], a slow descent in bilirubin levels (starting from 25 mg/dL pre-LT) and acute kidney injury. On post-LT day 12 ascites formation began with a daily drain output ranging from 10 0 0 to 20 0 0 mL. Pleural effusion causing respiratory distress required pleural drainage at day 14 (1500 mL of clear fluid was drained). A biopsy-proven acute rejection at day 15 was treated with steroid pulses, followed by improvement in liver function tests.
After a failed trial of high-dose diuretics for ongoing ascites,HVPG revealed an 18 mmHg porto-systemic gradient, which confirmed PHT. No caval stenosis was detected. A repeated liver biopsy was inconclusive in regards of a possible cause for RA. Due to persistent mild renal failure, a non-contrast enhanced CT was performed to rule out gross intra-abdominal abnormalities. In the following days, liver function tests continued to improve slowly whilst ascites was still not responsive to medical treatment. Repeat HCV-RNA polymerase chain reaction (PCR) tests remained negative.Concern grew around renal failure being aggravated by diuretics and forcing us to minimize calcineurin inhibitors dose. The MDT board decided for TIPS positioning which took place at post-LT day 68. The technique was similar to that used in Case #1 except that the shunt was created between the middle hepatic vein and the right portal branch using a 10 mm polytetrafluoroethylene (PTFE)stent graft.
In the following days after TIPS the volume of ascites and renal function were slowly improved and there were no complications related to the TIPS. However, two weeks after TIPS, and after more than 60 days of hospitalization, the patient developed sepsis from multi-resistant microorganisms (as demonstrated by blood and bile culture positivity) and symptomaticClostridiumdifficileinfection,in absence of undrained potential sources of sepsis. Immunosuppression was lowered and broad-spectrum antibiotics were administered. Despite all efforts, the patient developed multi-organ failure and passed away at post-LT day 100 and post-TIPS placement week 5.
Case #3: A 60-year-old male patient underwent LT in 2018 for hepatocellular carcinoma (HCC) and alcoholic cirrhosis with an MELD score of 13. During the operation, a grade-1 Yerdel portal vein thrombosis was found and treated with eversion thrombectomy. During the postoperative course the patient developed acute rejection responding to steroid pulses. The patient was discharged at post-LT day 14, with good graft function and general condition.Six weeks later after discharge, during a follow-up appointment,he was found with ascites and 5 kg weight gain. An ultrasound scan and a subsequent CT scan confirmed the presence of ascites without vascular abnormalities of the graft. The patient was admitted and treated with diuretics. After failure of medical management, HVPG revealed a 16 mmHg porto-systemic gradient which confirmed PHT. At the same time, a transjugular liver biopsy was taken. Histopathology showed no obvious intrahepatic cause for the recurrent PHT. At post-LT day 68, a TIPS was placed (right hepatic to right portal vein linked through two endoprosthesis) after demonstrating no caval anastomosis outflow impairment. At the end of the procedure, the portal pressure decreased from 35 to 20 mmHg. The patient was discharged on low-dose diuretics, after a 4 kg body weight reduction and resolution of ascites. At 12-month follow-up post-TIPS positioning, the patient is still on lowdose diuretics and free from ascites and TIPS-related side effects.A CT for HCC recurrence surveillance revealed absence of free intraperitoneal fluid and a patent TIPS.
RA is a negative prognostic factor in patients with CLD, due to its detrimental effect on renal and respiratory functions, electrolyte imbalance and infectious risks. Similarly, RA can be debilitating and life-threatening when occurring after LT. In the majority of cases, PHT and ascites after LT are due to the recurrence of CLD or to other (probably multifactorial) mechanisms [6]. RA prompts symptomatic and possibly causal treatment, as LT recipients can be particularly susceptible to complications due to their recently incepted immunosuppression and postoperative status. In addition,diuretics are used with caution in patients on calcineurin inhibitors in order not to impair their renal function [7]. When the underlying cause for RA is recurrent PHT (confirmed with HVPG measurement), the use of TIPS in the post-LT setting has been adopted successfully. Multicenter data indicate resolution of post-LT RA in 57% of cases [8]. On such basis, in our center we consider TIPS a viable option while retransplantation is kept as a rescue option when all other treatments fail and in cases with severe liver damage on graft biopsy. TIPS placement after piggyback LT can be more challenging due to the anatomical changes of the hepatic veins and difficulty in cannulating the graft cava. These issues have been overcome thanks to the increased confidence in endovascular techniques, achieving technical success in 98% of cases [8].
Piggyback caval anastomosis has been by far the most widespread cava reconstruction technique in LT for the last two decades. A well-known drawback of the piggyback caval reconstruction is impaired venous outflow, although in a small number of recipients. In such cases, endovascular dilatation of the stenotic anastomosis can be utilized to resolve PHT. Surgical refashioning of the anastomosis or adding a second anastomosis using the caudal end (i.e., the ligated end) of the graft cava has been practiced too. In our series, on phlebography, none of the cases had stenosis of the cavo-caval anastomosis and therefore we decided to treat PHT with a TIPS. The use of TIPS in LT recipients has a reported incidence of 33% compared to 29%–55% in non-LT cirrhotic patients [8]. Our patients did not develop encephalopathy, which is a favorable outcome we explain with the underlying preserved liver function in LT recipients compared to cirrhotic patients. Nevertheless, Jahangiri et al reported higher incidence of encephalopathy due to the neurotoxic effect of calcineurin inhibitors that are not metabolized by the first pass metabolism of the liver [9].
In the last decade, there has been growing experience with TIPS in LT recipients and several authors reported their case series. Saad et al. [10]described the largest series to date of 39 cases of post-LT TIPS, of whom 90% had RA and the remaining 10% had variceal bleeding, and reported a median decrease of porto-systemic gradient of 10 mmHg and a median MELD score increase of 6 points after TIPS placement. King et al. [11]reported a series comparing 22 LT recipients with 44 matched non-transplanted controls undergoing TIPS, and demonstrated that the mortality of the patients with a pre-TIPS MELD score>15 was significantly higher in the LT group. The median time from LT to TIPS placement was of 45 months and 50% of the patients underwent TIPS due to PHT from vascular origin and 32% had recurrent CLD.
The patients from our center differ from those of Saad et al. and King et al. as they did not have recurrent CLD, and neither of them had demonstrable vascular outflow impairment and required TIPS much earlier. The use of TIPS in LT recipients without recurrent CLD is an infrequent scenario.
In our report, we achieved a complete resolution of RA in Cases#1 and #3; patients progressed to hospital discharge and returned to their normal lives. Conversely, in Case #2, TIPS helped resolve RA and consequently renal function, however it is not known whether and to what extent this contributed to impair the recovery of graft function and susceptibility to infections. When RA from recurrent PHT is demonstrated and all possible correctable rootcauses have been ruled out, TIPS can be a safe option once medi-cal treatment fails. Caution must be taken in cases with borderline liver function as TIPS can interfere with graft function recovery.
Acknowledgments
None.
CRediT authorship contribution statement
Giuseppe Bianco:Conceptualization, Data curation, Formal analysis, Writing - original draft.Marco Maria Pascale:Conceptualization, Data curation, Formal analysis, Writing - original draft.Francesco Frongillo:Supervision, Writing - review & editing.Erida Nure:Supervision, Writing - review & editing.Salvatore Agnes:Supervision, Writing - review & editing.Gabriele Spoletini:Conceptualization, Data curation, Formal analysis, Writing - original draft.
Funding
None.
Ethical approval
Informed consent to publish this report was obtained, although no information that makes the subjects identifiable is provided in the manuscript. The patient described in case #2 is deceased and no consent was sought from the relatives as no identifiable information is provided in the report.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- BCLC staging system and liver transplantation: From a stage to a therapeutic hierarchy
- Multidisciplinary management of patients with post-inflammatory pancreatic necrosis
- Abdominal drainage systems in modified piggyback orthotopic liver transplantation
- Liver transplantation for liver failure in kidney transplantation recipients with hepatitis B virus infection
- Giant pseudoaneurysm of the splenic artery within walled of pancreatic necrosis on the grounds of chronic pancreatitis
- Hepatic isolated ectopic adrenocortical adenoma mimicking metastatic liver tumor
