香菇多糖对氨氮胁迫下泥鳅消化酶、肠道菌群、肌肉成分及组织结构的影响
2021-02-26刘成荣刘丽香
刘成荣 刘丽香
摘 要:研究飼粮添加香菇多糖对氨氮胁迫下泥鳅消化酶、肠道菌群、肌肉成分、组织结构的影响。选取初始体重为(8.9±0.3)g的健康泥鳅,随机分为7个处理组,对照组饲喂基础饲料,试验组饲喂基础饲料附加1.0 mg·g-1香菇多糖,对照组及试验组均分别养殖在相应氨氮浓度100、200、300 mg·L-1的水中;另设无氨氮胁迫组养殖于氨氮浓度为0 mg·L-1水中并饲喂基础饲料。每个处理3次重复,每次重复60尾鱼,养殖试验为35 d,分别于第7、14、21、28和35 d采样。结果表明:各试验组泥鳅消化道蛋白酶、淀粉酶和脂肪酶活性均分别高于对照组(P<0.05);在第28 d,100 mg·L-1试验组泥鳅消化道蛋白酶、淀粉酶和脂肪酶活性均达到最高,分别为(1953.8±39.31)、(21.5±3.37)、(9.64±0.32)U·mL-1。在第35 d,各试验组泥鳅消化道乳酸杆菌和芽孢杆菌数量均显著高于对照组(P<0.05),而大肠杆菌数量显著低于对照组(P<0.05)。在第35 d,各试验组泥鳅肌肉水分含量显著低于对照组(P<0.05),而蛋白质含量和脂肪含量显著高于对照组(P<0.05)。100 mg·L-1氨氮胁迫下试验组泥鳅肝胰脏和鳃组织结构比较完整,细胞结构正常,而其对照组泥鳅组织细胞坏死,组织结构崩溃,组织细胞功能受损害。综合分析表明,香菇多糖能提高氨氮胁迫下泥鳅消化道的蛋白酶、淀粉酶和脂肪酶活性,提高消化道中乳酸杆菌、芽孢杆菌数量,降低大肠杆菌数量,提高肌肉蛋白质和脂肪含量、降低水分含量,提高泥鳅鳃、肝胰脏抗氨氮胁迫的能力,有利于鱼类的健康。
关键词:香菇多糖;泥鳅;消化酶;肠道菌群;肌肉成分;组织结构
中图分类号:S 966.4 文献标志码:A 文章编号:0253-2301(2021)12-0045-10
DOI: 10.13651/j.cnki.fjnykj.2021.12.008
Effects of Mushroom Polysaccharides on the Digestive Enzymes, Intestinal Flora, MuscleComposition and Tissue Structure of Loach Under the Ammonia-nitrogen Stress
LIU Cheng-rong1,2, LIU Li-xiang3
(1. College of Environment and Bioengineering, Putian University, Putian, Fujian 351100, China;
2. Fujian Key Laboratory of Eco-toxicological Effects and Control of New Pollutants, Putian, Fujian 351100, China;
3. Finance Department, Putian University, Putian, Fujian 351100, China)
Abstract: In order to study the effects of mushroom polysaccharides added into the diets on digestive enzymes, intestinal flora, muscle composition and tissue structure of loach under the ammonia-nitrogen stress, the healthy loach with initial weight of (8.9±0.3) g was selected and randomly divided into seven treatment groups. The control group was fed with basal diet, while the experimental group was fed with basal diet supplemented with 1.0 mg·g-1 mushroom polysaccharides. The control groups and experimental groups were cultured in the corresponding water with the ammonia-nitrogen concentrations of 100 mg·L
-1, 200 mg·L-1, and 300 mg·L-1, respectively. In addition, the group without the ammonia-nitrogen stress was cultured in the water with the ammonia-nitrogen concentration of 0 mg·L-1 and fed with basal diet. Each treatment was repeated for 3 times with 60 fish in each replicate. The experiment lasted for 35 days, and the samples were taken on the 7th, 14th, 21st, 28th and 35th day, respectively. The results showed that: the activities of protease, amylase and lipase in the digestive tract of loach in each experimental group were higher than those in the control group (P<0.05). On the 28th day, the activities of protease, amylase and lipase in the digestive tract of loach in the experimental group with 100 mg·L-1 ammonia nitrogen stress were the highest, which were (1953.8±39.31) U·mL-1, (21.5±3.37) U·mL-1 and (9.64±0.32) U·mL-1, respectively. On the 35th day, the number of Lactobacillus and Bacillus in the digestive tract of loach in each experimental group was significantly higher than that in the control group (P<0.05), while the number of Escherichia coli was significantly lower than that in the control group (P<0.05). On the 35th day, the muscle moisture content of loach in each experimental group was significantly lower than that in the control group (P<0.05), while the protein content and fat content were significantly higher than those in the control group (P<0.05). Under the ammonia-nitrogen stress of 100 mg·L-1, the tissue structure of hepatopancreas and gills of loach in the experimental group was relatively complete, and the cell structure was normal, while the tissue cell in the control group was necrotic, the tissue structure collapsed and the tissue cell function was impaired. The comprehensive analysis showed that mushroom polysaccharides could improve the activities of protease, amylase and lipase in the digestive tract of loach under the ammonia-nitrogen stress, increase the number of Lactobacillus and Bacillus in the digestive tract, reduce the number of Escherichia coli, increase the contents of protein and fat in muscle, reduce the water content, and improve the ability of resisting the ammonia-nitrogen stress in the gills and hepatopancreas of loach, which was beneficial to the health of fish.
Key words: Mushroom polysaccharides; Loach; Digestive enzymes; Intestinal flora; Muscle composition; Tissue structure
水产养殖业已经成为最具前景和发展最快的行业之一,养殖水产动物能提供高质量的动物蛋白而深受人们的喜爱[1]。随着市场对水产品需求的增加,水产养殖大多为集约化高密度养殖,但鱼类采用集约化高密度养殖方式池塘底部会出现很多残饵粪便,极易引起氨氮增高[2]。研究表明,当氨氮浓度过高时,大多数硬骨鱼体内含氮产物的排泄被破坏,最终导致氨氮胁迫、氨中毒[3-4]。当鱼类出现氨氮胁迫时,会出现包括生长性能下降[5-6]、鳃组织和肝脏病变[7],免疫抑制和易感染疾病[8-9]。目前,有关氨氮胁迫对鱼类生理功能影响的研究已有一些报道,如许齿弹涂鱼Periophthalmodon schlosseri、薄氏大弹涂鱼Boleophthalmus boddaerti[10]、尼罗罗非鱼Oreochromis niloticus[11]、鳙鱼Hypophthalmythys nobilis[12]、草鱼Ctenophynodon idellus[13]和鲫鱼Carassius auratus[14]等。目前生产上大多使用抗生素和化学药物治疗鱼病,易造成环境污染并产生病菌抗药性和药物残留等问题[15]。有研究表明免疫增强剂可以代替抗生素或药物来治疗病害的发生[16]。
在免疫增強剂中,多糖可促进动物生长发育、提高免疫反应、提高动物肠道有益菌群数量、增加水生动物的抗病力且不污染环境,不产生抗药性、无药物残留等[17-21]。香菇多糖是从香菇提取的一类有抗氧化、抗肿瘤、提高免疫力、抗微生物和代谢调节作用的重要生理功能的生物活性物质[22-23]。泥鳅是我国一种重要的底栖鱼类,其营养丰富、味道鲜美,受到人们的欢迎,其在养殖过程中也往往会受到氨氮胁迫的危害。前人对泥鳅在氨氮胁迫方面展开了相关研究,如郝小凤等[24]研究氨氮对泥鳅Misgurnus anguillicaudatus的急性毒性和胁迫后对其肝鳃超微结构的影响; 刘洋等[25]研究了氨氮胁迫对泥鳅肝、鳃和肌肉组织SOD和GSH-Px的影响;张云龙等[26-27]研究了氨氮胁迫对大鳞副泥鳅Paramisgurnus dabryanus组织谷氨酰胺含量的影响以及氨基酸代谢调控的作用。但迄今未见香菇多糖对氨氮胁迫下泥鳅生理功能影响的报道,本研究探索在基础饲料中添加香菇多糖对氨氮泥鳅泥鳅消化酶活性、肠道菌群,肌肉成分和肝胰脏、鳃等组织结构的影响,以期为泥鳅健康养殖提供参考依据。
1 材料与方法
1.1 供试材料及试剂
供试泥鳅购买于莆田超市,体质量(8.9±0.3)g。
试剂:3,5二硝基水杨酸、氢氧化钠、酒石酸钾钠、亚硫酸钠、葡萄糖、碳酸钠、硫酸铜、酒石酸钾钠及聚乙烯醇均为分析纯试剂,麦康凯培养基、乳酸杆菌选择培养基、芽孢杆菌选择培养基为化学纯试剂配制。
1.2 试验方法
将水放在太阳光下或暗处静置48 h,以去除水中的有害气体,将泥鳅放养在装有上述30 L的容器内暂养3 d,无病害后正式开始试验,选取初始体重为(8.9±0.3)g的健康泥鳅,随机分为7个处理组,对照组与试验组分别饲喂基础饲料及在基础饲料中添加1.0 mg·g-1香菇多糖的饲料且分别养在相应氨氮浓度(100、200、300 mg·L-1)(香菇多糖添加量是根据香菇多糖对泥鳅最佳生理效应的用量而定的;氨氮浓度由96 h LC50而定)的水中;另设0 mg·L-1组养殖于氨氮浓度为0 mg·L-1水中并饲喂基础饲料。每个处理3次重复,每个重复60尾鱼,共420尾。投喂量以30 min内摄食完成为宜,每2 d换同浓度的氨氮水溶液,试验周期35 d,在试验第7、14、21、28、35 d取样,测定各处理组泥鳅消化道蛋白酶、淀粉酶和脂肪酶活性;在试验第35 d测定各处理组泥鳅消化道菌群数量,各组泥鳅肌肉成分和泥鳅肝胰脏、鳃组织结构的变化情况。
1.2.1 泥鳅消化酶活性的测定 (1)泥鳅消化道消化酶的酶液制备。试验第7、14、21、28、35 d,分别从每个处理组中随机取3尾泥鳅,并将泥鳅断头处死,后分离出泥鳅肠道,对各肠道组织加入10倍质量的去离子水,匀浆,4℃,12000 r·min-1离心10 min,所得上清液即为酶液。将所得酶液于冰箱4℃储存,24 h内测定泥鳅淀粉酶、蛋白酶和脂肪酶活性。每个指标测定3次。(2)消化酶活性测定。消化酶活性测定参考丁茜的方法[28]。淀粉酶活性测定采用DNS法,蛋白酶活性测定采用福林酚试剂法,脂肪酶活性测定采用聚乙烯醇橄榄油乳化液水解法。
1.2.2 泥鳅消化道大肠杆菌、乳酸杆菌和芽孢杆菌数量测定 在试验第35 d, 从处理组中分别随机取3尾泥鳅将泥鳅断头处死后解剖取出肠道,将肠道外表面的附着物小心地全部去除干净,分别用生理盐水轻擦肠道和用75%乙醇对肠道外表面进行消毒,然后在无菌环境下研磨成浆,用无菌水稀释成10-1至10-6稀释梯度,将后3个稀释浓度样品(200 μL)接种到大肠杆菌选择培养基(麦康凯培养基)、乳酸杆菌选择培养基、芽孢杆菌选择培养基,后倒置放在37℃培养48 h后进行消化道大肠杆菌、乳酸杆菌和芽孢杆菌的计数。
1.2.3 泥鳅肌肉成分测定 泥鳅肌肉成分测定方法参考蒲宗旺等[29]的方法。在试验第35 d, 从处理组中,分别随机取3尾泥鳅,将泥鳅断头处死,将泥鳅进行解剖后,取出泥鳅体背部的肌肉用于泥鳅肌肉蛋白质、脂肪和水分含量测定。蛋白质测定采用凯式定氮法,脂肪测定采用索氏抽提法,水分测定采用常压烘干法。
1.2.4 泥鳅肝胰脏和鳃组织结构观察 在试验第35 d,从处理组中分别随机取3尾泥鳅,断头处死后解剖取泥鳅鳃和肝胰脏,经PBS漂洗后用固定液固定过夜, 制作石蜡切片。具体方法参考吴利敏等的方法[30]。
1.2.5 数据处理 试验数据均采取平均值±标准差表示,采用SPSS 13.0软件对数据进行单因素方差分析和双因素方差分析,当P<0.05即认为有显著性差异。
2 结果与分析
2.1 香菇多糖对氨氮胁迫下泥鳅消化道蛋白酶、脂肪酶和淀粉酶活性的影响
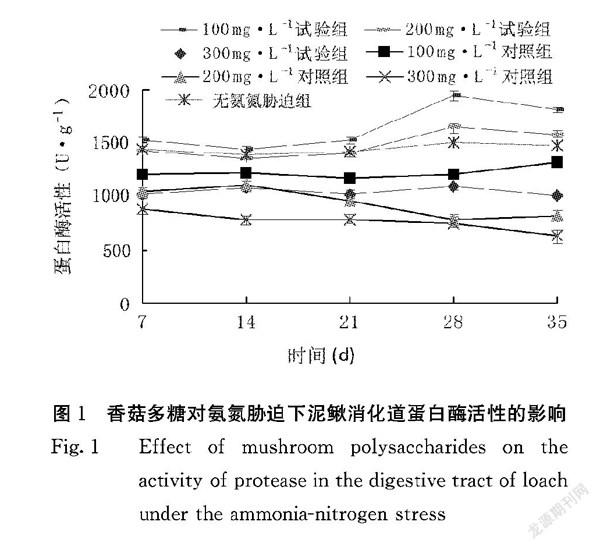
2.1.1 香菇多糖对氨氮胁迫下泥鳅消化道蛋白酶活性的影响 香菇多糖对氨氮泥鳅消化道蛋白酶活性的影响见图1。从第7~14 d,各试验组泥鳅消化道蛋白酶活性一直下降,但从第14 d之后到第28 d,试验组泥鳅消化道蛋白酶活性不断上升,到第28 d时,100 mg·L-1试验组泥鳅消化道蛋白酶活性达到最高,为(1953.8±39.31)U·mL-1,显著高于其他各组(P <0.05)。100、200、300 mg·L-1试验组泥鳅消化道蛋白酶活性均分别显著高于100、200、300 mg·L-1对照组(P<0.05),由此说明香菇多糖可以提高氨氮胁迫泥鳅消化道蛋白酶的活力。
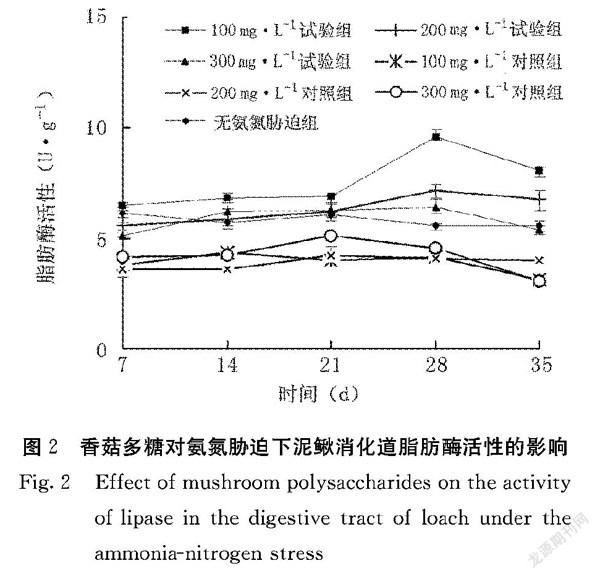
2.1.2 香菇多糖对氨氮胁迫下泥鳅消化道脂肪酶活性的影响 香菇多糖对氨氮胁迫泥鳅消化道脂肪酶活性的影响见图2。第7~28 d,100、200、300 mg·L-1试验组泥鳅消化道脂肪酶活性均在不断上升,到第28 d时达到最大,其中100 mg·L-1试验组泥鳅消化道脂肪酶活性达到最高,为(9.64±0.32)U·mL-1,显著高于其他处理组(P<0.05)。100、200、300 mg·L-1试验组泥鳅消化道脂肪酶活性分别高于100、200、300 mg·L-1对照组,差异均达显著水平(P<0.05)。由此说明,香菇多糖能显著提高氨氮胁迫下泥鳅消化道的脂肪酶活性。
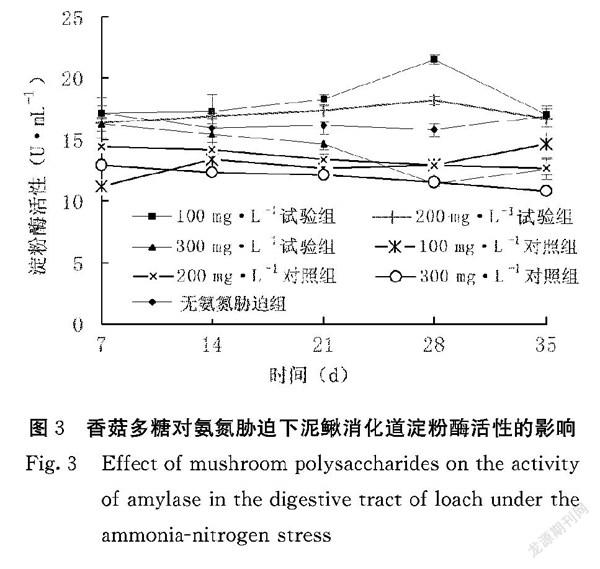
2.1.3 香菇多糖对氨氮胁迫下泥鳅消化道淀粉酶活性的影响 香菇多糖对氨氮胁迫泥鳅消化道淀粉酶活性的影响见图3。200、300 mg·L-1对照组泥鳅消化道淀粉酶活性在试验第7~35 d均呈下降趋势。100、200 mg·L-1试验组泥鳅消化道淀粉酶活性均高于100、200 mg·L-1对照组,且差异达显著水平(P<0.05),在试验第28 d,100 mg·L-1试验组泥鳅消化道淀粉酶活性达到最高,为(21.5±3.37)U·mL-1,说明香菇多糖能明显提高氨氮胁迫泥鳅消化道淀粉酶活性。
2.2 香菇多糖对氨氮胁迫下泥鳅消化道菌群的影响
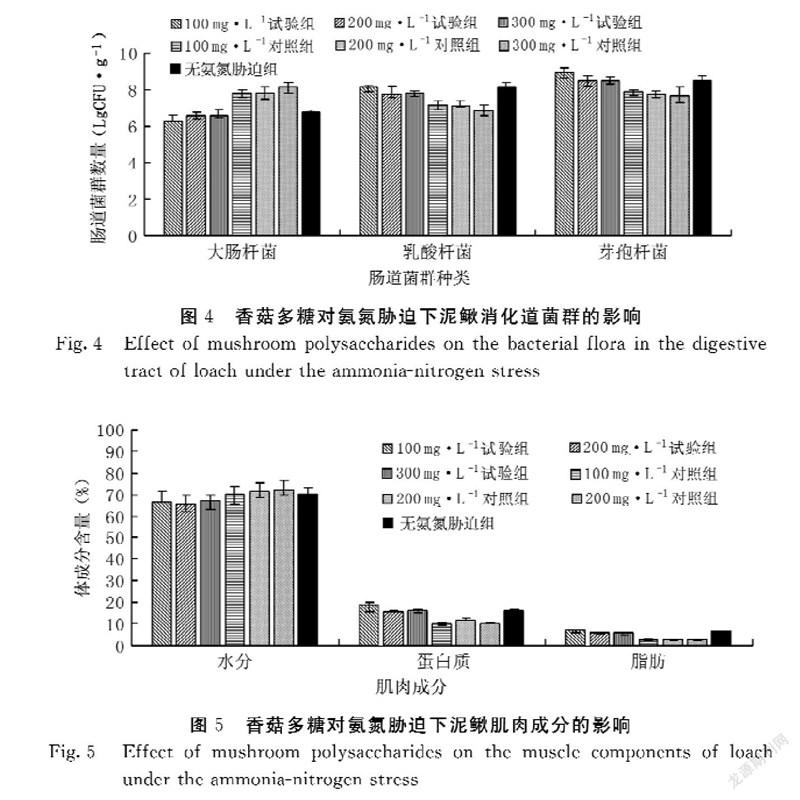
香菇多糖对氨氮胁迫泥鳅消化道菌群的影响见图4。在试验第35 d,100、200、300 mg·L-1试验组泥鳅消化道乳酸杆菌和芽孢杆菌数量均显著高于其对照组(P<0.05),而消化道大肠杆菌数量显著低于其对照组(P<0.05)。其中100 mg·L-1试验组泥鳅消化道乳酸杆菌和芽孢杆菌数量均显著高于其他试验组(P<0.05),大肠杆菌数量显著低于其他试验组(P<0.05),分别为8.12±0.19、8.96±0.27、6.24±0.29 LgCFU·g-1,說明香菇多糖能提高氨氮胁迫下泥鳅消化道乳酸杆菌和芽孢杆菌数量、降低大肠杆菌数量。
2.3 香菇多糖对氨氮胁迫下泥鳅肌肉成分的影响
香菇多糖对氨氮胁迫泥鳅肌肉成分的影响见图5。100、200、300 mg·L-1试验组泥鳅肌肉水分含量显著低于其对照组(P<0.05),而蛋白质含量和脂肪含量均显著高于其对照组(P<0.05)。100、200、300 mg·L-1试验组水分含量和脂肪含量之间无显著差异(P>0.05),而100 mg·L-1试验组泥鳅肌肉蛋白质含量显著高于200 mg·L-1试验组、300 mg·L-1试验组(P<0.05),说明香菇多糖能明显降低氨氮胁迫泥鳅肌肉水分含量,提高蛋白质、脂肪含量和泥鳅肌肉品质,并增强口感。
2.4 香菇多糖对氨氮胁迫下泥鳅鳃、肝胰脏组织结构的影响
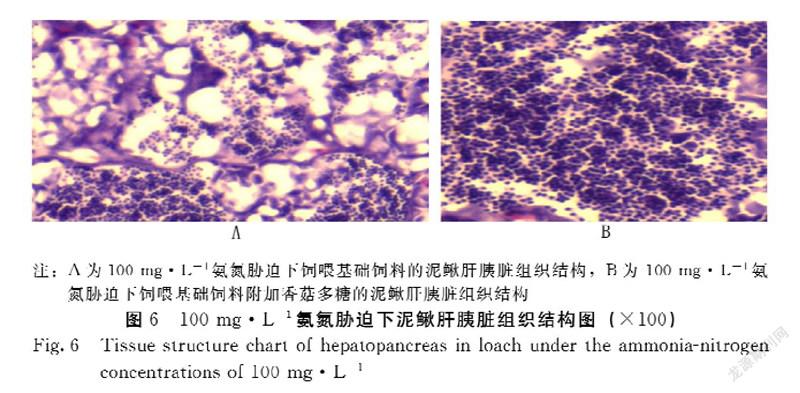
2.4.1 香菇多糖对氨氮胁迫下泥鳅肝胰脏组织结构的影响 香菇多糖对氨氮胁迫泥鳅肝胰脏组织结构的影响见图6。100 mg·L-1对照组泥鳅肝细胞轮廓模糊不清,肝组织充血松散无序,肝细胞核溶解,肝细胞肿大、坏死,血窦扩张;而100 mg·L-1试验组泥鳅肝组织细胞形态结构正常,肝细胞排列整齐,细胞轮廓清晰正常,由此可以看出,香菇多糖能使氨氮胁迫下泥鳅肝的组织结构和功能保持正常,促进泥鳅的健康成长。
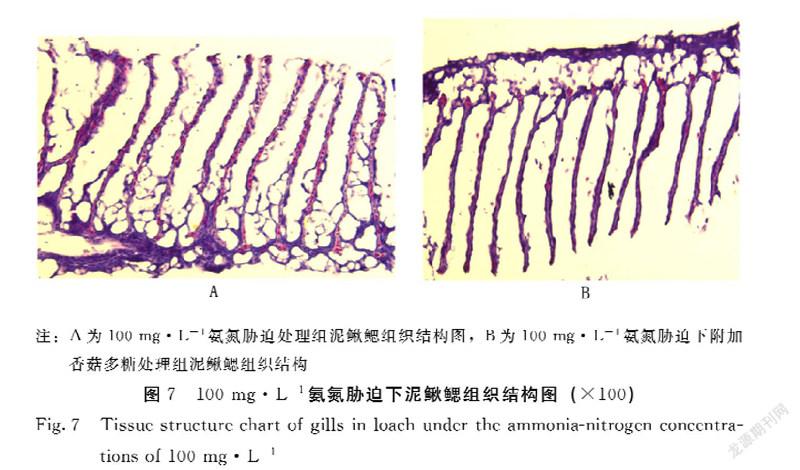
2.4.2 香菇多糖对氨氮胁迫下泥鳅鳃组织结构的影响 香菇多糖对氨氮胁迫下泥鳅鳃组织结构的影响见图7。在100 mg·L-1氨氮胁迫下饲喂基础饲料的泥鳅鳃小片细胞肿大,出现点状出血,鳃小片上皮细胞肿大坏死,毛细血管破裂;在100 mg·L-1氨氮胁迫下饲喂基础饲料附加多糖的泥鳅鳃丝结构比较整齐,鳃小片细胞排列整齐有序,鳃丝功能正常。由此可知,香菇多糖对氨氮胁迫下泥鳅的鳃具有保护作用。
3 讨论与结论
鱼类为人类提供超过世界总人口所需要的蛋白质一半以上,但鱼类的自然生态环境严重恶化使每年全世界鱼类养殖单位遭受最大的经济损失[31]。虽然人们可以利用某些疫苗和抗生素等来应对由于环境恶化而产生的环境胁迫,但在大规模使用方面不合适、不经济甚至效果不好而限制它们的使用[32-33],在饲料中添加免疫增强剂来增强鱼类的免疫力以应对各种环境胁迫产生的疾病是另一种选择,已经有一些免疫增强剂应用在水产养殖中[34-36]。近10年来,高等真菌产生的具有如抗氧化、抗癌、抗菌、免疫增强作用等多种生物学活性的多糖受到人们的高度关注[37],许多高等真菌如灰树花Grifola frondosa、香菇Lentinula edodes、杂色灵芝Mottled ganoderma lucidum、平菇Oyster mushroom和猴头菇Hericium erinaceus的子实体、发酵液可以产生具有生物活性功能的多糖[38],多糖可以提高动物的免疫力及对环境胁迫的能力[39]。在日粮中添加多糖可提高鱼类肠道消化酶活性,促进动物生长。强俊等[40]表明,在饲料中添加低聚木糖可显著提高奥尼罗非鱼Orni tilapia肠道淀粉酶和脂肪酶活力。任国锐等[41]研究表明,在日粮中添加壳聚糖,鲤鱼Cyprinus carpio肠道淀粉酶和蛋白酶比对照组都有显著升高。Eman等[42]在尼罗罗非鱼Tilapia nilotica饲料中添加黄芪多糖,发现尼罗罗非鱼的生长性能显著提高。Silvia等[43]研究了甘露寡糖对欧洲鲈鱼European bass的影响,结果表明甘露寡糖能显著提高肠道黏膜禪皱高度、宽度。本研究结果表明,香菇多糖能提高氨氮胁迫泥鳅消化管消化酶的活性,与其他研究者的结论一致,原因是功能性多糖能被某些有益菌如双歧杆菌Bifido bacterium、乳酸杆菌Lacto bacillus等利用,降低肠道pH,增强动物肠道消化酶活力[44-46]。
在饲料中添加多糖能促进动物有益菌产生而抑制有害菌繁殖,促进动物生长发育。孙盛明等[47]研究结果表明,饲料中添加200~400 mg·kg-1甘露寡糖能提高Megalobrama amblycephala幼鱼的抗氧化能力,改善肠道菌群结构。Hoseinifar等[48]研究发现果寡糖能明显提高鲤鱼Cyprinus carpio肠道中的异养好氧细菌和乳酸菌数量。本研究中,氨氮胁迫下泥鳅饲喂基础饲料添加香菇多糖,泥鳅肠道乳酸菌和芽孢杆菌数量显著高于仅饲喂基础饲料,其有害菌大肠杆菌数量则显著低于仅饲喂基础饲料。多糖能提高动物有益菌群的原因被认为是香菇多糖可减少动物肠道大肠杆菌等有害菌的繁殖,提高乳酸菌等有益菌数量,从而维持动物肠道的健康[49]。Mao等[50]用香菇多糖治疗发生腹泻的仔猪,发现治疗后仔猪肠道乙酸、丙酸、丁酸含量及乳酸菌双歧杆菌数量增加,促进动物肠道健康。环境胁迫使鱼类生长受到抑制,原因可能是产生大量自由基,使动物氧化应激和脂质过氧化,迫使鱼体消耗大量物质来抵抗氧化应激,使鱼体储存的物质如糖原、脂类和蛋白质被降解,体重下降,生长受阻。鱼类体成分与环境因素有关,这可能是由于环境胁迫降低鱼体摄食频率,不能从外界摄取过多的物质转换为自身能量,另一方面,鱼类为了保证自身代谢需要而消耗大量的糖类、脂肪和蛋白质,致使自身物质含量降低。在鱼类糖代谢过程中,胰岛素(insulin,INS)可促进鱼体内血糖的转化,抑制糖异生,促进肝脏、肌肉组织糖原的合成,有降血糖功能[51]。David等[52]发现两个ins基因,它们均能够编码产生能与胰岛素受体的INS分子。Polakof等研究表明INS能促进虹鳟鱼糖原合成并抑制糖原分解[53]。环境污染物可通过改变血糖水平、糖原含量和激素水平影响鱼类糖代谢[54-55]。Tintos等[56]研究发现虹鳟鱼在含有萘胁迫水体中时,鱼体肝糖原、肌糖原含量均下降。
多糖能够改变胁迫动物的代谢,从而影响动物的体成分。段正星[57]研究发现酸解氧魔芋葡甘露聚糖(AOKGM)干预后齐口裂腹鱼Schizothorax prenati对Cd胁迫有耐受力,表明AOKGM对镉胁迫引起的代谢紊乱有调节作用。可能原因是多糖使鱼类肝胰脏中PPARα、PPARβ、PPARγ、GPDH和HFABP的表达量显著下降。许多研究结果显示,多糖饲料添加剂能促进水生动物的生长、改善体成分、促进营养物质的消化吸收。黄智漩[58]研究表明,灵芝Lucid ganoderma多糖對小鼠具有明显的降血糖作用,黑果枸杞Lycium ruthenicum多糖能明显降低患糖尿病小鼠的血糖含量,加速体内葡萄糖转变为肝糖原。中草药多糖添加剂对动物体成分的影响主要体现在调整动物机体氨基酸的组成,促进氨基酸总量、必需氨基酸及鲜味氨基酸含量的增加等方面[59-60]。多糖等免疫增强剂由于具有强大去除自由基的能力及保护处于胁迫状态下的动物内脏,因此,受到人们的关注。郭苏兰等[61]在研究由苦白蹄Fomes officinalis Ames制备的阿里红多糖对用LPS诱导的急性肺损伤小鼠肺组织的影响时发现,阿里红多糖可通过作用于 TLR-4/NF-κB 通路,减少炎症因子的产生,从而对小鼠肺组织起保护作用。柴艳等[62]在研究Atractylodis macrocephalae多糖对仔猪肠道的影响时发现,白术多糖能提高仔猪小肠绒毛高度与隐窝深度的比值,提高肠道消化吸收功能。陈梦梦等[63]研究表明黄芪Astragalus spp.多糖可通过参与DNA的修复和复制过程,促进大鼠结肠黏膜细胞的增生及组织修复过程。黄玉章[64]研究结果表明,日粮中添加黄芪多糖可增加小肠绒毛表面积,提高机体对营养物质的消化和吸收。本研究表明,香菇多糖能恢复由于氨氮胁迫对泥鳅鳃和肝脏的损伤,有益鱼类的健康,这与上述其他研究者的结果类似。
本研究结果表明,香菇多糖能显著提高氨氮胁迫泥鳅消化道蛋白酶、淀粉酶和脂肪酶活性,可提高泥鳅消化道乳酸菌和芽孢杆菌数量并抑制消化道大肠杆菌等有害菌的繁殖,提高泥鳅体成分中蛋白质和脂肪含量,香菇多糖可保护氨氮胁迫下泥鳅肝胰脏、鳃等组织器官,避免组织器官受到氨氮胁迫的损伤,促进泥鳅的健康生长。
参考文献:
[1]KANNAN M,SAMUTHIRAPANDING R,THIRUNAVUKKARASU M,et al.Application of marine-derived polysaccharides as imunostimulants in aquaculture:A review of current knowledge and further Perspectives[J].Fish and Shellfish Immunology,2019,86(1):1177-1193.
[2]SHRIVASTAVA J,SINHA A K,DATTA S N,et al.Preacclimation to low ammonia improves ammonia handling in common carp(Cyprinus carpio)when exposed subsequently to high environmental ammonia[J].Aquat Toxicol,2016,18(4):334-344.
[3]CLAIBORNE J B,EVANS D H.Ammonia and acid-base balance during high ammonia exposure in a marine teleost(Myoxocephalus octodecimspinosus)[J].Journal of Experimental Biology,1988,140(1):89-105.
[4]MEADE G C.Microbes of the avian cecum:types present and substrates utilized[J].Journal of Experimental Zoology,1989,252(2):48-54.
[5]THRAN V R,THRANE A S,WANG F S,et al.Ammonia triggers neuronal disinhibition and seizures by impairing astrocyte potassium buffering(Article)[J].Nature Medicine,2013,19(12):1643-1648.
[6]HEGAZI M M,HASANEIN S S.Effects of chronic exposure to ammonia concentrations on brain monoamines and ATPases of Nile tilapia(Oreochromis niloticus)[J].Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology,2010,151(4):420-425.
[7]LEASE H M,HANSEN J A,BERGMAN H L.Structural changes in gills of Lost River suckers exposed to elevated pH and ammonia concentrations[J].Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology,2003,134(4) :491-500.
[8]邱德全,周鮮娇,邱明生.氨氮胁迫下凡纳滨对虾抗病力和副溶血弧菌噬菌体防病效果研究[J].水生生物学报,2008,32(4):455-461.
[9]HURVITZ A,BERCOVIER H,VAN RJV.Effect of ammonia on the survival and the immune response of rainbow trout (Oncorhynchus mykiss,Walbaum) vaccinated against Streptococcus iniae[J].Fish & Shellfish Immunology,1997,7(1):45-53.
[10]IP Y K,LEONG MWF,SIM M Y,et al.Chronic and acute ammonia toxicity in mudskippers,Periophthalmodon schlosseri and Boleophthalmus boddaerti:brain ammonia and glutamine contents,and effects of methionine sulfoximine and MK801[J].Journal of Experimental Biology,2005,208(10):1993-2004.
[11] 陈家长,臧学磊,胡庚东,等.氨氮胁迫下罗非鱼( Oreochromis niloticus)机体免疫力的变化及其对海豚链球菌易感性的影响[J].生态环境学报,2011,20(4):629-634.
[12]SUN H J,LU K,MINTER E J A,et al.Combined effects ammonia of and microcystin on survival,growth,antioxidant responses,and lipid peroxidation of bighead carp Hypophthalmythys nobilis larvae[J].Journal of Hazardous Materials,2012,221(1):213-219.
[13]XING X D,LI M,YUAN L X,et al.The protective effects of taurine On acute ammonia toxicity in grass carp Ctenopharynodon idellus[J].Fish & shellfish immunology,2016,56(1):517-522.
[14]REN Q Y,LI M,YUAN L X,et al.Acute ammonia toxicity in crucian carp Carassius auratus and effects of taurine on hyperammonemia[J].Comparative Biochemistry and Physiology Part C:Toxicology and Pharmacology,2016,190(1):9-14.
[15]REILLY A,KAFERSTEIN F.Food safety hazards and the application of the principles of the Hazard Analysis and Critical Control Point (HACCP) system for their control in aquaculture production[J].Aqucult Res,1997, 28(10):735-752.
[16]SAKAI M.Current research status of fish immunostimulants[J].Aquaculture,1999,172(2):63-92.
[17]RINGO E,OLSEN R E,GIFSTAD T O,et al.Prebiotics in aquaculture:a review,Aquacult[J].Nutrition,2010,16 (1) :117-136.
[18]MOHAN H,PADMANABAN A M,UTHAYAKUMAR H,et al.Effect of dietary Ganoderma lucidum polysaccharides on biological and physiological responses of the giant freshwater prawn Macrobrachium rosenbergii[J].Aquaculture,2016,464 (1):42-49.
[19]XIU Y W,WEN J W,LONG M W,et al,Lentinan modulates intestinal microbiota and enhances barrier integrity in a piglet model challenged with lipopolysaccharide[J].Food Function,2019,10(1):479-489.
[20]TZIANABOS A O.Polysaccharide immunomodulators as therapeutic agents:structural aspects and biologic function[J].Clin Microbiol Rev,2000,13(3):523-533.
[21]HAN S B,PARK S K,AHN H J,et al.Characteriaztion of B cell membrane receptors of polysaccharide isolated from the root of Acanthopanax koreanum[J].International Immunopharm acol,2003,3(5):683-691.
[22]MING L,NA Y,JIAN G.et al.Effects of ammonia stress,dietary linseed oil and Edwardsiella ictalurid challenge on juvenile darkbarbel catfish Pelteobagrus vachelli[J].Fish & Shellfish Immunology,2014,38(1):158-165.
[23]GUANGMING R,LIMING X,TONGYAN L,et,al.Protective effects of lentinan on lipopolysaccharide induced inflammatory response in intestine of juvenile taimen(Hucho taimen,Pallas)[J].International Journal of Biological Macromolecules,2019(121) :317-325.
[24]郝小鳳,刘洋,凌去非.氨氮对泥鳅的急性毒性及对其肝、鳃组织超微结构的影响 [J].水生态学杂志,2012,33(5):101-107.
[25]刘 洋, 凌去非,于连洋,等.氨氮胁迫对泥鳅不同组织SOD和GSH-PX活性的影响[J].安徽农业科学, 2011,39(2):1069-1072.
[26]张云龙,张海龙,王凌宇,等.氨和空气暴露对大鳞副泥鳅组织中谷氨酰胺含量的影响 [J].中国水产科学,2017,24(5):1115-1122.
[27]张云龙,王光毅,金慧,等.氨基酸代谢调控在大鳞副泥鳅应对氨暴露中的作用[J].水生生物学报,2019,43(5):1013-1020.
[28]丁茜,田宝军,耿丽娜,等.温度对泥鳅肠道消化酶活性的影响[J].齐鲁渔业,2009,26(6):4-7.
[29]蒲宗旺,王永明,张运邦,等.台湾泥鳅含肉率及肌肉营养成分分析与评价[J].食品工业科技,2017,38(18):300-311.
[30]吴利敏,徐瑜凤,李永婧,等.急性氨氮胁迫对淇河鲫幼鱼脑、鳃、肝、肾组织结构的影响[J].中国水产科学,2020,27(7):789-800.
[31]MOSTAK A,NOORLIDAH A,ADAWIYAH S,et al.Influence of raw polysaccharide extract from mushroom stalk waste on growth and pH perturbation induced-stress in Nile tilapia,Oreochromis niloticus[J].Aquaculture,2017,468(1):60-70.
[32]DARWISH A M.Laboratory efficacy of florfenicol against Streptococcus iniae infection in sunshine bass[J].Journal of Aquatic Animal Health,2007,19(1):1-7.
[33]CHEN M,WANG R,LI L P.et al.Screening vaccine candidate strains against Streptococcus agalactiae of tilapia based on PFGE genotype[J].Vaccine, 2012,30(42):6088-6092.
[34]CHEN D,AINSWORTH A J.Glucan administration potentiates immune defence mechanisms of channel catfish,Ictalurus punctatus Rafinesque[J].Journal of Fish Dis,1992,15(4):295-304.
[35]SIWIC K I,ANDERSON D P,RUMSEY G L.et al.Dietary intake of immunostimulants by rainbow trout affects non-specific immunity and protection against furunculosis[J].Veterinary Immunology and Immunopathology,1994,41(1):125-139.
[36]SAKAI M.Current research status of fish immunostimulants[J].Aquaculture,1999,172(1):63-92.
[37]COHEN R,PERSKY L,HADAR Y.Biotechnological applications and potential of wood-degrading mushrooms of the genus Pleurotus[J].Appl.Microbiol Biotechnol,2002(5):582-594.
[38]WASSER S P.Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides[J].Appl Microbiol Biotechnol,2002,60(3):258-274.
[39]VETVICKA L,VANNUCCI L,SIMA P.The effects of β-glucan on fish immunity[J].North American Journal of Medical Sciences,2013,5(10):580-588.
[40]強俊,王辉,李瑞伟,等.低聚木糖对奥尼罗非鱼幼鱼生长、体成分和消化酶活力的影响[J].淡水渔业,2009,39(6):63-68.
[41]任国锐,李瑞金,王兰.低分子质量壳聚糖对鲤鱼生长和消化酶活性的影响[J].食品科学,2013,34(1):8-12.
[42]EMAN Z,ENGY R,FATMA A,et al.Effects of dietary Astragalus polysaccharides(APS)on growth performance,immunological parameters,digestive enzymes,and intestinal morphology of Nile tilapia(Oreochromis niloticus)[J].Fish & Shellfish Immunology,2014,38(1) :149-157.
[43]SILVIA T,ALEX M,TIBIABIN B S,et al.Reduced gut Bacterial translocation in European sea bass(Dicentrarchus Labrax)fed mannan oligosaccharides(MOS)[J].Fish & Shellfish Immunology,2011,30(2):674-681.
[44]ZOKAEIFARA H,BALCAZARB J L,SAADA C R,et al.Effects of Bacillus subtilis on the growth performance,digestive enzymes,immune gene expression and disease resistance of white shrhnp,Litopenaeus Vannamei[J].Fish & Shellfish Immunology,2012,33(4):683-689.
[45]CRISTINA F C,LUCIA H A ,LICIA M L,et al.Responses of digestive enzymes of tambaqui(Colossoma macwpomum)to dietary cornstarch changes and metabolic inferences[J].Molecular & Integrative physiology,2007,147(4):857-862.
[46]PEDERSEN B H, UGELSTAD I,HJELMELANDK K.Effects of atranshory,low food supply in the early life of larval herring(Clupea harengus)on mortaliy,growth,and digestive capacity[J].Marine Biology,1990,107(1):61-66.
[47]孙盛明,谢骇,朱健,等.饲料中添加甘露寡糖对团头妨幼鱼生长性能、抗氧化能力和肠道菌群的影响[J].动物营养学报,2014,26(11):3371-3379.
[48]HOSEINIFAR S H,SOLEIMANI N,EINAR R.Effects of dietary fructo Oligosaccharide supplementation on the growth performance,haemato,immunological parameter,gut microbiota and stress resistance of Common Carp(Cyprinus carpio)fry[J].British Journal of Nutrition,2014,112(1):1296-1302.
[49]REN G,XU L,LU T,et al.Protectiive effects of Lentinan On lipopolysaccharide induced inflammatory response in intestine of Juvenile taimen(Hucho taimen,pallas)[J].International Journal of Biological Macromolecules,2019,121(1):317-325.
[50]MAO X B,HU H Y,XIAO X C,et al.Lentinan administration relieves gut barrier dysfunction induced by rotavirus in a weaned piglet model[J].Food & Function,2019,10(4):2094-2101.
[51]CARUSO M A,SHERIDAN M A.New insights into the signaling system and function of insulin in fish[J].General and Comparative Endocrinonoly,2011,173(2):227-247.
[52]DAVID M I.A Second insulin gene in fish genomes[J].General and Comparative Endocrinonoly,2004,135(1):150-158.
[53]POLAKOF S,SKIBA-CASSY S,CHOUBERT G,et al.Insulin-induced hypoglycaemia is co-ordinately regulated by liver and muscle during acute and chronic insulin stimulation in rainbow trout (Oncorhynchus mykiss)[J].The journal of Experimental Biology,2010,213(9):1443-1452.
[54]ZHAO F,JIANG G,WEI P,et al.Bisphenol S exposure impairs glucose homeostasis in male zebrafish (Danio rerio)[J].Ecotoxicology and Environmental Safety,2018,147(1):794-802.
[55]YANG Y,LIU W,LI D,et al.Altered glycometabolism in zebrafish exposed to thifluzamide[J].Chemosphere,2017,183(1):89-96.
[56]TINTOS A,GESTO M,GUEZ J M,et al.Naphthalene treatment alters liver intermediary metabolism and levels of steroid hormones in plasma of rainbow trout (Oncorhynchus mykiss)[J].Ecotoxicology and Environmental Safety,2005,66(2):139-147.
[57]段正星.镉致齐口裂腹鱼氧化胁迫、脂质代谢紊乱及酸解氧化魔芋葡甘露聚糖干预研究[D].成都:四川农业大学,2019.
[58]黄智漩.灵芝多糖降血糖作用的研究[J].食用菌,2009(1):60-61.
[59]MA D Y,SHAN A S.Effects of Chinese herbal drugs on animal growth,endocrine and immunt[J].China Animal Husbandry & Veterinary Medicine,2004(31):162-169.
[60]XIE H Y.Research and application of Chinese herbs as feed addivites[J].J Anim Vet Sci,2004(34):322-334.
[61]郭苏兰,李佳娜,肖水秀.阿里红多糖对用脂多糖诱导的急性肺损伤小鼠肺组织的保护作用及相關机制[J].当代医药论丛,2019,17(10):127-130.
[62]柴艳,李琦华,贾俊静,等.白术多糖对断奶仔猪生产性能及肠道组织形态的影响[J].中国饲料,2020(15):49-54.
[63]陈梦梦,朱曙东.黄芪多糖对溃疡性结肠炎大鼠结肠黏膜组织再生、 修复的影响[J].中医临床研究,2019,11(31):1-6.
[64]黄玉章.黄芪多糖对奥尼罗非鱼生长性能和免疫功能的影响[D].福州:福建农林大学,2009.
(责任编辑:林玲娜)
收稿日期:2021-10-25
作者简介:刘成荣,男,1964年生,教授,主要从事动物生理方面研究。
基金项目:福建省自然科学基金高校联合面上项目(2018J01468)。
