Two-way predation between immature stages of the hoverfly Eupeodes corollae and the invasive fall armyworm (Spodoptera frugiperda J.E.Smith)
2021-02-25LIHuiJIANGShanshanZHANGHaowenGENGTingKrisWYCKHUYSWUKongming
LI Hui ,JIANG Shan-shan ,ZHANG Hao-wen ,GENG Ting ,Kris A.G.WYCKHUYS ,WU Kong-ming
1 Department of Entomology,College of Plant Protection,China Agricultural University,Beijing 100193,P.R.China
2 State Key Laboratory for Biology of Plant Diseases and Insect Pests,Institute of Plant Protection,Chinese Academy of Agricultural Sciences,Beijing 100193,P.R.China
3 Institute of Plant Protection,Chinese Academy of Agricultural Sciences,Beijing 100193,P.R.China
Abstract Since its 2018 invasion of eastern Asia,the fall armyworm Spodoptera frugiperda (Lepidoptera:Noctuidae) has become a key pest in local maize production.Though pesticides have been widely used to mitigate the initial S.frugiperda attack,biological control is receiving ample attention as a desirable,environmentally-sound alternative to chemical control.Hoverflies (Diptera:Syrphidae) are abundant natural enemies in Chinese maize fields and have been observed to consume S.frugiperda larvae.In this study,we use laboratory assays to study the two-way interaction between immature stages of S.frugiperda and the endemic syrphid Eupeodes corollae.To mimic natural conditions,assays were performed in the presence of fresh maize leaves.Those 2nd or 3rd instar larvae of E.corollae preyed on 1st and 2nd instar S.frugiperda larvae with a Holling type III response,consuming a respective theoretical maximum of 43.48 and 83.33 larvae over a 24-h period.Conversely,once S.frugiperda larvae reached 3rd instar,they exhibited aggressive behavior and equally preyed on syrphid larvae with a Holling type III response.Those 5th and 6th instar larvae of S.frugiperda consumed a respective 16.39–19.23,6.02–19.61 and 6.76–8.26 of 1st,2nd and 3rd instar E.corollae larvae per day.Though our results await field-level validation,S.frugiperda agonistic (i.e.,defensive) and consumptive behavior towards resident natural enemies such as E.corollae possibly degrades biotic resistance and raises its invasion potential.Our findings shine new light on the interaction between lepidopteran herbivores and their natural enemies,and can help advance the development of conservation biological control and other integrated pest management (IPM) strategies against S.frugiperda in China and abroad.
Keywords:Eupeodes corollae,Spodoptera frugiperda,predation,functional response,conservation biological control,invasion biology,trophic ecology
1.Introduction
The fall armyworm (FAW),Spodopterafrugiperda(J.E.Smith) (Lepidoptera:Noctuidae) is a migratory pest that is threatening agricultural production,food security and farmer livelihoods across the globe (Earlyet al.2018).As a polyphagous herbivore,S.frugiperdalarvae feed on more than 350 plant species including several prime food and agro-industrial crops e.g.,maize,sorghum,millet,sugarcane,and vegetables (Montezanoet al.2018).Spodoptera frugiperdaadults possess exceptional dispersal abilities,engage in long-distance migration and can fly up to 100 km per night by relying upon low-level jet-stream air currents (Johnsonet al.1987).Native to the Neotropics,S.frugiperdamade its arrival in West Africa in early 2016 and subsequently spread across the African continent in two years (Goergenet al.2016).Following its arrival in India in mid-2018 (Sharanabasappaet al.2018),S.frugiperdainvaded Yunnan Province,China,in December 2018 (Sunet al.2021) and other Southeast Asian countries in early 2019 (Daoet al.2020).By October 2019,S.frugiperdahad been recorded in 26 provinces (autonomous regions,municipalities) in China,causing vast economic damage to lacal maize production (Jianget al.2019).
In addition to numerous studies onS.frugiperdabiology,population genetics and flight performance (Wuet al.2019),research has been carried out to devise sustainable pest management strategies.This has involved the validation of synthetic pesticides (Zhao S Yet al.2019),geneticallymodifiedBacillusthuringiensis(Bt) maize (Zhang and Wu 2019),and biological control using entomopathogenic fungi,viruses and various arthropod natural enemies.More specifically,scientists have evaluated the potential of endemic egg and larval parasitoids (Chenet al.2019; Huoet al.2019; Liet al.2019; Zhuet al.2019),and examined opportunities to deploy predatory Hemiptera,Neuroptera and Dermaptera (Tanget al.2019; Xu Q Xet al.2019;Zhao Y Jet al.2019).In addition to the scientifically-guided introduction of exotic natural enemies for invasive pest control (Neuenschwander and Herren 1988; Messing and Wright 2006; Van Driescheet al.2010; Wyckhuyset al.2018),the in-field conservation or augmentation of locallyoccurring biota can be particularly rewarding (Jonssonet al.2010; Balzan and Wäckers 2013).As the oldest form of pest control,conservation biological control constitutes a ‘green’,economically-viable and practicable alternative to pesticide-based measures for crop protection (Naranjoet al.2015; Gurret al.2017; Chaplin-Krameret al.2019).
Among the endemic natural enemies present in China’s maize fields,syrphids are abundant,polyphagous predators that can assume a role inS.frugiperdabiological control.While syrphid larvae commonly prey on aphids,thrips and other plant-sucking insects (Kotwalet al.1984; Stahlset al.2003; Mengualet al.2008a,b),they equally attack small-sized lepidopteran larvae (Wrattenet al.1995).The syrphidEupeodescorollaeis a widely-distributed species that is dominant in maize ecosystems of northeastern China(Lanet al.2011; Puet al.2017).Spring generations ofE.corollaedevelop in non-crop habitats (e.g.,weed strips),with the adults subsequently colonizing summer field crops(e.g.,maize) and vegetables (Lanet al.2011).Throughout its larval stage,E.corollaecan consume up to 2 000 aphids(Liuet al.2005; Lanet al.2011),though its predation rates of other prey items remain unquantified.Given the recentS.frugiperdainvasion of local maize fields,an in-depth understanding ofE.corollaepredation on this pest can help ascertain its relative contribution to biotic resistance and potential use in conservation biological control (Horrockset al.2019).
Invasion processes often entail the permanent settlement of exotic species in novel environments,resulting in interspecific competition,species displacement,genetic erosion or ecosystem degradation (Rowles and O’Dowd 2007; Crowder and Snyder 2010).Some of the above processes can be pronounced following the arrival of nonnative polyphagous predators (Royet al.2016),but also occur among herbivores such asBemisiatabaciwhitefly or the tiger mosquitoAedesalbopictus(Edgerlyet al.1993;Perringet al.1993; Liuet al.2007).Following its arrival in Asia’s agro-ecosystems,invasiveS.frugiperdaare expected to compete with resident lepidopteran species for floral resources (Sáezet al.2017; Grahamet al.2019) or trigger apparent competition among parasitic hymenopterans (Holt and Bonsall 2017),possibly over extensive spatial scales(Frostet al.2016).Yet,to what extent immatureS.frugiperdawill act as a supplementary prey resource forE.corollaeor instead engage in predation on syrphid larvae is unknown.
In this study,we examined the two-way interactive predation between different developmental stages of the endemicE.corollaeand the invasiveS.frugiperda.First,we quantified the level of predation ofE.corollaelarvae on differentS.frugiperdalarval instars,calculating attack rate,handling time,and theoretical maximum prey consumption.Next,we assessed the degree to whichS.frugiperdalarvae preyed uponE.corollaelarvae in the presence of maize foliar tissue (i.e.,the primary food item ofS.frugiperda).Our study illuminates important aspects ofS.frugiperdaecology in its newly-invaded range and adds to a future development of integrated pest management (IPM) packages.
2.Materials and methods
2.1.Insect rearing
Larval stages ofS.frugiperdawere initially collected from maize fields at Dehong Autonomous Prefecture,Yunnan Province,China (24.43°N,98.58°E) in January 2019.Larvae were transferred to the laboratory,reared on an artificial diet of soybean flour and wheat bran (Greeneet al.1976) and maintained in a climatic chamber at (25±1)°C,50–70% RH and 16 h L:8 h D.Upon pupal eclosion,S.frugiperdaadults were fed with a 10% honey-water solution.
Adults ofE.corollaewere initially collected from wheat fields at the Langfang Experimental Station of Chinese Academy of Agricultural Sciences (CAAS) in Hebei Province,China (39.53°N,116.70°E) in June 2018.A laboratory population was maintained in a controlled environment room at (25±1)°C,30–70% RH,and 16 h L:8 h D.Larvae were fed withMegourajaponicaaphids on laboratory-grown broad bean plantlets,while syrphid adults were fed with 10% honey-water solution and pollen,with the latter being a 3:1 mixture (by weight) of commercially-available rape and maize pollen.
2.2.Predation assays
Two-way interactive predation ofS.frugiperdaandE.corollaeimmature stages was assessed under laboratory conditions.More specifically,a given larval instar of one species termed ‘predator’ was placed in a ventilated Petri dish (5 cm in diameter,1.3 cm high) together with a specific life stage of the 2nd species,i.e.,‘prey’.Newly-molted (<12-h old) individuals of the ‘predator’ species were starved for 12 h prior to testing,and presented singly with a given development stage of the ‘prey’ species.For the ‘predator’species,we tested 1st until 3rd instar stages forE.corollaeand 3rd until 6th instar larvae forS.frugiperda.As such,one singleE.corollae‘predator’ individual was paired with one egg batch (approximately 20 eggs) or one single 1st,2nd or 3rd instar larva ofS.frugiperdaprey in a ventilated Petri dish.Conversely,one singleS.frugiperda‘predator’was either paired with 20 eggs or one single 1st,2nd or 3rd instar larva ofE.corollaeprey in a ventilated Petri dish.The above ratios did not allow forad-libitumpredation but permitted a baseline assessment of prey acceptance;with each predator–prey combination replicated 30 times.Moisture was provided through a water-soaked cotton ball.Predation by either ‘predator’ species was recorded after 24 h,by counting the number of consumed ‘prey’ items (i.e.,eggs) or mortality of the presented ‘prey’ larvae.Assays were carried out in the presence of several 4-or 5-leaf stage maize leaves (i.e.,natural food of the herbivorousS.frugiperda),placed at the bottom of each Petri dish.All experiments were carried out under laboratory conditions at (25±1)°C,(60±5)% RH and 16 h L:8 h D.
2.3.Functional response
To assess functional response of eitherE.corollaeorS.frugiperdato different prey,a similar experimental setup was used as above though a given ‘predator’ species was now presented with varying numbers of ‘prey’ items.Experiments were conducted with one newly-molted(<12-h old) and 24 h starved individual of the ‘predator’species.ForE.corollae,2nd and 3rd larval stages were placed individually in 1.5 cm×14.5 cm plastic containers with varying densities of 1st instar (20,40,60,80,120,160 or 200 individuals) or 2nd instar (20,40,60,80 or 120) larvae ofS.frugiperda.ForS.frugiperda,5th and 6th instar larvae were placed individually with varying densities(i.e.,10,20,30,40 or 50 individuals) of 1st,2nd,3rd instar larvae ofE.corollae.In all predation assays,several fresh maize leaves were placed at the bottom of the Petri dish to provide the natural food forS.frugiperdaand avoid larval cannibalism.Each predator–prey combination was replicated 5 times.AsS.frugiperdalarvae consumed maize leaves in addition to preying uponE.corollae,we equally measured differences in the weight of maize leaves over the 24-h experimental period.To account for eventual loss of water (i.e.,due to evaporation),we assessed weight loss of maize leaves in 30 Petri dishes withoutS.frugiperdalarvae over a 24-h period.
2.4.Data analysis
In the above assays,predation rates were recorded after 24 h.If ‘prey’ larvae or eggs disappeared within the experimental arena,‘prey’ was considered to be consumed by the ‘predator’ species.The resulting predation rate was computed and differences in predation rates between developmental stages were assessed using Chi-square test (P<0.05).
The predation rate of syrphid larvae onS.frugiperda‘prey’,S.frugiperdalarvae on syrphid ‘prey’,and extent ofS.frugiperdamaize leaf feeding were calculated.For either predator–prey interaction,functional response data were analyzed and plotted following protocols outlined by Juliano (2001).First,the type of functional response was determined by using a binomial logistic regression of the proportion of prey eaten as a function of prey offered.A polynomial function was fitted using the glm function in R version 2.0.1 (R Development Core Team 2008):
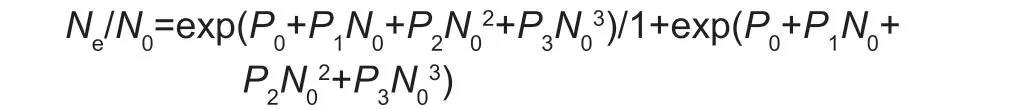
whereNeis the number of prey items eaten andN0is the initial prey density;P0,P1,P2andP3are the intercept,linear,quadratic,and cubic coefficients,respectively.If estimates of the linear coefficient in the original model were not significantly different from 0,the model was reduced by omitting the cubic term until coefficients were significant(Juliano 2001).Based on the sign of the linear coefficient,the shape of the curve of the functional response (i.e.,type II or III) was distinguished (De Clercqet al.2000).Second,Holling’s disc equation was used to obtain estimates for handling time (Th) and attack rate (a).For a type II response,the respective eq.is:
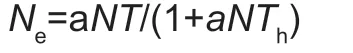
whereais the attack rate,Nis the initial prey density,Tis the total time available for the predator,andThis the handling time.For a type III response,ais no longer assumed to be constant,but instead increases with prey density.We fitted a modela=(d+bN)/(1+cN),in whichaincreases asymptotically withN,in each equation,a type III functional response can be obtained:
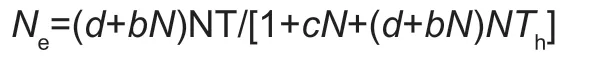
whereb,c,anddare fitted constants (Juliano 2001).The parametersb,c,dandThwere then obtained by fitting observed data to the above models using non-linear least square regression with the nls function in R.Maximum number of attacked prey (T/Th) was also determined.
Differences in the weight of maize leaves consumed by different instars ofS.frugiperdalarvae were assessed using LSD multiple range test.All analyses were performed using the R Programming Language version 2.0.1 and SPSS Statistics package 21.0.
3.Results
3.1.Two-way predation between S.frugiperda and E.corollae
Syrphid larvae readily preyed upon early life stages ofS.frugiperda,with 2nd instarE.corollaeconsuming 63.3 and 46.7% of the respective 1st and 2nd instar larvae ofS.frugiperda,and 3rd instarE.corollaeconsuming 100 and 80% of the respectiveS.frugiperdastages(Table 1).Predation rate ofE.corollaethus increased with developmental stage (Table 1).
OnceS.frugiperdalarvae reached 3rd instar,they equally preyed upon 1st and 2nd instarE.corollae.No predation was observed between 3rd instar larvae ofS.frugiperdaandE.corollae(Table 1).4th,5th and 6th instarS.frugiperdalarvae preyed upon all immature stages ofE.corollae,with 5th and 6th instarS.frugiperdaconsuming a respective 53.3 to 90% of presentedE.corollaeprey items.Overall,whileE.corollaeimmatures preyed on the early life stages ofS.frugiperda,only olderS.frugiperdalarvae preyed onE.corollaelarvae (Fig.1).
3.2.Functional response
Both interactions (i.e.,E.corollaepreying uponS.frugiperdalarvae andvice versa) yielded a positiveP1value and a negativeP2value,with the response of either species to prey density conform with a type III functional response (Tables 2 and 3).For some predation data,estimates of the linear coefficients in the original cubic model did not differ from 0 (P>0.05),and a reduced model was thus chosen.The proportion ofS.frugiperdaandE.corollaeconsumed by predators increased initially and then decreased with prey density increasing,which is the typical pattern of a type IIIfunctional response (Figs.2 and 3).Based on the Holling III equation,handling time,attack rate,and the theoretical maximum number were derived forE.corollae(Table 4) andS.frugiperda(Table 5).
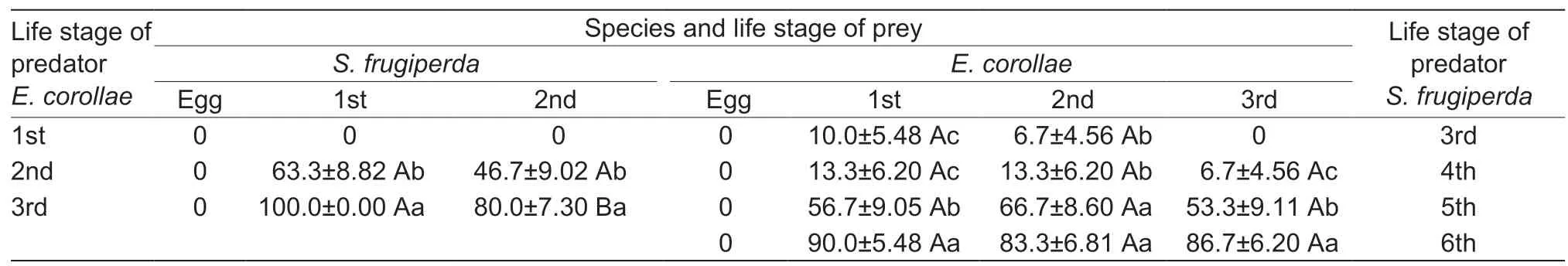
Table 1 Two-way predation rate between immature stages of the endemic Eupeodes corollae and Spodoptera frugiperda1)
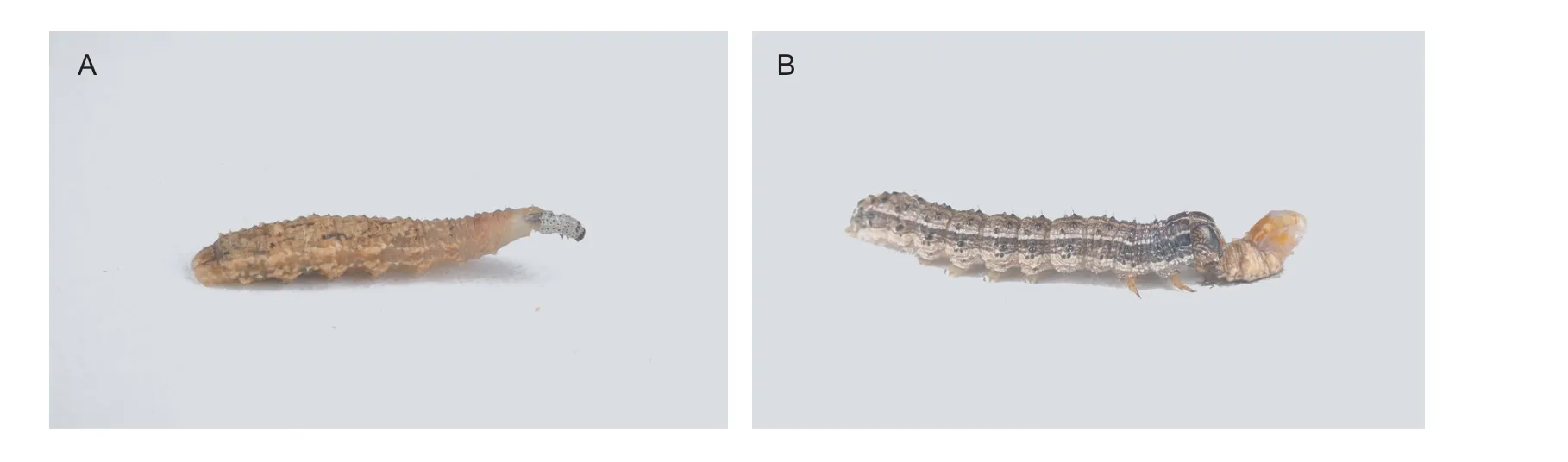
Fig.1 Photographs showing two-way predation between Spodoptera frugiperda and Eupeodes corollae larvae.A,3rd instar larvae of E. corollae attacking 1st instar of S. frugiperda.B,5th instar of S. frugiperda consuming 3rd instar larvae of E.corollae.
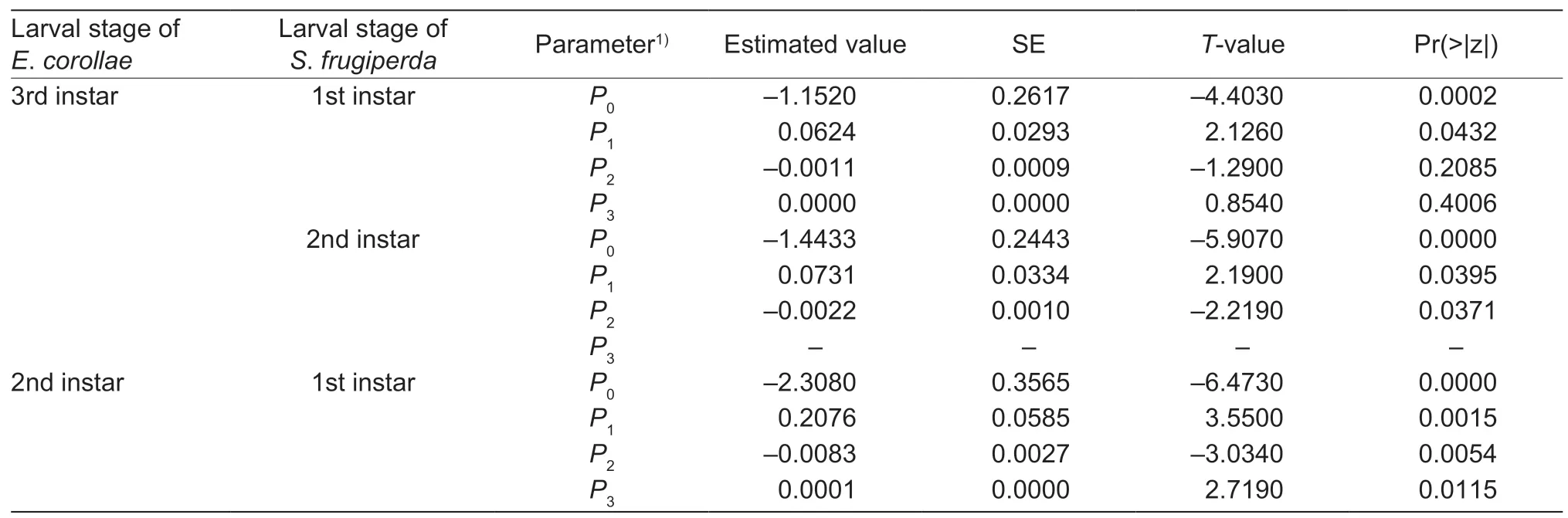
Table 2 Logistic regression describing the proportion of Eupeodes corollae preying on different Spodoptera frugiperda larval instars as a function of prey density
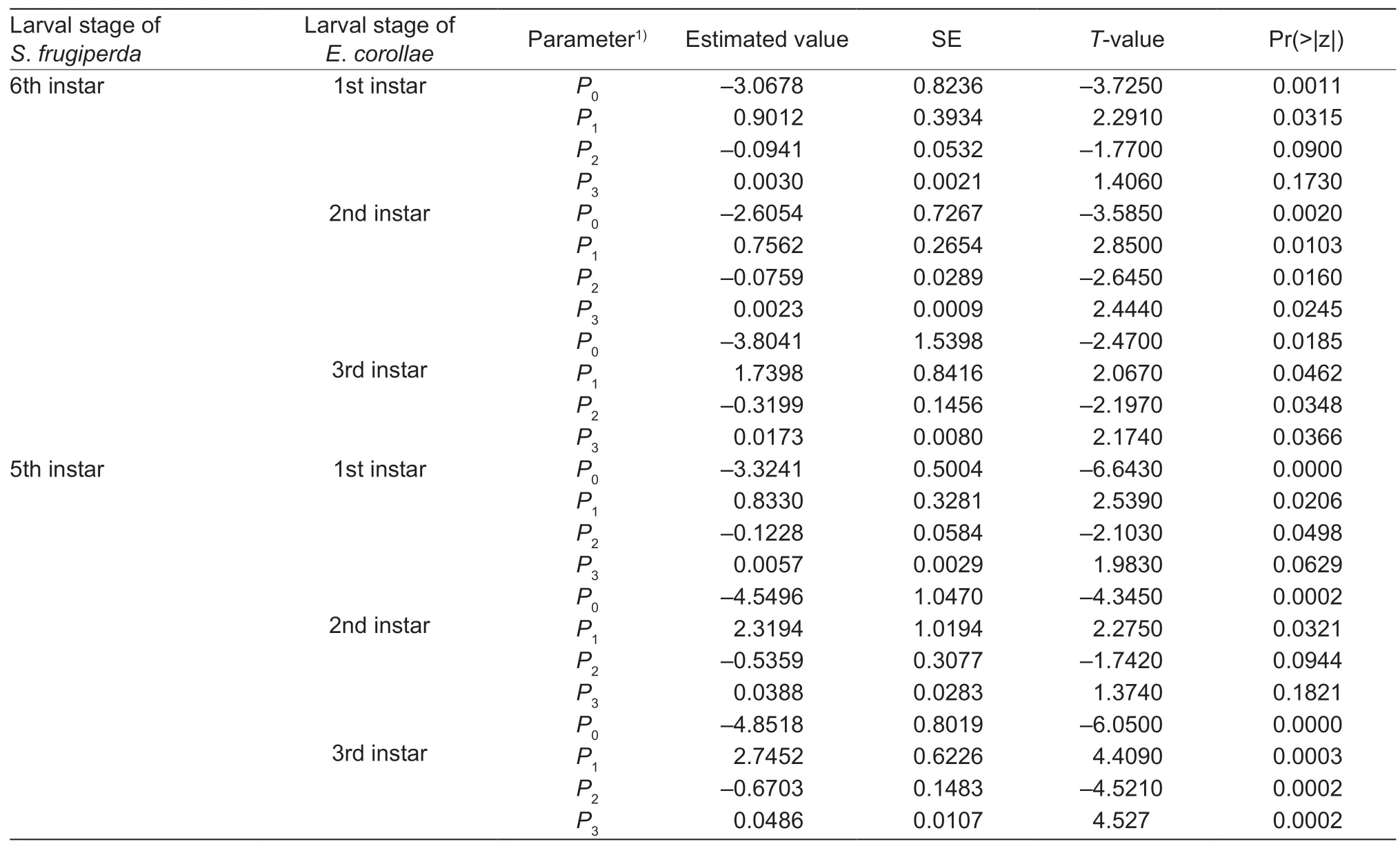
Table 3 Logistic regression describing the proportion of Spodoptera frugiperda preying on different Eupeodes corollae larval instars as a function of prey density
Among syrphid predators of different ages,the highest theoretical maximum prey consumption (Nmax) was 83.33(i.e.,3rd instar syrphid larvaevs.1st instarS.frugiperdaprey) (Table 4).ForS.frugiperdapredators,5th and 6th instar larvae attained the highest theoretical maximum prey consumption on 1st and 2nd instar syrphids,i.e.,19.23 and 19.61,respectively (Table 5).Hence,in terms of theoretical maximum prey consumption,the predation ability of syrphid onS.frugiperda‘prey’ items was higher than that ofS.frugiperdalarvae on syrphid ‘prey’.
ForE.corollaelarvae,handling time was the lowest for 3rd instarE.corollaepreying upon 1st instarS.frugiperda.When preying uponE.corollae,handling time of 5th and 6th instarS.frugiperdawas higher than that ofE.corollaelarvae attackingS.frugiperdalarvae (Tables 4 and 5).Attack rate (a) ofS.frugiperdaincreased with developmental stage(i.e.,attack rate of 6th instarS.frugiperdabeing higher than that of 5th instar larvae) (Table 5).
During the functional response trials ofS.frugiperdaprey onE.corollaelarvae,we also assessed the weight of maize leaves consumed by 5th and 6th instarS.frugiperda.Though later developmental stages ofS.frugiperdaconsumed a higher weight of maize leaves than early life stages,the extent of maize consumption was not affected by either the development stage or density ofE.corollae(i.e.,prey items) (Table 6).
4.Discussion
Invasive species can impact human health,threaten the survival of native biota and disrupt ecosystem functioning(Pejchar and Mooney 2009),imposing considerable socioeconomic costs (Bradshawet al.2016; Painiet al.2016).Biological invasions,e.g.,by the polyphagous herbivoreS.frugiperda,result from an ecological imbalance when nonnative species are liberated from their co-evolved natural enemies (‘enemy-release hypothesis’; Liu and Stiling 2006).In their native range,S.frugiperdapopulations are kept in check by insect-killing fungi,nucleo-polyhedrosis viruses and a broad complement of predators and parasitoids (Molina-Ochoaet al.2003; Wyckhuys and O’Neil 2006; da Silvaet al.2017) but those are often absent in novel environments.In invaded areas,e.g.,in Asia,such imbalance can either be restored through a careful introduction of exotic natural enemies (Van Driesche and Hoddle 1997; Messing and Wright 2006; Heimpel and Mills 2017) or through the deliberate conservation and population enhancement of endemic predators,e.g.,the syrphidE.corollae(Rutledgeet al.2004).Our work demonstrates how,under laboratory conditions,late-instarE.corollaelarvae consume large numbers of immatureS.frugiperda.Considering howE.corollaeare commonly encountered in China’s maize agro-ecosystems,they may contribute toS.frugiperdabiological control.On the other hand,our microcosm assays reveal facultative carnivory byS.frugiperdalarvae in the presence of plant food items (i.e.,maize leaves),providing unique insights intoS.frugiperdainter-specific aggression and ecology.Our work thus facilitates the development of IPM programs.Yet,given that our assays were performed in the laboratory,follow-up studies are essential to validate this interactive predation on (aphid-infested) live maize plants under field conditions.
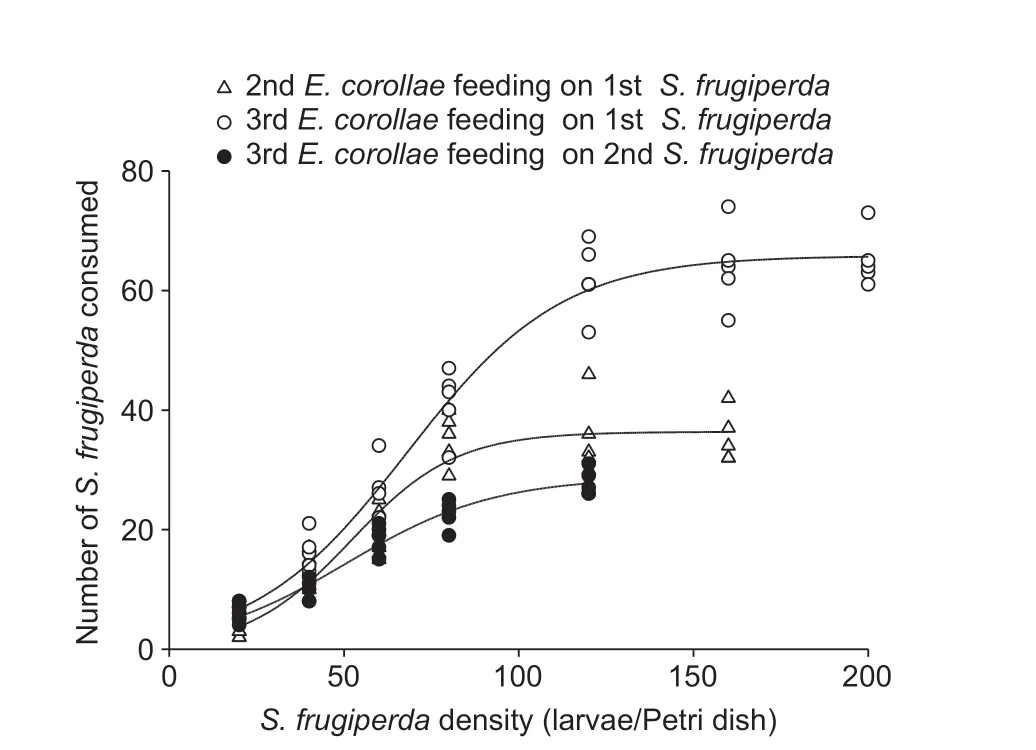
Fig.2 Functional response curves of different Eupeodes corollae development stages feeding on Spodoptera frugiperda.
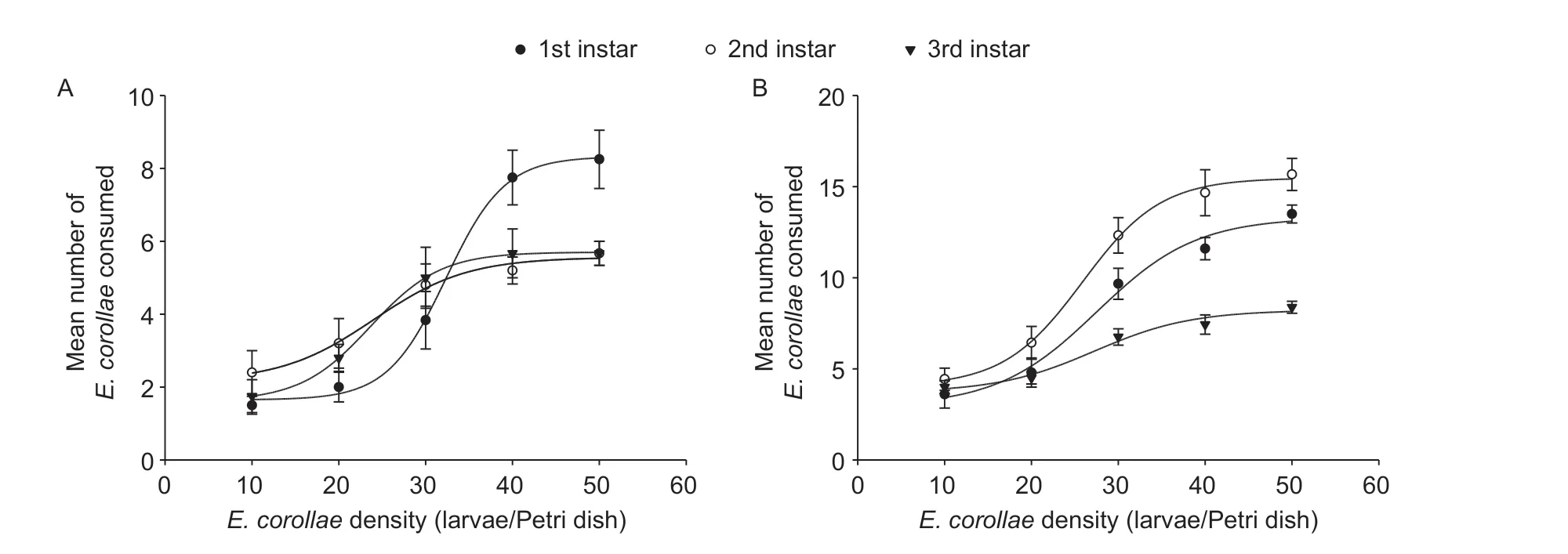
Fig.3 Functional response curves of 5th instar (A) or 6th instar (B) larvae of Spodoptera frugiperda feeding on different Eupeodes corollae larval instars,in the presence of maize leaves.
ThoughE.corollaelarvae did not prey upon 3rd instarS.frugiperdalarvae (i.e.,its interactions were limited to mouth hook probing),older syrphid larvae exhibited high levels of predation on early instarS.frugiperda.Our assays signal how native syrphid populations can play a role inS.frugiperdabiological control.Hence,by limiting the use of broad-spectrum insecticides in early-season maize filed,the in-filed establishment and population build-up of generalist predators such asE.corollaecan be encouraged andS.frugiperdaoutbreaks can possibly be avoided (Symondsonet al.2002; Yoo and O’Neil 2009).Other habitat manipulation tactics,e.g.,the establishment of flower strips,use of intercrops or maintenance of weedy field margins,can help conserveE.corollaepopulations and bolster natural biological control (Whiteet al.1995;Sutherlandet al.2001; Laubertieet al.2012).Yet,prior to implementing some of the above practice,it is essential to properly investigate syrphid-mediated biological control under field conditions and at varyingS.frugiperdapest pressure (or field colonization dynamics).
Throughout its native and invasive range,the fall armyworm is dreaded for its voracious consumption of food security crops such as maize,rice,sorghum,wheat or sugarcane (Andrewset al.1988; Baudronet al.2019; Xu P Jet al.2019).Within the Lepidoptera,feeding habitats however are not restricted to phytophagy; certain macrolepidopterans are facultative or obligate carnivores,with larentiine geometrids capturing flies or lycaenid butterflies engaging in myrmecophagy (Pierce 1995; Powellet al..1998).Cannibalism is frequently recorded among earlyinstarS.frugiperdalarvae and accounts for 40–60%mortality in both laboratory cultures and field populations(Chapmanet al.2000),while inter-specific predation occurs with other plant-inhabiting herbivores,e.g.,Helicoverpazea(Bentivenhaet al.2017).Spodoptera frugiperdalarvae equally demonstrate strong defensive behavior towards piercing-sucking predators,e.g.,the pentatomidSupputius cincticepsor the reduviidZeluslongipes(Silvaet al.2012;Mukherjee and Heithaus 2013),with such interactions negatively impacting predator survival or fitness (Mukherjee and Heithaus 2013).Our work shows how late-instarS.frugiperda,even in the presence of their natural food,routinely bite and consumeE.corollaelarvae.Though our microcosm assays did not fully mimic natural conditions (i.e.,S.frugiperdafeeding within the wrapped maize leaves or whorl; Labatte 1993),this type of defensive (or consumptive)behavior possibly can aidS.frugiperdainvasion processes,expand its impact on local agro-ecosystems and hamper conservation biological control.
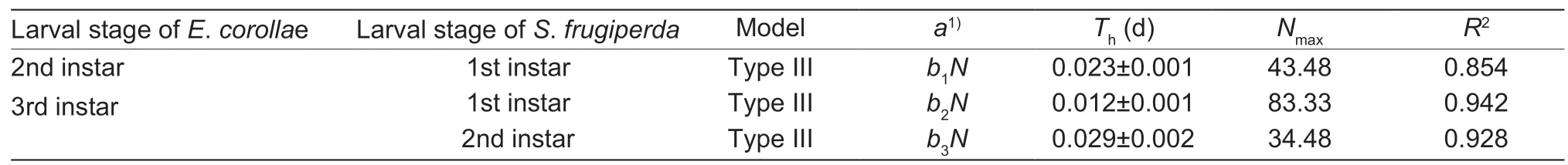
Table 4 Attack rate (a),handling time (Th),and theoretical maximum prey consumption (Nmax) of different Eupeodes corollae life stages perying on 1st or 2nd instar larvae of Spodoptera frugiperda in the presence of maize leaves
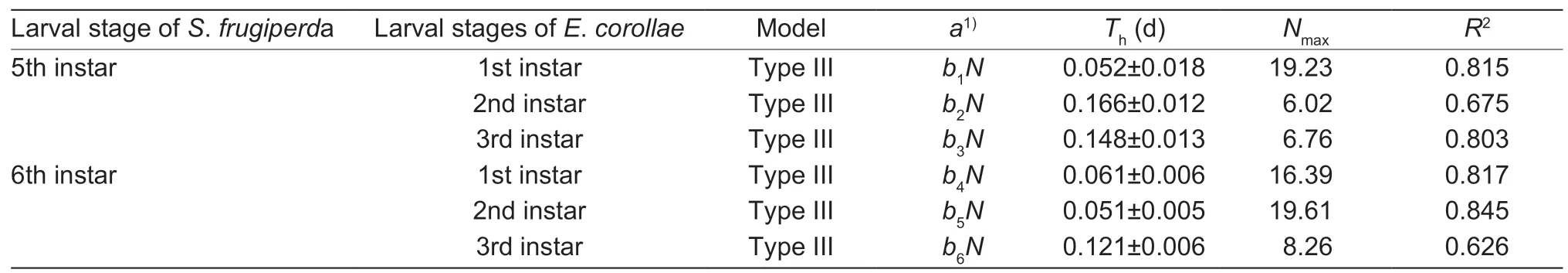
Table 5 Attack rate (a),handling time (Th),and theoretical maximum prey consumption (Nmax) by different Spodoptera frugiperda life stages preying on Eupeodes corollae larvae in the presence of maize leaves
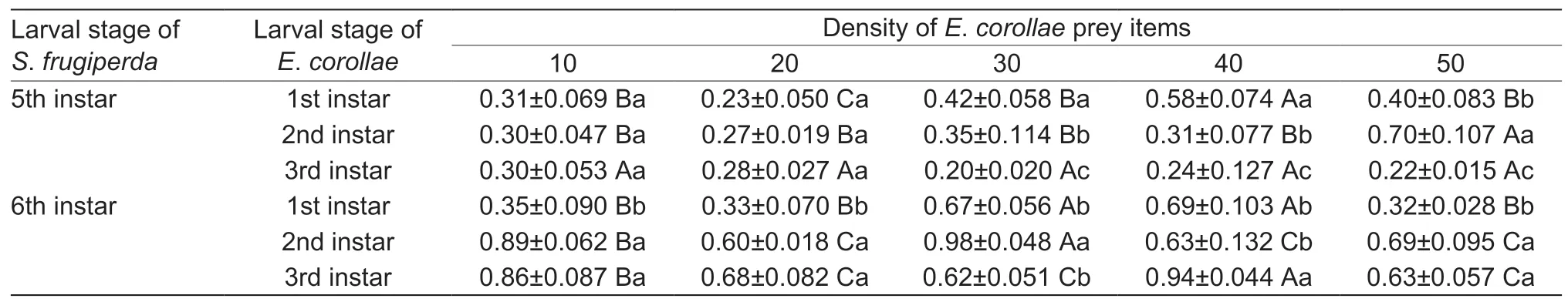
Table 6 Weight of maize leaves consumed by different Spodoptera frugiperda life stages,when presented with varying densities of Eupeodes corollae larvae
When assessing the potential contribution of a given natural enemy to biological control,its predation behavior is routinely characterized under laboratory conditions (Lucaset al.1997; De Clercqet al.2000; Mohagheghet al.2001).As such,functional response assays have been performed for numerous predatory insects,including syrphids (Putra and Yasuda 2006).Natural enemy predation rates are affected by prey density and often negatively impacted by the defensive behavior of prey such asS.frugiperda(Zanuncioet al.2008).In this study,E.corollaelarvae exhibited a type III functional response to early life stages ofS.frugiperda,thus accentuating their potential use as biological control agents ofS.frugiperda.Similarly,when provided with maize leaves,S.frugiperdalarvae exhibited a type III functional response towardsE.corollaelarvae,which can possibly alter in the presence of other host plants(Messina and Hanks 1998) or when performing assays on live plants.
Caution needs to be adopted when extrapolating data from our interactive predation trials to field conditions,as laboratory assays do not adequately account for ‘realworld’ environmental conditions (Murdoch 1973).Biotic and abiotic factors,numerical response,foraging behavior and interference competition all impact the role of a given predator in suppressing pest populations (Chenet al.2014;Madahiet al.2015).Hence,observational studies,field surveys,molecular gut content analyses and cage exclusion trials,e.g.,on whorl-stage maize will be essential to properly interpret laboratory-derived results (Desneuxet al.2006;Macfadyenet al.2015).The latter will be especially important to validate and quantitatively assessS.frugiperdaconsumptive (and non-consumptive) effects onE.corollaepopulations in China’s maize fields.Irrespective of the outcome of those studies,we are confident thatE.corollaecan complement the action of other resident predators e.g.,Armachinensis(Tanget al.2019; Wang Yet al.2019)and parasitic hymenopterans such asTrichogrammaandTelenomusspp.and thereby contribute toS.frugiperdabiological control in its newly-invaded range in eastern Asia.
5.Conclusion
Here,by using laboratory assays,we demonstrate a strong predation ability of endemicE.corollaeon early life stages ofS.frugiperdaand concurrently reveal howS.frugiperdalarvae attack and consume small numbers of syrphid larvae.This agonistic (and consumptive) behavior towards native insects and resident natural enemies can degrade biotic resistance,raiseS.frugiperdainvasion potential and broaden its environmental impacts in invaded settings.Aside from illuminating important aspects ofS.frugiperdainvasion ecology,our work confirms howE.corollaeplay a role in biological control ofS.frugiperdain eastern Asia.
Acknowledgements
We would like to thank Ms.Shao Mingyue from the College of Plant Protection,Hebei Agricultural University,for her assistance with insect rearing.This work was supported by the earmarked fund for China Agriculture Research System(CARS-15-19).
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Research on the invasive pest of fall armyworm (Spodoptera frugiperda)in China
- Migration of invasive Spodoptera frugiperda (Lepidoptera:Noctuidae)across the Bohai Sea in northern China
- Fitness of fall armyworm,Spodoptera frugiperda to three solanaceous vegetables
- Susceptibility and tissue specificity of Spodoptera frugiperda to Junonia coenia densovirus
- Analysis of phototactic responses in Spodoptera frugiperda using Helicoverpa armigera as control
- Genome editing of the SfABCC2 gene confers resistance to Cry1F toxin from Bacillus thuringiensis in Spodoptera frugiperda
