Analysis of phototactic responses in Spodoptera frugiperda using Helicoverpa armigera as control
2021-02-25LIUYingjieZHANGDandanYANGLiyuDONGYonghaoLIANGGemeiPhilipDONKERSLEYRENGuangweiXUPengjunWUKongming
LIU Ying-jie ,ZHANG Dan-dan ,YANG Li-yu ,DONG Yong-hao ,LIANG Ge-mei ,Philip DONKERSLEY,REN Guang-weiXU Peng-junWU Kong-ming
1 Key Laboratory of Tobacco Pest Monitoring Controlling & Integrated Management,Tobacco Research Institute,Chinese Academy of Agricultural Sciences,Qingdao 266101,P.R.China
2 State Key Laboratory for Biology of Plant Diseases and Insect Pests,Institute of Plant Protection,Chinese Academy of Agricultural Sciences,Beijing 100193,P.R.China
3 Lancaster Environment Centre,Lancaster University,Lancaster,LA1 4YQ,UK
Abstract Light traps are widely utilized to monitor and manage insect pest populations.In late 2018,the fall armyworm (FAW),Spodoptera frugiperda,invaded China through Yunnan Province representing a huge threat to grain production.To estimate the efficiency of light traps on FAW moths,we first identified the opsin genes from FAW by using the transcriptome.Phylogenetic analysis indicated that the four opsins of FAW were clustered with those of other Noctuidae species.The expressed levels of opsins in S.frugiperda were lower than in Helicoverpa armigera,suggesting a different phototactic response between the two species.Then,we determined the phototactic behavior of FAW using H.armigera as a control,which is widely monitored and managed using light traps in China.Our results indicated that the two moths species showed significantly different phototactic behavior and both female and male FAW displayed faster flight-to-light speed than H.armigera.This may be due to a faster flight capacity in FAW compared to H.armigera.However,the capture rate of both female and male of S.frugiperda was significantly lower than that of H.armigera,which was consistent with the expression levels of opsins.These results support the positive phototaxis of S.frugiperda moths and suggest light traps could be used for monitoring and managing the pests,but with a lower efficiency than H.armigera.
Keywords:Spodoptera frugiperda,Helicoverpa armigera,light performance,opsin genes,light trap
1.Introduction
Visual cues based on light are correlated with the ecology and behaviors of some insects,including mating (Jigginset al.2001),foraging (Cutleret al.1995; Weiss 2001),hostplant recognition (Kelber 1999) and migration (Merlinet al.2009).Correspondingly,insects have evolved phototactic behaviors (Jinget al.2005; Ohet al.2011; Kimet al.2019).Photosensitive genes such as opsins play a key role in phototaxis in nocturnal moths (Yanet al.2014; Liuet al.2018).Opsins are a kind of G-protein-coupled receptor characterized by a seven-transmembrane domain structure(Terakita 2005).According to physiological and molecular phylogenetic data,insect opsins are generally divided into three subfamilies:long-wavelength sensitive opsin (LW)(>500 nm),blue-sensitive opsin (B) (400–500 nm) and ultraviolet-sensitive opsin (UV) (325–400 nm) (Briscoe and Chittka 2001; Henze and Oakley 2015).Due to differential adaptive evolution by gene duplication and loss,insect opsins are highly diverse,from type to copy number.For example,Drosophilaevolved a novel subfamily named blue-green-sensitive opsins (about 480 nm) (Salcedoet al.1999; Briscoe and Chittka 2001; Henze and Oakley 2015),dragonflies have many opsins derived from multiplication events (Futahashiet al.2015),and most beetles have lost B-opsin (Jackowskaet al.2007; Sharkeyet al.2017).
The fall armyworm (FAW),Spodopterafrugiperda(Lepidoptera:Noctuidae),is native to tropical and subtropical areas and distributed widely throughout the Americas(Sparks 1979; Pogue 2002).As a polyphagous pest with a broad range of over 300 host plant species (Pogue 2002;Montezanoet al.2018),S.frugiperdaprefers to feed on corn and causes serious damage and yield reduction to grains in the Americas (Nagoshiet al.2007,2008; Hardkeet al.2011; Omotoet al.2016).Spodoptera frugiperdamoths are highly migratory and spread rapidly worldwide; this species was first reported in Nigeria and Ghana in early 2016(Goergenet al.2016),and has subsequently been reported in more than 40 countries in sub-Saharan Africa (Stokstad 2017; Earlyet al.2018; Otimet al.2018).In early 2018,S.frugiperdainvaded Southwest India and its presence was confirmed in at least six Asian countries within one year(Aliet al.2018; Kalleshwaraswamyet al.2018; Mallapuret al.2018; Jinget al.2020).FAW was reported in Yunnan Province in December 2018 (Sunet al.2021),and after six months was found in more than 22 provinces (autonomous regions,municipalities),significantly threatening grain production in China (Wanget al.2019; Jinget al.2020).Previously,there have been no reports about the opsins of FAW,and few studies focused on the responses of FAW moths to light for monitoring or management.Therefore,we identified the opsins ofS.frugiperdaand determined the phototactic response of the moths using moths ofHelicoverpaarmgieraas the control.
The cotton bollworm,Helicoverpa armigera(Lepidoptera:Noctuidae),is one of the most significant migratory crop pests throughout Africa,Asia,Australia,and Europe (Fenget al.2005,2009).Transgenic crops,e.g.,Bt cotton containing insecticidal toxins fromBacillusthuringienisis(Bt),have been widely used to control larval populations ofH.armigerasince the 1990s (Wuet al.2008).On the other hand,as a model for surveying and management,light traps have also been widely used for the control and detection of population dynamics ofH.armigeramoths in China and Australia (Zaluckiet al.1986; Maelzer and Zalucki 1999;Fenget al.2010).
Herein,we identified the opsin genes in the FAW.We also comparatively analyzed the expression levels of opsins and the phototactic responses of FAW moths withH.armigeramoths.
2.Materials and methods
2.1.Experimental materials and experimental design
InsectsSpodoptera frugiperdaandH.armigerapupae were supplied by the Institute of Plant Protection,Chinese Academy of Agricultural Sciences.Each pupa was separately placed in a 25-mL plastic box with a filter paper at the bottom and then reared in the insect chamber with controlled conditions of (25±1)°C,(70±5)% relative humidity and a 14 h L:10 h D photoperiod.Adult moths were provided with 10% sugar water.Two-day old unmated moths were used in this study.
Identifying the opsins and determining their expression levels by transcriptomeTo identify opsin genes and determine their expression levels inS.frugiperda,we collected two-day-old female and male adults and performed RNA-seq upon them (with three individuals each group and three replicates).We determined the expression levels of opsins inH.armigerausing the same method.cDNA libraries were constructed and sequenced,and the data were assembled and annotated by Majorbio (Shanghai,China) as described previously (Xuet al.2016).The opsin genes ofS.frugiperdaandH.armigerawere extracted with BLASTx using the four opsins ofH.armigeraas reference genes.Then,we designed specific primers to confirm the open reading frames (ORFs) of opsins inS.frugiperdausing cDNA as a template,with PCR and Sanger sequencing(Appendix A).We also amplified the long wavelength sensitive opsin 2 (LW2) using DNA templates.The PCR program was as follows:30 s at 94°C,30 s at 55°C,and 90 s at 72°C,for 40 cycles.Phylogenetic analysis was performed with MEGA7 using the neighbor-joining (NJ)method with Poisson distribution (Kumaret al.2016).For gene expression analysis,the number of expressed tags was calculated and then normalized to number of transcripts per million tags (TPM) by using RSEM software packages(Li and Dewey 2011).
Phototactic behavior testThe phototactic responses of moths were tested in a chamber within a dark room,which consisted of two opaque nylon net cages (60 cm×60 cm×60 cm),and a plastic pipe (20 cm in diameter and 200 cm in length) to connect the two cages.An entrance hole(4 cm in diameter) was made at the top center of the response pipe.An LED (380–780 nm,650 lx) light was placed in the top center of the cage as a light source on the light side (Fig.1).The time of per moth to the LED light from the hole was recorded (time to LED light).
Both male and female adults (2 d old) ofS.frugiperdaandH.armigerawere tested at (25±1)°C.A single moth was released into the chamber through the entrance hole.To keep moths moving within the chamber,the plastic pipe was turned artificially with a speed of approximately 20 r min–1.The selection of the light or dark side was noted for each individual.All tests were performed between 8:00 p.m.to 10:00 p.m.
LED light trapLED light traps were supplied by Tianyi New Energy Co.,Ltd.(TYF-40; Xinxiang,China),which had light luminance intensity of 1 650 lx and a moth-sensitive spectral wave length ranging from 365 to 550 nm.A nylon cloth bag was connected to the bottom of the trap to collect the adult moths attracted by the light trap.The light trap was put in a dark room (400 cm×700 cm×300 cm) at (25±1)°C.Twoday-old moths were released at 8:00 p.m.each day and the results were investigated after 10 h.
2.2.Statistical analysis
All experimental data were analyzed using GraphPad 3 and SPSS ver 16.0 (SPSS Inc.,Chicago,IL,USA).Theχ2test was used to analyze differences in light-dark selection behavior.The unpairedt-tests were used to analyze the time spent,captured percentage by light trap,expression levels of opsins betweenS.frugiperdaandH.armigera.
3.Results
3.1.Opsin genes in S.frugiperda
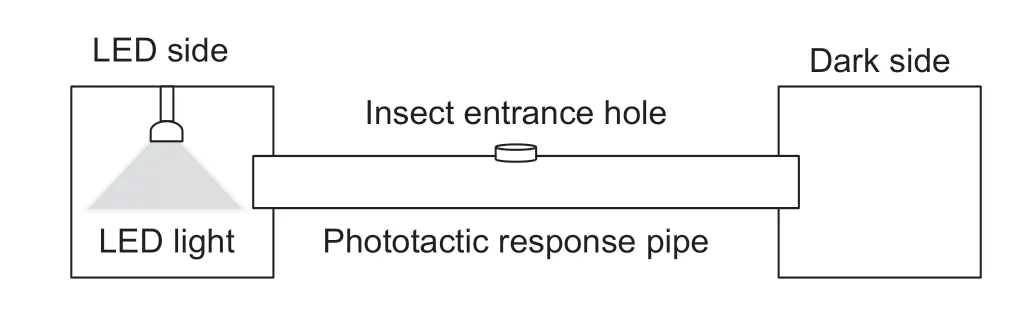
Fig.1 Schematic diagram of the phototactic response test chamber.
We obtained a total of 49.56 gigabases (Gb) of clean data fromS.frugiperda.An overview of the sequencing and assembly data are shown in Appendix B.The RNA-seq data were submitted to Sequence Read Archive (SRA) database(accession number:SRP220973).Using functional annotation,we obtained BLAST hits for 69 951 unigenes(30.45% of transcripts) using the E-value cutoff (1e-5) and NR database.With functional annotation and a BLAST search,using four opsins fromH.armigeraas reference sequences,we found five contigs which showed high identities with these reference sequences.We then determined these sequences by PCR and Sanger sequencing and obtained four opsin genes (Appendix A; Genbank accession numbers:MN442089–MN442092).An NJ tree was constructed with amino acid sequences of 22 opsins fromS.frugiperdaand six other Noctuidae species.The results indicated that the four opsins ofS.frugiperdaclustered with long-wavelength sensitive opsin 1 (LW1),LW2,Blue and UV,respectively(Fig.2).There was no intron in LW2,suggesting that as in opsins of other Noctuidae species,the LW ofS.frugiperdaoccurred due to a duplication event by retrotransposon.
3.2.The expression levels of opsin genes in S.frugiperda and H.armigera
Previously,we proved that LW2 genes were expressed at very low levels in adult moths in Noctuidae (Xuet al.2016).Therefore,we focused solely on the expression levels of LW1,Blue,and UV in this study.We obtained 47.49 Gb data fromH.armigera(Appendix C) and the data were submitted to the SAR database (accession number:SRP220973).The TPM values of contigs standing for opsins were used to analyze the expression levels in the two species.Interestingly,all these opsins were expressed with lower average levels inS.frugiperdathan inH.armigera.Moreover,LW1 in females (t4=6.352,P=0.0031),UV in both females (t4=14.292,P=0.0001) and males (t4=7.670,P=0.0016),and Blue in males (t4=3.251,P=0.0313) showed significant differences between the two species (Fig.3).However,there were no significant differences in the expression levels of LW1 in males (t4=1.674,P=0.1694) and Blue in females (t4=0.1580,P=0.8821) (Fig.3).
3.3.Selection behavior towards LED light and capture rate by light traps
All moths were significantly attracted to the light cage,suggesting positive phototaxis in both species (S.frugiperda:female,χ2=70.124,n=89,P=0.001; male,χ2=49.028,n=71,P=0.001;H.armigera:female,χ2=88.615,n=104,P=0.001;male,χ2=101.319,n=113,P=0.001) (Fig.4-A).In individuals attracted to the light,both female and maleS.frugiperdamoved faster thanH.armigera(females:t182=7.217,P=0.0001,n=84 forS.frugiperda,n=100 forH.armigera,Fig.4-B; males:t173=4.359,P=0.0001,n=65 forS.frugiperda,n=110 forH.armigera,Fig.4-C).However,the capture rates by light traps of both female and maleS.frugiperdawere significantly lower than those ofH.armigera(females:t4=4.498,P=0.0108,n=88 forS.frugiperda,n=85 forH.armigera,Fig.5-A; males:t4=6.589,P=0.0027,n=71 forS.frugiperda,n=99 forH.armigera,Fig.5-B).
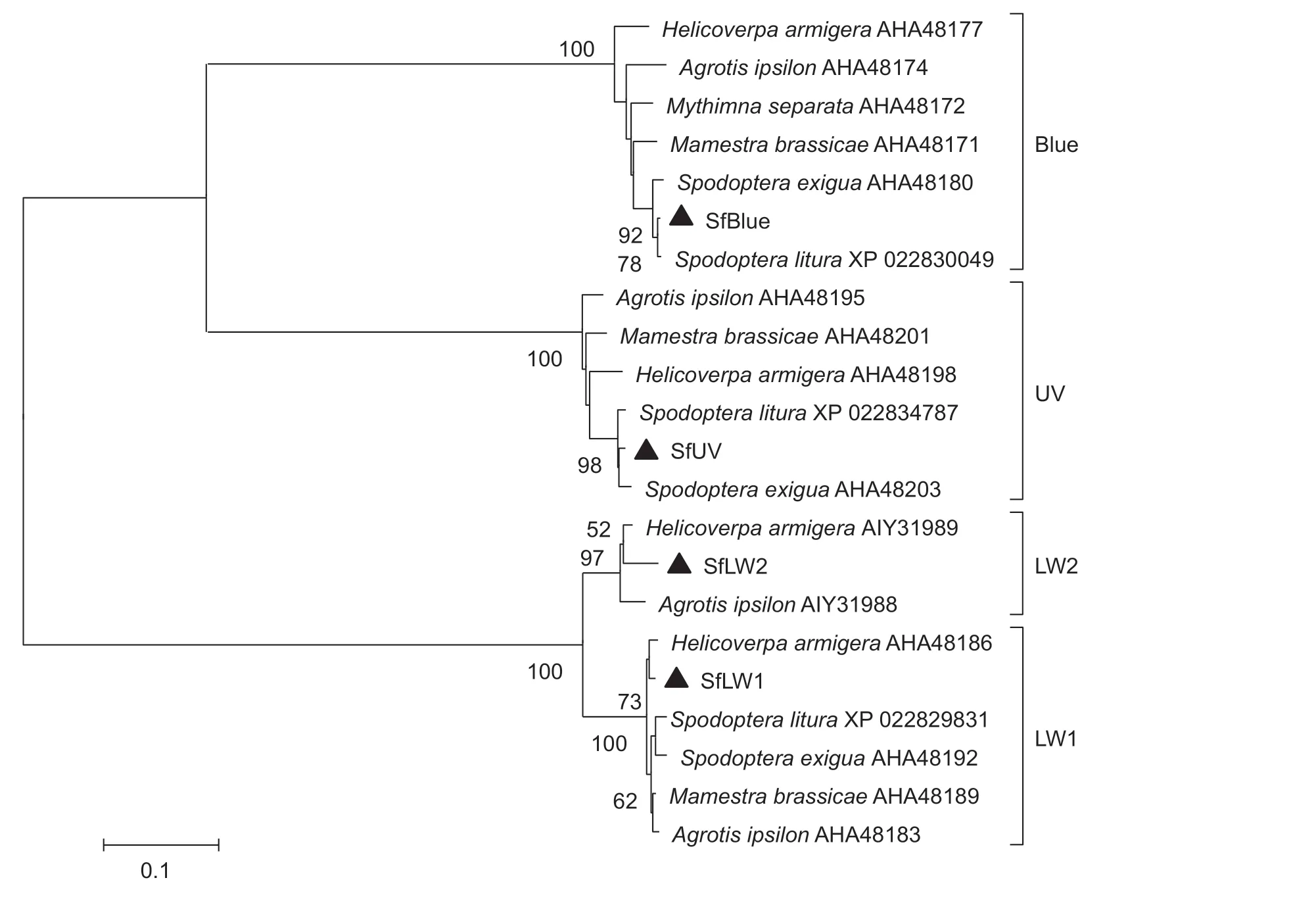
Fig.2 Neighbor-joining (NJ) tree with Poisson distribution constructed using the amino acid sequences of 22 opsins from Spodoptera frugiperda and six other species in Noctuidae.Blue,blue sensitive opsin; UV,ultraviolet sensitive opsin; LW1,long wavelength sensitive opsin 1; LW2,long wavelength sensitive opsin 2.▲ stands for opsins from S.frugiperda.The bootstrap values (1 000 pseudo replicates)>50% are indicated on the nodes.
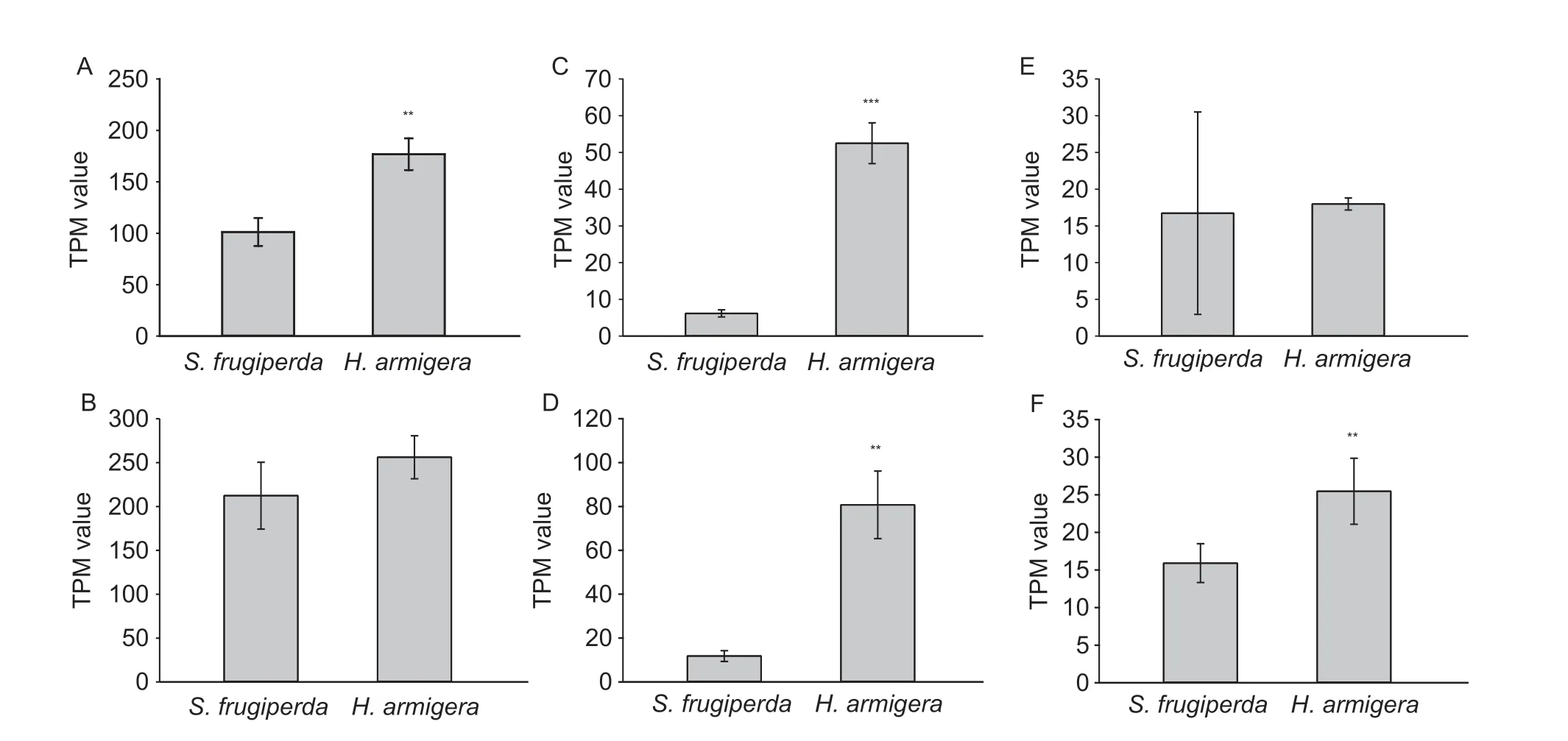
Fig.3 The expression levels of opsins in moths of Spodoptera frugiperda and Helicoverpa armigera.A,long-wavelength sensitive opsin 1 (LW1) in females.B,LW1 in males.C,ultraviolet-sensitive opsin (UV) in females.D,UV in males.E,blue-sensitive opsin (Blue) in females.F,Blue in males.TPM, transcripts per million tags.Data are mean±SD.** and *** stand for P<0.01 and P<0.001,respectively.
4.Discussion
Many insects,especially nocturnal insects,show positive phototaxis to artificial lights.Light traps are widely utilized to monitor and manage pest populations (Johansenet al.2011; Ohet al.2011; Tokushimaet al.2016; Park and Lee 2017; Kimet al.2019).The sensitivities of insects to light are diverse among species (Peitschet al.1992; Briscoe and Chittka 2001).FAW invaded China in late 2018 and became a huge threat to grain production (Sunet al.2021).Herein,for the first time,we describe the opsins inS.frugiperda,which possessed four opsins,including two LWs from retrotransposon replication as species from Noctuidae (Xuet al.2013,2016).
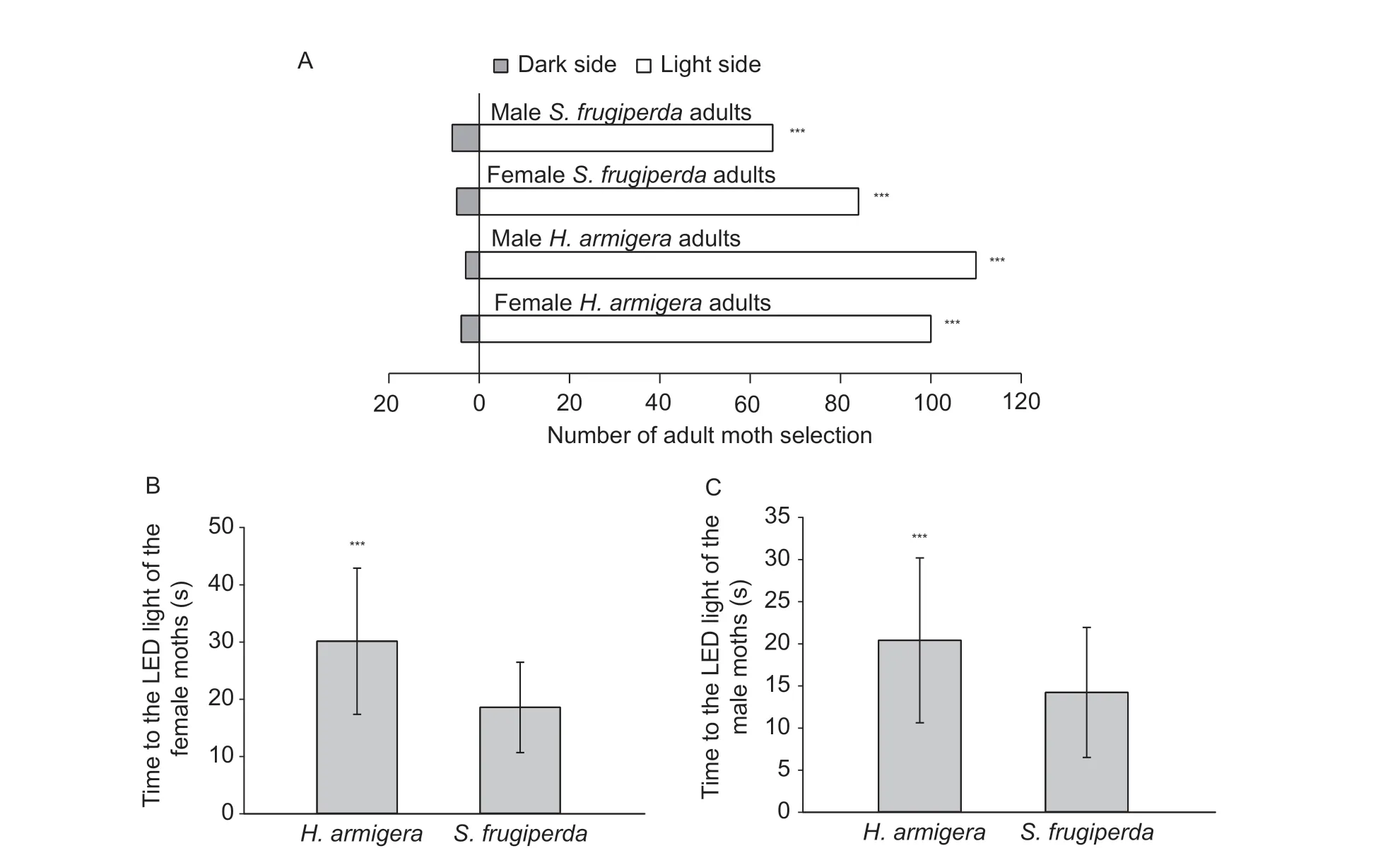
Fig.4 Selection behavior of Helicoverpa armigera and Spodoptera frugiperda moths to LED light.A,selection behavior of both females and males from H.armigera and S.frugiperda (χ2 test).B,time to the LED light of female moths.C,time to the LED light of male moths.Data are mean±SD.*** stands for P<0.001.
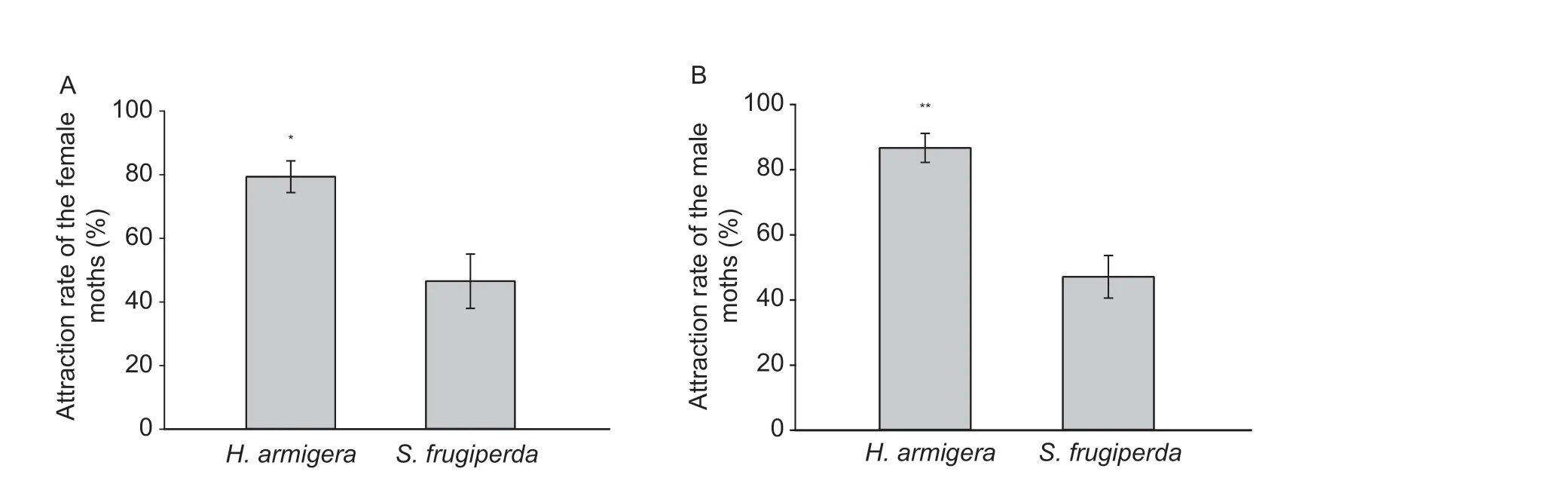
Fig.5 Captured rates of females (A) and males (B) from Helicoverpa armigera and Spodoptera frugiperda by light traps.Data are mean±SD.* and ** stand for P<0.05 and P<0.01,respectively.
The expression levels of opsins were positively related to the phototaxis of nocturnal moths (Liuet al.2018).We had previously described moth opsins and proved the occurrence of LW duplication events in Noctuidae species,where LW2 was expressed with very low levels (Xuet al.2013,2016).Hence,we only focused on the expression levels of LW1,Blue,and UV,which might be responsible for the phototactic behavior of insects.It is difficult to precisely compare homologous genes in different species by qPCR.However,next generation sequencing,e.g.,RNA-seq by Illumina,may provide relatively precise results based on huge quantities of data.Interestingly,our RNA-seq results suggested that the expression levels of opsins inS.frugiperdawere lower than inH.armigera,which suggested different phototactic behavior between the two species.
To estimate the efficiency of monitoring and management ofS.frugiperdausing light traps,we first determined the light-sensitiveS.frugiperdausingH.armigeraas a control,as there had formed a maturely forecast and control technology forH.armigeraby light traps in China (Fenget al.2004,2005,2009).Environmental conditions such as light and the physiological status of the insect can impact the phototactic behavior of insects (Park and Lee 2017; Kimet al.2018; Liuet al.2018).Our results revealed that moths ofS.frugiperdaandH.armigerashowed positive phototaxis to the light source,which were the same with other nocturnal insects (Land 1997; van Grunsvenet al.2014; Zhenget al.2014).Surprisingly,the flight-to-light speed ofS.frugiperdamoths was faster than moths ofH.armigera,which was not consistent with the opsin expression levels in the two species.
To determine whether the faster flight-to-light behavior of FAW thanH.armgierarepresented stronger phototactic behavior,we performed the light trap experiment.Interestingly,the light trap capture rates for both female and maleS.frugiperdawere significantly lower than those ofH.armigera,which was consistent with the opsin expression levels in the two species.These results suggested that the faster flight-to-light speed of FAW may be due to the better flight capacity of the FAW compared toH.armigera,however,the phototactic behavior ofH.armigerawas stronger than FAW.
5.Conclusion
We identified four opsins inS.frugiperdaand found that the expression levels of opsins inS.frugiperdawere lower than inH.armigera,suggesting different phototactic responses in the two species.Subsequently,we proved that although both female and maleS.frugiperdawere faster in flight-tolight speed that the those ofH.armigera,the capture rate ofS.frugiperdawas significantly lower thanH.armigera,which was consistent with opsin expression levels in these two species.These results indicated the positive phototaxis ofS.frugiperdamoths and suggested light traps could be used for monitoring and managing FAW,but with a lower efficiency than forH.armigera.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2017YFB0403905),the Central Public-interest Scientific Institution Basal Research Fund,China (Y2019YJ06),and the Agricultural Science and Technology Innovation Program of China (ASTIP-TRIC04).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper can be available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
杂志排行
Journal of Integrative Agriculture的其它文章
- Research on the invasive pest of fall armyworm (Spodoptera frugiperda)in China
- Migration of invasive Spodoptera frugiperda (Lepidoptera:Noctuidae)across the Bohai Sea in northern China
- Fitness of fall armyworm,Spodoptera frugiperda to three solanaceous vegetables
- Susceptibility and tissue specificity of Spodoptera frugiperda to Junonia coenia densovirus
- Two-way predation between immature stages of the hoverfly Eupeodes corollae and the invasive fall armyworm (Spodoptera frugiperda J.E.Smith)
- Genome editing of the SfABCC2 gene confers resistance to Cry1F toxin from Bacillus thuringiensis in Spodoptera frugiperda
