Genome editing of the SfABCC2 gene confers resistance to Cry1F toxin from Bacillus thuringiensis in Spodoptera frugiperda
2021-02-25JINMinghuiTAOJiahuiLIQiCHENGYingSUNXiaoxuWUKongmingXIAOYutao
JIN Ming-hui ,TAO Jia-hui ,LI Qi ,CHENG Ying ,SUN Xiao-xu ,WU Kong-ming,XIAO Yu-tao
1 Agricultural Genomics Institute at Shenzhen,Chinese Academy of Agricultural Sciences,Shenzhen 518120,P.R.China
2 State Key Laboratory for Biology of Plant Diseases and Insect Pests,Institute of Plant Protection,Chinese Academy of Agricultural Sciences,Beijing 100193,P.R.China
Abstract ATP-binding cassette transporter C2 (ABCC2) is known to be a receptor for Bacillus thuringiensis (Bt) toxins in several lepidopteran insects.Mutations in the ABCC2 gene have been genetically linked to field-evolved resistance to the Cry1F toxin from Bt in Spodoptera frugiperda.Here we generated a SfABCC2 knockout strain of S. frugiperda using the CRISPR/Cas9 system to provide further functional evidence of the role of this gene in susceptibility and resistance to Cry1F.Results from bioassays showed that the SfABCC2 knockout S.frugiperda strain displayed 118-fold resistance to Cry1F compared with the parental DH19 strain,but no resistance to Vip3A toxin from Bt.These results provide the first reverse genetic evidence for SfABCC2 as a functional receptor for Cry1F.
Keywords:Spodoptera frugiperda,ABCC2,CRISPR/Cas9,Bt receptor,Cry1F
1.Introduction
The fall armyworm (Spodoptera frugiperda) is an important noctuid polyphagous pest native to the subtropical and tropical regions in the Americas (Nagoshiet al.2017a).As a major pest of maize,S.frugiperdahas a wide host range of more than 80 plant species,causing serious economic damage (Gouinet al.2017).In 2016,S.frugiperdawas detected as an established invasive pest in sub-Saharan Africa,and outbreaks in western Africa were reported within two months (Goergenet al.2016; Nagoshiet al.2017b).In early May–June 2018,this pest was first observed in India(Sharananasappaet al.2018),and was reported to invade China in December,2019 (Liet al.2019; Sunet al.2021).Currently,S.frugiperdais confirmed in most eastern and southern Asian countries (FAO 2019).
Transgenic crops expressing insecticidal proteins from the bacteriumBacillus thuringiensis(Bt) have been used for pest control in some crops such as cotton,corn,and soybean (Wanget al.2017).The modes of action of these Bt proteins include binding to specific receptors on midgut cells and formation of toxin pores that lead to osmotic cell death (Vachonet al.2012).Recognition and binding to receptors is known to be a critical step in the modes of action of Bt toxins,and high levels of resistance to these toxins are inevitably related to altered binding (de Bortoli and Jurat-Fuentes 2019).A number of Bt toxin receptors have been reported,including cadherin,aminopeptidases,alkaline phosphatases,and ABC transporter proteins (Adanget al.2014).Within ABC transporter genes,mutations in the ABCC2 family are linked to Bt resistance in several lepidopteran pests,such asHeliothis virescens(Gahanet al.2010),Plutella xylostella(Baxteret al.2011),Bombyx mori(Atsumiet al.2012),Spodoptera exigua(Parket al.2014),andHelicoverpa armigera(Xiaoet al.2014).
Transgenic corn producing the Cry1F toxin was planted commercially from 2003 for controllingS.frugiperda(Siebertet al.2008).However,field-evolved resistance to maize producing the Cry1F toxin was detected in populations ofS.frugiperdafrom Puerto Rico in 2010 (Storeret al.2010).Genetic linkage results showed that resistance to Cry1F inS.frugiperdafrom Puerto Rico was linked to a mutation in anSfABCC2gene,which resulted in reduced Cry1F toxin binding (Banerjeeet al.2017).This resistance allele(SfABCC2mut) was found to be at high frequency (0.55) inS.frugiperdapopulations in Puerto Rico,but was not detected outside the island (Banerjeeet al.2017).
Recent reports demonstrated the use of the CRISPR/Cas9 genome editing system as a tool to study gene function inS.frugiperda(Wuet al.2018).In the present study,we used the CRISPR/Cas9 genome editing system to generate a SfABCC2 knockout strain ofS.frugiperda.Bioassay results showed that theSfABCC2knockout strain exhibits resistance to Cry1F,but no resistance to Vip3A.This work providesin vivofunctional evidence forSfABCC2as a receptor for Cry1F.
2.Materials and methods
2.1.Insects
Spodoptera frugiperdastrain DH19 was collected from Dehong,Yunnan Province of China in January 2019 and reared in the laboratory for two generations on an artificial diet (the unpublished feed formula contains wheat germ meal as the main component) without exposure to any insecticides or Bt toxins.Insects were kept at (27±2)°C and (75±10)% relative humidity (RH) with 14 h light:10 h dark photoperiod.Adults were provided access to a 10%sucrose solution for nutrition.
2.2.Bt toxins and bioassays
The Cry1F and Vip3A toxins used in this study were obtained from the Institute of Plant Protection,Chinese Academy of Agricultural Sciences (CAAS).Toxicity of each Bt toxin to DH19 and theSfABCC2knockout strain was determined with diet overlay bioassays.Liquid artificial diet (900 mL)was dispensed into a 24-well plate (surface area=2 cm2).Gradient concentrations of Bt toxin solution were prepared by diluting the stock suspensions with PBS (pH=7) solution.After the diet cooled,50 µL of Bt toxin solution was applied to the surface of diet in each well.A single newly-hatched larval was put in each well with dried diet,and mortality was recorded after 7 days.The LC50values (the concentration of Bt protein killing 50% of larvae) and the corresponding 95% fiducial limits were calculated through probit analysis of the mortality data using SPSS.
2.3.Design and preparation of sgRNA
The small guide RNA (sgRNA) for editing theSfABCC2gene (GenBank accession:AUO38740.1) was designed using the sgRNAcas9 design tool (Xieet al.2014).After on-target and off-target analysis,a sgRNA-ABCC2 target sequence (5´-CGCATGAGTCAAGTGTCAGTGGG-3´) was selected at exon 4 (Fig.1-A).The selected sgRNA was checked by comparing it against theS.frugiperdagenome(https://bipaa.genouest.org),and no potential off-target sites were identified.The template DNA forin vitrotranscription of the sgRNA was made using PCR-based fusion of two oligonucleotides with the T7 promoter.In vitrotranscription was performed with the GeneArt Precision gRNA Synthesis Kit (Thermo Fisher Scientific,MA,USA),according to the manufacturer’s instructions.Cas9 protein was purchased from Thermo Fisher Scientific,USA.
2.4.Egg collection and microinjection
Freshly laid eggs (within 2 h after oviposition) were washed with distilled water and then placed on a microscope slide and fixed with double-sided adhesive tape.About a 1-nL mixture of sgRNA (200 ng µL–1) and Cas9 protein (50 ngµL–1) was injected into individual eggs using the Nanoject III(Drummond,Broomall,USA).After injection,eggs were incubated at 25°C and 65% RH for hatching.
2.5.Analysis of CRISPR/Cas9-induced mutations of SfABCC2
For analysis of mutations,PCR fragments flanking the targeted sites were amplified with ABCC2 primers(Appendix A).The genomic DNA was extracted with a Multisource Genomic DNA Miniprep Kit (Axygen,NY,USA) according to the manufacturer’s instructions.The PCR conditions were:94°C for 5 min,34 cycles of 94°C for 30 s,60°C for 30 s,72°C for 35 s,followed by a final extension period of 72°C for 10 min.PCR amplicons were then analyzedviaagarose gel electrophoresis.Direct sequencing of PCR products was conducted and double sequencing peaks,indicating a mutation event,were identified.PCR products were then recovered and cloned into pEASY-T3 vector (TransGen,Beijing,China) and sequenced by Sangon Biotech (Shanghai,China).
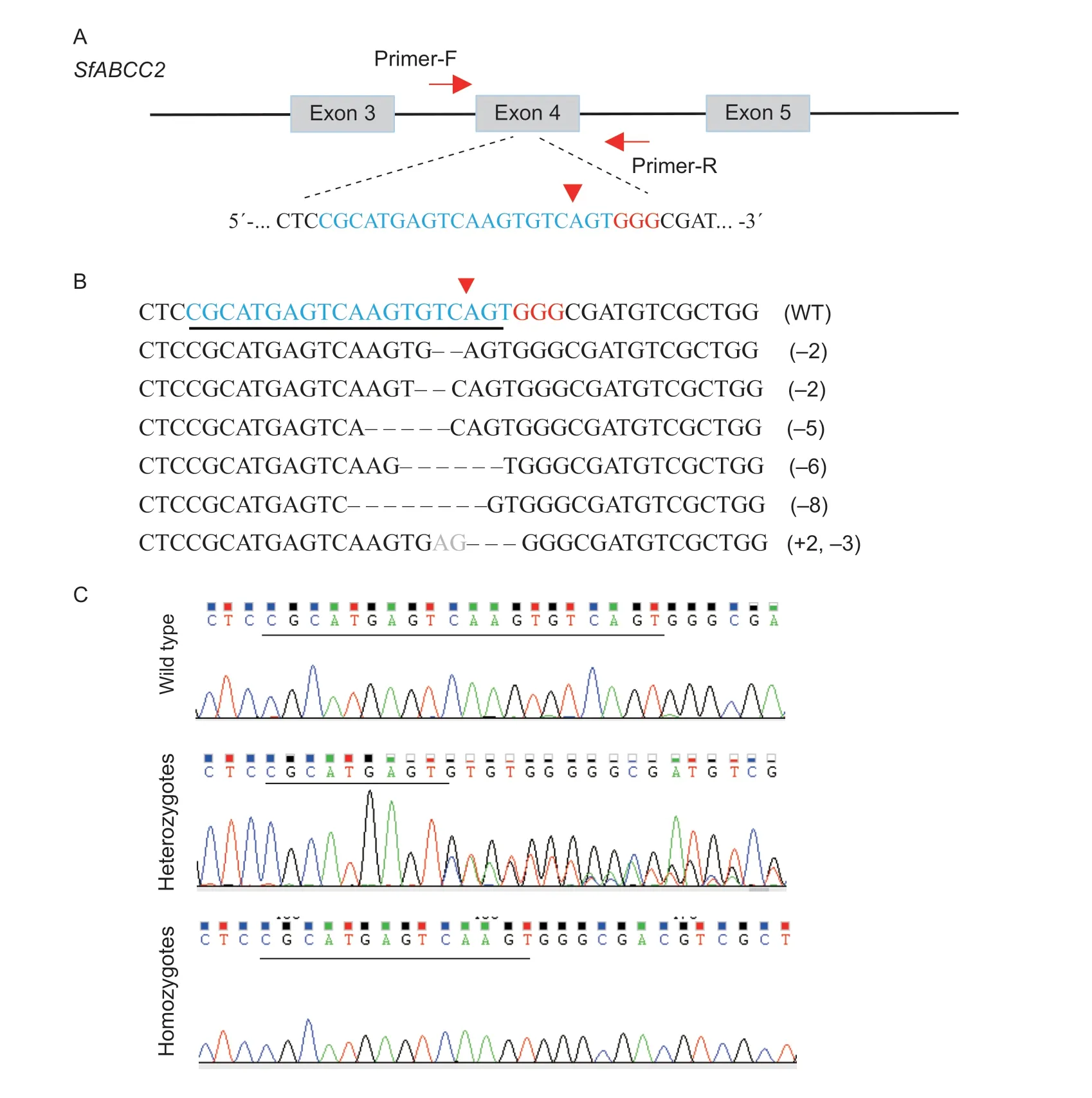
Fig.1 The CRISPR/Cas9-induced mutation types of SfABCC2 of Spodoptera frugiperda.A,schematic diagram of the sgRNAtargeting sites.The sgRNA-targeting site was located on exon 4,and sgRNA-targeting sequence is in blue.B,sequences of indel mutations flanking the target site in G0 larvae.The wild type (WT) sequence is shown at the top.The protospacer adjacent motif (PAM) sequence is highlighted in red,and cleavage site is indicated by a red triangle.C,representative chromatograms of sequences of wild type,mutant heterozygotes and mutant homozygotes.
3.Results
3.1.CRISPR/Cas9 mediates efficient SfABCC2 knockouts
We selectedSfABCC2to check the mutation efficiency of the CRISPR/Cas9 genome editing system when editing S.frugiperda.To targetSfABCC2,a mixture ofin vitrotranscribed sgRNA and Cas9 protein were co-injected into 200 newly laid eggs.After injection,21.5% (43/200) of injected eggs hatched and 81.4% (35/43) of the neonates develop to adults (G0).The G0male and female moths were mass backcrossed with strain DH19 to produce the next generation in single pair mating (G1).
After enough eggs were collected,genomic DNA samples of individual G0moths were prepared,andSfABCC2genotypes screened by direct sequencing of PCR products.The results showed that 31% (11/35) of the G0moths were mutagenized.PCR products were TA-cloned and sequenced to identify the exact mutations.In total,six types of indel mutations were detected (Fig.1-B and C).
3.2.Mutation inheritance
Fig.2 Flow diagram of the crossing scheme used to obtain the homozygous strain.
From the detected mutations,we selected a 6-bp deletion to generate a homozygous knockout strain (Fig.2).G1larvae(progeny crosses of the 6-bp deletion G0moth and stain DH19) were reared to pupation,and 96 exuviates of the final instar larvae were used to prepare genomic DNA,identifying 26 pupae carrying the 6-bp deletion allele.Adults from these pupae were mass-crossed to obtain a G2generation.Ninety-six individuals from the G2were genotyped using final instar exuviate,and 17.7% (17/96) were identified as homozygotes.These homozygous individuals were pooled to produce aSfABCC2knockout strain (ABCC2-KO).
3.3.Susceptibility to Bt toxins in the SfABCC2 knockout strain
Susceptibility to Cry1F and Vip3A was compared between the progenitor strain DH19 and the knockout strain (ABCC2-KO).Bioassay results showed that the knockout strain had decreased susceptibility to Cry1F but not to Vip3A (Fig.3).The LC50value obtained for Cry1F in the knockout strain(39.2 (29.2–54.2) µg cm–2) was 118-fold higher than that of the control strain DH19 (0.33 (0.25–0.44) µg cm–2).In contrast,the bioassay results with Vip3A showed no significant change in susceptibility between the DH19 (0.07(0.052–0.095) µg cm–2) and ABCC2-KO strains (0.051(0.038–0.070) µg cm–2).
4.Discussion
Fig.3 Nonlinear Log dose-response curves for Spodoptera frugiperda larvae from the strain DH19 (DH19-S) and knockout stain (ABCC2-KO) to Bt toxins Cry1F and Vip3A.
The CRISPR/Cas9 genome editing system has been successfully used to manipulate different target genes in different non-model species (Huanget al.2016; Liet al.2016; Wanget al.2016; Zhuet al.2016; Jinet al.2018),includingS.frugiperda(Wuet al.2018).In this study,we obtained 31% mutation frequency in G0S.frugiperdamoths,similar to the mutation frequency of theSePgpgene inS.exigua(Zuoet al.2017) and some reports in the silkworm,but lower than the 87.5%SlitPBP3gene mutagenesis inSpodoptera litura(Zhuet al.2016),or the 55% cadherin gene mutagenesis inH.armigera(Wanget al.2016).The mutation frequency may be influenced by several factors,such as target gene selection,sgRNA or Cas9 dose,or forms of injected Cas9 (plasmid,mRNA or Cas9 protein)(Zhuet al.2016).
Mutations inABCC2genes have been reported to be associated with Bt resistance in several lepidopteran insects (Gahanet al.2010; Atsumiet al.2012; Parket al.2014; Xiaoet al.2014; Banerjeeet al.2017).In a resistant strain ofS.frugiperdaoriginated from Puerto Rico,Cry1F resistance was geneticly linked to a two base pairs insertion(GC) in theSfABCC2gene.Heterologous expression of the mutantSfABCC2in cell cultures demonstrated thatSfABCC2serves as a functional Cry1F and Cry1A toxin receptor (Banerjeeet al.2017).However,no directin vivofunctional validation of SfABCC2 as Cry1F receptor has been conducted.
The insertion mutation inSfABCC2that is linked to Cry1F resistance in the field population ofS.frugiperdain Puerto Rico was not detected inSfABCC2in the DH19 strain (Appendix B),which validated that this DH19 strain can be used for genome editing studies.We knocked out theSfABCC2gene in DH19 using the CRISPR/Cas9 system,and the resulting knockout strain displayed 118-fold resistance to Cry1F.These levels of resistance are in agreement with resistance levels observed in field-resistantS.frugiperda(Storeret al.2010),and provide reverse genetics evidence forSfABCC2as a functional receptor of Cry1F.Furthermore,the susceptibility of the SfABCC2-KO strain to Vip3A toxin was similar to that of DH19 strain,revealing the lack of a common receptor between Cry1F and Vip3A toxins (Jianget al.2018).
Nonsense-mediated mRNA decay is an important cellular surveillance pathway in the degrading mRNAs that causes premature translation of termination codons.This mechanism can prevent the synthesis of potentially harmful and truncated proteins (Conti and Izaurralde 2005;Weischenfeldtet al.2005).In this study,artificially induced 6-bp deletion in ABCC2 may also induce this degradation mechanism or other degradation mechanism.Besides,the ABCC2 protein functions as a Bt receptor that located on the membrane of midgut.Previous study showed that a single point mutation in cadherin (a receptor of Cry1Ac),resulted its mis-localization and led to resistance to Cry1Ac(Xiaoet al.2017).The 6-bp deletion in this study may also result in inaccurate protein localization.However,these two hypotheses need further validation.
Resistance to Bt toxins is the main threat for the longterm use of Bt products and transgenic crops producing these insecticidal proteins.The growing relevance ofS.frugiperdaworldwide highlights the need for efficient control methods.Information on resistance mechanisms is critical to develop efficient management practices and improved Bt-based technologies against this pest.
5.Conclusion
The results of this study provide new functional evidence forSfABCC2as a receptor of Cry1F,and also demonstrate that the CRISPR/Cas9 genome editing system is an efficient genome editing tool for studying the function of candidate genes inS.frugiperda.
Acknowledgements
We thank Prof.Juan Luis Jurat-Fuentes (University of Tennessee,Knoxville,USA) for his comments and revision on an earlier draft of the manuscript.This study was financially supported by the Key Project for Breeding Genetic Modified Organisms of China (2016ZX08012004003).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper can be available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
杂志排行
Journal of Integrative Agriculture的其它文章
- Research on the invasive pest of fall armyworm (Spodoptera frugiperda)in China
- Migration of invasive Spodoptera frugiperda (Lepidoptera:Noctuidae)across the Bohai Sea in northern China
- Fitness of fall armyworm,Spodoptera frugiperda to three solanaceous vegetables
- Susceptibility and tissue specificity of Spodoptera frugiperda to Junonia coenia densovirus
- Two-way predation between immature stages of the hoverfly Eupeodes corollae and the invasive fall armyworm (Spodoptera frugiperda J.E.Smith)
- Analysis of phototactic responses in Spodoptera frugiperda using Helicoverpa armigera as control
