Valorization of AZ91 by the hydrolysis reaction for hydrogen production(Electrochemical approach)
2021-02-24AlBhAubertZkhourNkhlBobet
S.Al Bh ,I.Aubert ,M.Zkhour ,M.Nkhl ,J.L.Bobet
a LCPM/PR2N(EDST),Lebanese University,Faculty of Science II,90656 Jdeidet El Metn,Lebanon
b Université de Bordeaux,ICMCB,CNRS,UMR 5026,F-33600 Pessac,France
c University of Bordeaux,CNRS,Arts et Métiers,Institute of technology,Bordeaux INP,INRAE,I2M Bordeaux,F-33400 Talence,France
Abstract The hydrolysis of Mg-based materials appears to be an ideal solution for clean energy production.Green hydrogen was produced by the hydrolysis reaction of a“standard”AZ91 alloy(called AZ91 in the following)in“model”seawater solution.Two milling speeds(i.e.250rpm and 350rpm)were tested to enhance the reactivity of AZ91.Graphite and AlCl3 were used as ball milling additives.Milling at higher rotational speed is more energetic,hence it ameliorates the most the hydrolysis performance of AZ91.Comparing both milling additives,AlCl3 enhances the most the hydrolysis of AZ91 with a generation of 65% of its theoretical H2 generation capacity.The best material was obtained by milling AZ91 at 350rpm with graphite for 2h followed by a further milling with AlCl3 for 2h–a yield of 75% of its theoretical H2 generation capacity was reached within a few minutes.The corrosion behavior of milled AZ91 was investigated by anodic polarization and electrochemical impedance spectroscopy(EIS).The calculated electrochemical parameters from EIS fitting of two materials milled under different conditions but with the same milling additive are approximately the same.This suggests that,in order to fully evaluate the reactivity of AZ91,hydrolysis,anodic polarization and EIS must be considered.
Keywords: Ball milling;AZ91;Mg17Al12;Corrosion;Hydrogen;Electrochemical impedance spectroscopy.
1.Introduction
The way we use fossil fuels as our main source of energy since the industrial revolution has caused a massive increase in the levels of CO2and other greenhouse gases in our atmosphere,which is the main cause of global warming [1].Decarbonizing the energy supply using clean,sustainable and renewable alternative energies is essential for future energy sustainability,global security and climate change [2–7].Renewable energy resources will play a key role in the transition to a clean and sustainable energy system [8,9].In fact,hydrogen as an energy carrier is among the most promising solutions to the problems mentioned above [1,10–12].
Hydrogen can be produced using diverse domestic resources,including nuclear,natural gas,coal,biomass,and other renewable sources [13].The latter include solar,wind,hydroelectric,or geothermal energy.The production of hydrogen can be achieved via various process technologies,namely electrolysis,photolysis,biolysis,thermolysis,plasma arc decomposition of methane,coal gasification,fermentation,etc.[1,9,13–15].Nowadays,hydrogen is mainly produced by steam reforming of natural gas,a process that leads to massive emissions of greenhouse gases [16,17].However,all these production techniques are limited for economic,environmental reasons and dependence on resources(especially for production from biomass).
Conversely,hydrogen production by the hydrolysis reaction of magnesium can occur spontaneously under normal conditions of temperature and pressure without any catalyst.However,magnesium hydrolysis is rapidly interrupted by the formation of a passivation layer of solid magnesium hydroxide on the surface of Mg,which limits the hydrolysis by blocking the diffusion of H2O to Mg matrix.Recently,many highly reactive Mg-based materials have been developed to improve the hydrogen production by the hydrolysis reaction(e.g.Mg2Si [18],Mg-Mg2X(X=Cu,Sn)[19],Mg-Ni-Ce[20],MgNdNi15[21],Mg-Mg2Si [22]Mg–Graphite composites elaborated by plasma-assisted milling [23])and have been reviewed elsewhere [24].On the other hand,it has been demonstrated that the hydrolysis of magnesium can be replaced by that of Mg-based alloys and waste [25–33].This can enable the low-cost production of pure hydrogen by the hydrolysis reaction.On the other hand,ball milling has been shown to be beneficial in improving the reactivity of Mgbased materials.Several milling strategies were investigated such as using various additives,changing the milling atmosphere,varying the rotational speed,using different milling devices [25–38].In this work,we present the production of hydrogen by the hydrolysis reaction of an AZ91 alloy.This“standard”alloy has schematically an Mg matrix and an Mg17Al12intermetallic phase [39–41].
Previously [35],we showed that ball milling for 5h at 250rpm under inert atmosphere(i.e.Ar)enhances the hydrolysis reaction performance of Mg17Al12(from 6% to 11% of the theoretical hydrogen production capacity in 60 min).Upon all the tested additives(i.e.graphite,NaCl,MgCl2and AlCl3),AlCl3showed the best performance due to the beneficial effect of its exothermic dissolution.The addition of graphite and AlCl3simultaneously during ball milling of Mg17Al12further enhances its hydrolysis with a yield of 16% reached in less than 5 min.
In our prior works [32,33],Mg alloys waste(AZ31 mixed with Al scraps in a proportion closer to that existing in AZ91)provided from the sacrificial anode industry was ball milled to enhance its hydrolysis reactivity.Milling under H2was found to be less efficient than that under Ar.When using a single additive(i.e.graphite or AlCl3),the best hydrolysis properties are obtained with graphite(yield of 95% of its theoretical capacity reached in 5min)due to the formation of the protective graphite layer.By incorporating both additives sequentially,the best material from the hydrogen production point of view was obtained by milling Mg alloy with G for 2h and with AlCl3for 2 extra hours(complete hydrolysis reached in 5 min).
In this present investigation,a“standard”AZ91(i.e.in which Al forms with Mg the intermetallic Mg17Al12)is ball milled under Ar using the same mill device following two strategies:The first was used to enhance the hydrolysis performance of Mg17Al12[35]and the second was applied to improve the reactivity of Mg alloy waste [33].The comparison between both strategies was done by reproducing the best“additive+milling duration”combination on the present AZ91.Hydrogen production by AZ91 is an electrochemical reaction where Mg is oxidized to Mg2+and water is reduced to produce H2gas [28].Thus,the higher hydrolysis performance of Mg-based materials corresponds to higher oxidationof magnesium.As a consequence,both anodic polarization(corresponding to the oxidation of Mg)and hydrolysis tests estimate the reactivity of the powder from an electrochemical and chemical aspects respectively.Hydrolysis test,anodic polarization and electrochemical impedance spectroscopy are used to evaluate the corrosion rate of the milled materials and to investigate the effect of milling strategy on the hydrolysis properties of the materials [42].
2.Experimental details
2.1.Ball milling
The plate of AZ91(from a local supplier in Pessac,France)was crushed by face milling to reduce it into pellets.AZ91 milling was performed under Ar at room temperature in a Fritsch Pulverisette 7 planetary mill.The milling strategies used in this work will be named according to the rotational speed.The milling conditions for both strategies are summarized in Table 1.
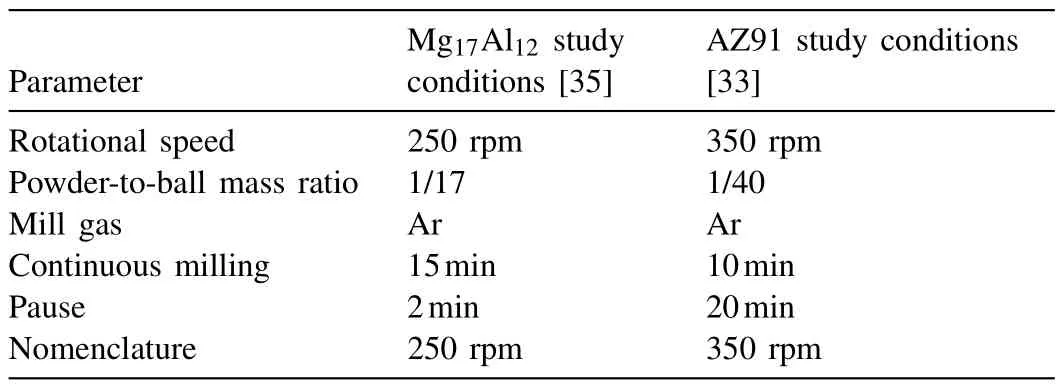
Table 1 Milling conditions used in each strategy.
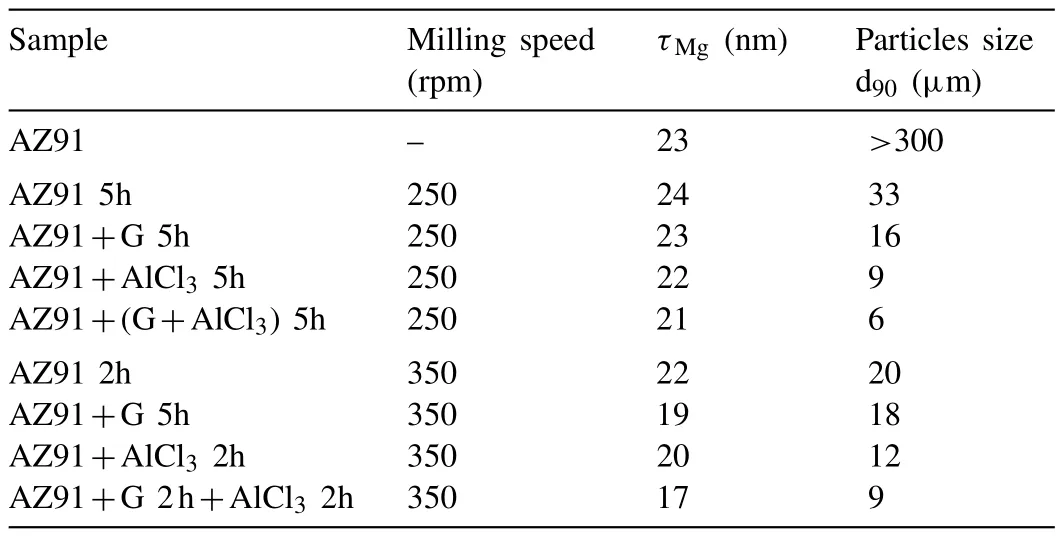
Table 2 Mg crystallite size(τMg)and particles size d90 of all the materials involved in this study.
Based on our previous studies[25,32–35],we have adopted the addition of 5wt.% of additives(i.e.graphite G(Aldrich)and anhydrous aluminum chloride powder AlCl3(98.5%,Acros Organics))during ball milling.The powder obtained after ball milling was passed through a sieve of 200 μm and was stored in Argon-filled glove box to avoid air exposure.
2.2.Materials characterization
The structural and morphological properties of AZ91 alloy and milled AZ91 are investigated by X-ray diffraction(Philips PANalyticalX’Pert(PW1820)diffractometer with Cu Kα1 radiation(λ=1.5405 ˚A)),scanning electron microscope(TESCAN VEGA3 SB microscope)equipped with energy dispersive X-ray spectrometer(EDS)and laser granulometry in absolute ethanol(MASTERSIZER2000 from Malvern®).Particles size data are expressed as number distribution where d90corresponds to the point in the size distribution,up to and including which,90% of the total number of particles in the sample is contained.
2.3.Hydrogen production and electrochemical measurements
The hydrolysis reaction was carried out in a“model seawater”solution(i.e.3.5wt.% NaCl)at room temperature(the experimental setup is detailed in reference [43]).Hydrogen production is presented as the conversion yield(%),which is defined as the volume of produced hydrogen over the theoretical volume of hydrogen if AZ91(Mg+Mg17Al12)powder is completely consumed.
Open circuit potential(OCP),anodic polarization curve and electrochemical impedance spectrum(EIS)were determined using an electrochemical workstation(Ametek Versa-STAT 3F type)at room temperature in 3.5wt.% NaCl solution with a classical three-electrode cell setup(described in Ref.[21]).The working electrode was a pellet of 0.80 cm2±0.05cm2(measured using ImageJ software)and was wet ground with ethanol to a 4000-grit finish before each test.The preparation of the working electrode and the details about the electrochemical tests conditions were performed following the procedure reported in references [21,42].EIS measurements were carried out over a frequency ranging from 100 kHz to 700 mHz with a 10 mV amplitude sinusoidal voltage at OCP.The EIS results were fitted using the commercial ZView 3.5f software.All above electrochemical measurements were repeated at least twice to ensure reproducibility.
Throughout this work,the materials will be named as follows:
-AZ91 mh:AZ91 milled for m hours
-AZ91+X mh:AZ91 milled with 5wt.% of X for m hours
-AZ91+(X+Y)mh:AZ91 milled with 5wt.% of X and 5wt.% of Y for m hours and
-AZ91+X mh+Y nh:AZ91 milled with 5wt.% of X for m hours followed by a milling with 5wt.% of Y for n hours,(X,Y=AlCl3,G).
3.Results
3.1.AZ91 cast alloy
AZ91,consisting mainly of 90wt.% Mg,9wt.% Al and 1wt.% Zn,is a part of AZ family used in the field of transportation,agriculture,chemical,construction and energy industries owing to its impressive efficient properties such as castability,weldability,good machinability,rigidity,toughness and lightness [44–46].
Fig.1.a shows the X-ray diffraction(XRD)patterns of the AZ91 used in this work.The difference plot of the experimental XRD patterns of AZ91 alloy(open circles)and the calculated XRD patterns of Mg(red line)allows highlighting the presence of the intermetallic Mg17Al12.In addition,the difference curve shows that the peaks of Mg are doubled.This is indicative of the existence of a solid solution of Zn into Mg(Mg0.97Zn0.03[47]).Indeed,the substitution of Mg atoms by Zn atoms in the hexagonal crystal lattice of Mg decreases the cell parameters by 0.6%(adecreases from 3.21 ˚A to 3.19 ˚A andcdecreases from 5.21 ˚A to 5.18 ˚A).In fact,Zn metallic radius(rZn=134 pm)is smaller than the one of Mg(rMg=160 pm).This results in a slight displacement of the peaks of Mg towards higher 2θangles(e.g.peak of the plane(101)of Mg at 36.62° while that of Mg0.97Zn0.03is at 36.85°).Since the alloy contains 1wt.% of Zn,the contribu-tion of Mg0.97Zn0.03in the hydrogen production/ corrosion behavior of the alloys is considered similar to that of Mg.
3.2.Ball milling at 250rpm
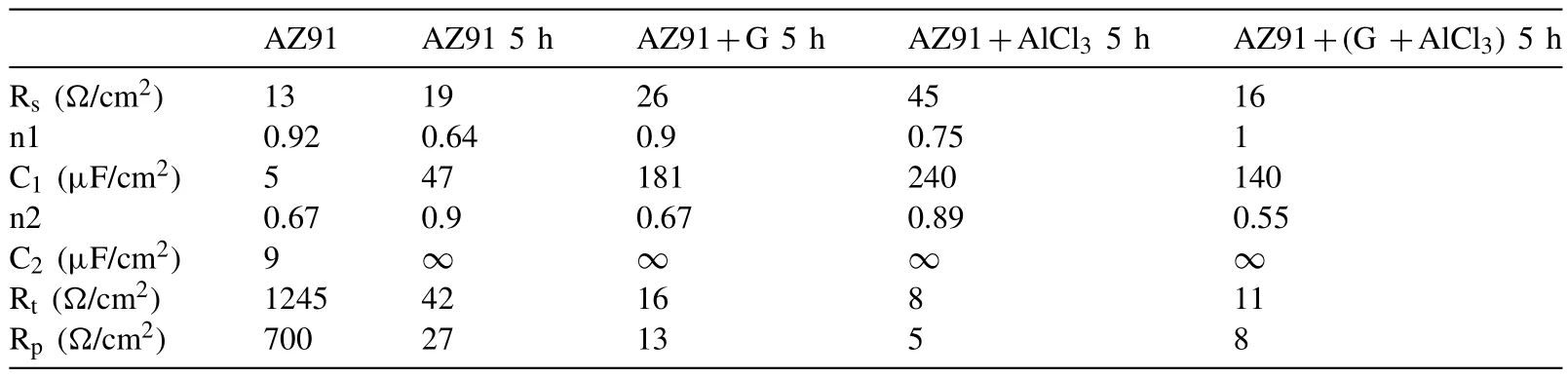
In our previous work [35],we stated that cheap additives(e.g.Graphite and AlCl3)can be used to improve the hydrolysis of Mg17Al12.In this section,we present the effect of ball milling at 250rpm for 5h without additives(named AZ91 5h),with 5wt.%G(named AZ91+G 5h),with 5wt.%AlCl3(named AZ91+AlCl35h)and with 5wt.% G and 5wt.%AlCl3(named AZ91+(G+AlCl3)5h).
3.2.1.Structural and morphological modifications
Fig.1.b shows the X-ray diffractograms of AZ91 alloys milled at 250rpm before hydrolysis and AZ91+(G+AlCl3)5h after hydrolysis.The slight variation of the width of the diffraction peaks indicates an insignificant variation in the size of Mg crystallites(τMgof approximately 23 nm,CfTable 2).Nevertheless,mechanical milling reduces the particles size from more than 300 μm for unmilled AZ91 to 6 μm for AZ91+(G+AlCl3)5h(Table 2).These observations indicate that the milling at 250rpm is not energetic enough to decrease Mg crystallite size.The reduction in particles size,on the other hand,is probably due to the effect of ball milling additives.
Indeed,the milling of AZ91 for 5 h without additives reduces the particles size to 33 μm(Fig.2.a and Table 2)while milling with 5wt.% of graphite for 5 h decreases the particles size to 16 μm(Fig.2.b and Table 2).Milling with AlCl3(i.e.AZ91+AlCl35h)and with both graphite and AlCl3(i.e.AZ91+(G+AlCl3)5h)further reduces the particles size and creates surface defects(Fig.2.c and d and Table 2).The beneficial effect of ball milling of Mg and Mg17Al12(i.e.the main components of the AZ91 alloy)with both additives has been reported in the literature [34,35,37].Indeed,graphite forms a protective layer on the surface of Mg [34,37]and Mg17Al12[35]while the addition of AlCl3reduces the particles size and favors the formation of surface defects(e.g.cracks).
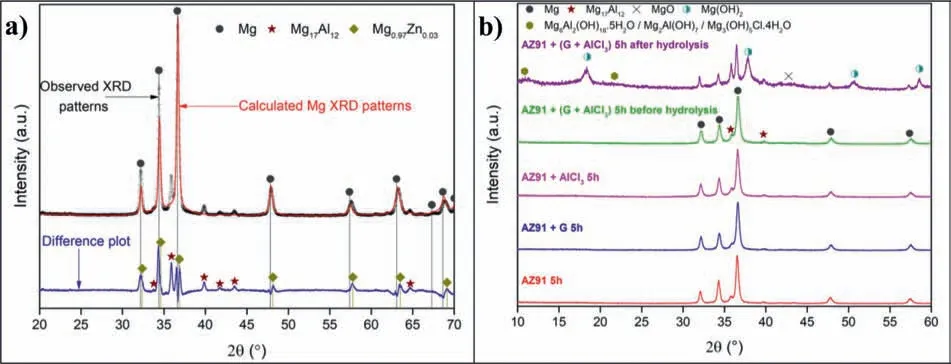
Fig.1.a)Observed(open circles)and calculated(red line)X-ray diffraction patterns of AZ91 with the difference(blue line)at the same scale plotted below and b)XRD patterns of AZ91 5h,AZ91+G 5h,AZ91+AlCl3 5h and AZ91+(G+AlCl3)5h milled at 250rpm before hydrolysis and AZ91+(G+AlCl3)5h after hydrolysis.
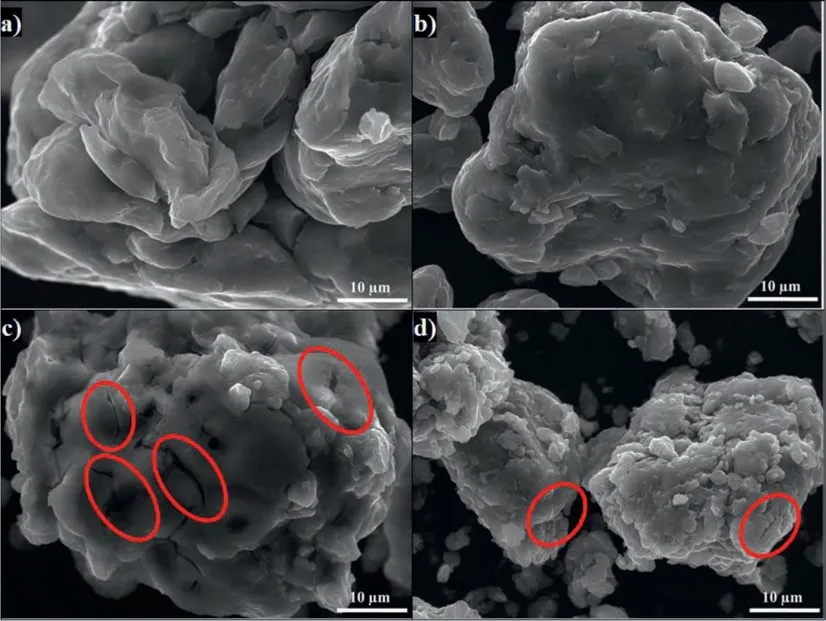
Fig.2.SEM images of a)AZ91 5h,b)AZ91+G 5h,c)AZ91+AlCl3 5h and d)AZ91+(G+AlCl3)5h milled at 250rpm.Red circles indicate surface defects formed during ball milling with AlCl3.
3.2.2.Hydrogen production
Fig.3.a and b show the hydrogen production evolution by the hydrolysis of the milled AZ91 at 250 and 350rpm,respectively.AZ91 flakes barely react with NaCl solution with only a production of 7% of its theoretical production capacity in 60 min of reaction.Note that the theoretical production capacity was calculated for each sample taking into account the equations for the hydrolysis of Mg and Mg17Al12[35]and the composition of the milled material(i.e.quantification of the 2 phases)evaluated from XRD refinements(78wt.% of Mg and 21wt.% of Mg17Al12).
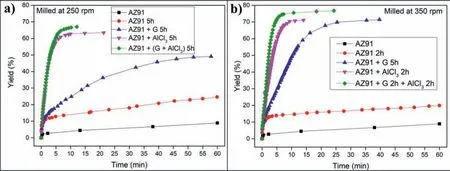
Fig.3.Hydrogen generation of a)AZ91,AZ91 5h,AZ91+G 5h,AZ91+AlCl3 5h and AZ91+(G+AlCl3)5h milled at 250rpm and b)AZ91,AZ91 2h,AZ91+G 5h,AZ91+AlCl3 2h and AZ91+G 2h+AlCl3 2h milled at 350rpm.
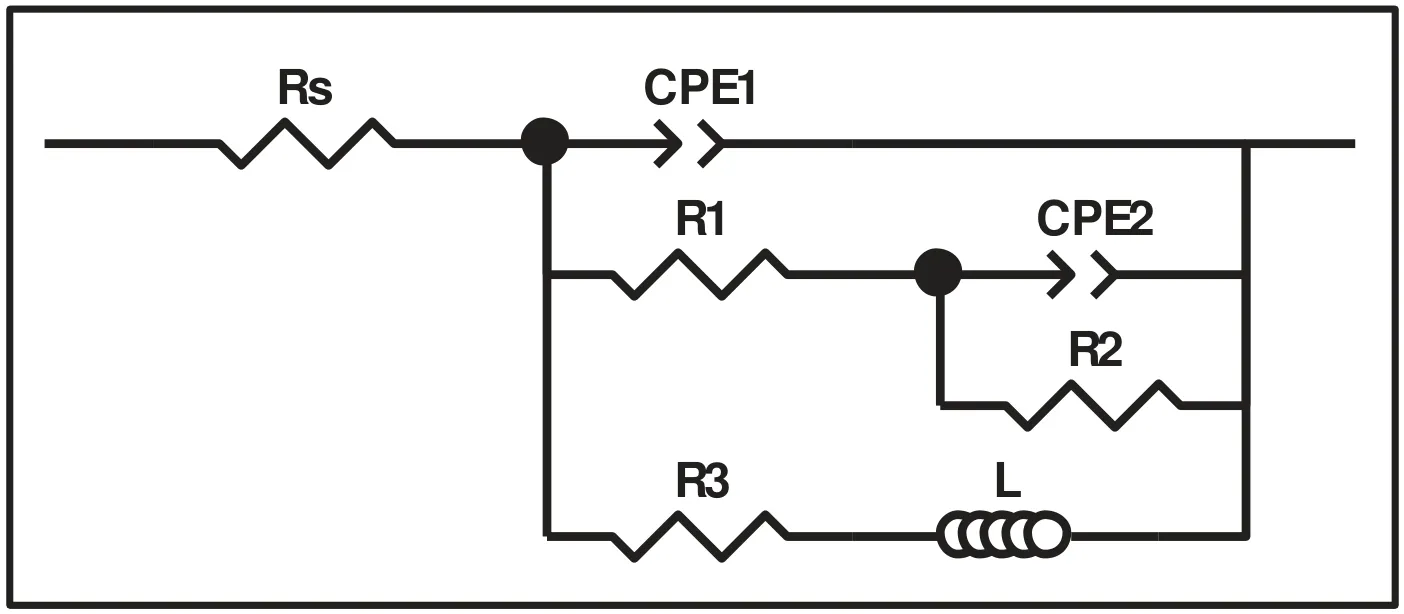
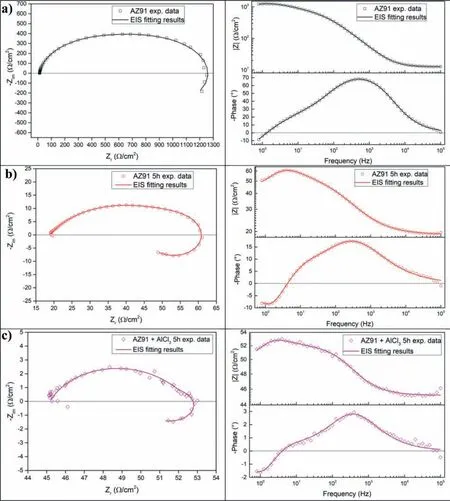
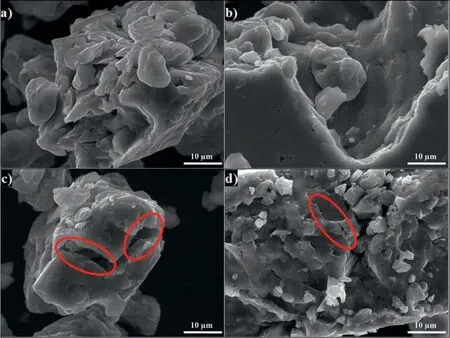
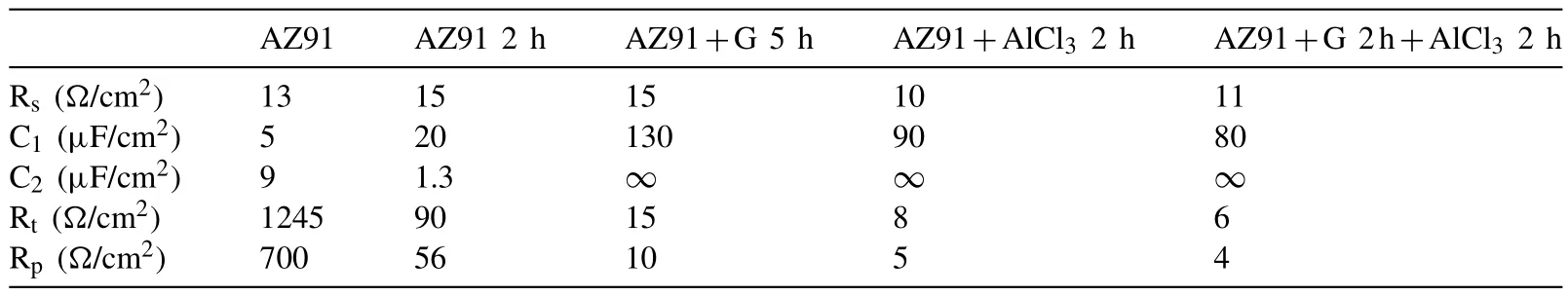
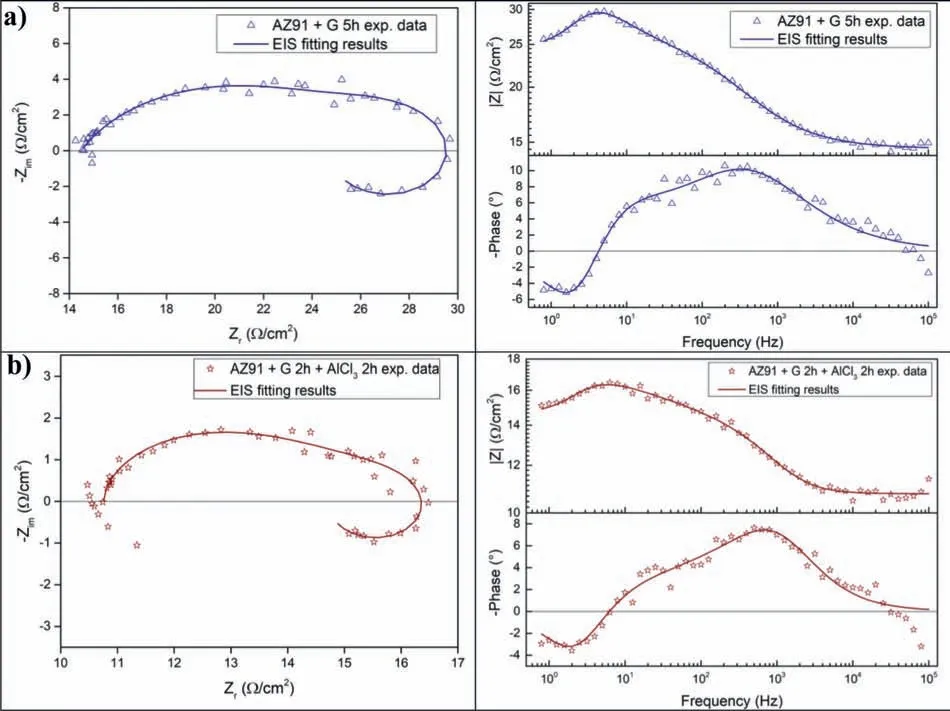
Milling AZ91 for 5h at 250rpm improves hydrolysis performance with a production of 20% of the theoretical H2generation capacity in 60 min while milling with graphite for the same duration(i.e.AZ91+G 5h)increases the production of hydrogen to 50%(Fig.3.a).This can be explained by the formation of the protective layer of graphite[25,34]which prevents the adhesion of the passive Mg(OH)2on the surface.However,the best performances were obtained for AZ91+AlCl35h and AZ91+(G+AlCl3)5h with a production of 65% of the theoretical capacity in approximately 25 min and 10 min of reaction respectively.The exothermic dissolution of AlCl3during the hydrolysis of AZ91 milled with AlCl3generates,at the materialsolution interface,H+and Al3+acidic ions which promotes the dissolution of Mg(OH)2[35,48–50].Previously,we have shown that ball milling improves the hydrolysis of Mg17Al12and the reactivity was reported as follow:Mg17Al12 XRD patterns of AZ91+(G+AlCl3)5h after the hydrolysis(Fig.1.b)reveal the presence of Mg peaks which confirm the incomplete reaction between AZ91 and the aqueous solution.Moreover,the formation of MgxAly(OH)zphases confirms that the milling strategy increases the hydrolysis reactivity of Mg17Al12since a part of Al(from Mg17Al12)contributes in the hydrogen production while the XRD patterns of AZ91 5h and AZ91+G 5h after the hydrolysis(CfSupplementary material Fig.1)reveals the presence of Mg,Mg17Al12and Mg(OH)2but no MgxAly(OH)zcan be detected. Open circuit potential(implying the corrosion potential Ecorr)for all the materials were recorded during 30 min of immersion in 3.5wt.% NaCl aqueous solution.The corrosionpotentials Ecorrpresented in Table 3 were obtained by averaging OCP values from 15min to 30min where the potential was the most stable.AZ91 alloy has an Ecorrof -1.63V/SCE(Table 3)while Singhet al.[51]reported an OCP of -1.5V/SCE.In fact,galvanic coupling exists between Mg and Mg17Al12[21,42].Mechanical and thermal treatments during the manufacture of Mg-Al alloys affect the precipitation of the intermetallic in Mg so that the distribution of Mg17Al12in the matrix varies [52].This affects the surface area between each phase as well as the OCP [53].Ball milling at 250rpm for 5h shifts the OCP toward higher potentials(Ecorr=-1.55V/SCE).The increase in Ecorrfor milled AZ91 indicates a higher reactivity to form the passivation layer Mg(OH)2[54].The variation of the OCP of AZ91+G is attributed to the galvanic coupling established between Mg-G and Mg17Al12-G Table 3 OCP values and corrosion current density(Jcorr)for all the materials involved in this study. The corrosion current densities(Jcorr)are determined from the anodic polarization curves [55].The anodic polarization curve(Fig.4.a)demonstrates the formation of a passivation film for AZ91,while the passivation is negligible when the alloy is milled with and without additives.AZ91 corrodes in the“model”seawater solution with a corrosion current density of 0.03 mA/cm2(Table 3)in total agreement with that reported previously(Jcorr=0.0255 mA/cm2according to ref.[51]).As evaluated by the hydrolysis tests,ball milling increases the corrosion rate of the milled materials to 4.1 mA/cm2for AZ91 5h and a maximum reactivity for AZ91+(G+AlCl3)5h with Jcorr=7.5 mA/cm2(Table 3). Fig.4.Anodic polarization curves for a)AZ91,AZ91 5h,AZ91+G 5h,AZ91+AlCl3 5h and AZ91+(G+AlCl3)5h milled at 250rpm and b)AZ91,AZ91 2h,AZ91+G 5h,AZ91+AlCl3 2h and AZ91+G 2h+AlCl3 2h milled at 350rpm in 3.5wt.% NaCl aqueous solution. Fig.5.Electrical equivalent circuit used to interpret the experimental impedance data. On the other hand,a dissimilarity between the hydrolysis test and anodic polarization is observed for AZ91+G 5 h:The hydrolysis test shows that AZ91+G 5h is more reactive than AZ91 5h while the anodic polarization test shows that AZ91 5h is more reactive.Such dissimilarity is due to the presence of the protective graphite layer on the surface of the pellets during the polarization test which changes the electrical properties of the surface. 3.2.3.Electrochemical impedance spectroscopy EIS is known as an efficient method to investigate the effect of ball milling additives on the corrosion behavior of materials [34].Therefore,we investigate the electrical properties of the milled materials surface during immersion in NaCl solution by electrochemical impedance spectroscopy measurements.The Nyquist EIS spectra of AZ91 and milled AZ91 at OCP display a capacitive loop(Zim<0)and an inductive loop(Zim>0,CfFig.5).Previously,Caoet al.[56]fitted the EIS experimental data of AZ91 by neglecting the presence of the inductive loop.King et al.[57]considered the contribution of the inductive loop(by adding an inductor to their electrical equivalent circuit)while fitting the EIS data of pure Mg in NaCl solution.The inductive loop can be interpreted by the formation of a reaction intermediate(i.e.multi-step reaction)[55,58–60]and its adsorption on the surface.It is worth noting that the inductive loop is not a measurement artefact. In the present study,we adopted the equivalent circuit of King et al.[57]to fit our experimental data and we used constant phase element(CPE)for the representation of the capacitive elements(Fig.5). Fig.6 shows relevant examples of the various impedance curves obtained for AZ91 and AZ91 milled at 250rpm.EIS spectra of AZ91+G 5h and AZ91+(G+AlCl3)5h(Supplementary material Fig.2.a and b,respectively)are similar to AZ91+AlCl35h(Fig.6.c).The effective capacitances for both CPE1 and CPE2 were calculated from the values of the capacitance Q(i),the CPE exponent order n(i)and the associated resistance R(i)[61].For CPE1,n1 values are greater than 0.5 while for CPE2,n2 decreases to 0.55 in the case of AZ91+(G+AlCl3)5h indicating that the corrosion mechanism becomes diffusional.The values of all electrochemical parameters of AZ91 and milled AZ91 at 250rpm determined using ZView software are summarized in Supplementary material Table 1. Fig.6.Nyquist(left)and Bode(right)plots for:a)AZ91,b)AZ91 5h,and c)AZ91+AlCl3 5h milled at 250rpm. From the EIS fitting results,equivalent circuit parameters were calculated and presented in Table 4.Rsis the solution resistance,C1and C2are the effective capacitances of CPE1 and CPE2 respectively,Rtthe charge transfer resistance and Rpthe polarization resistance.Based on the calculated values of the capacitances,C1is most likely a double layer capacitance on a roughened electrode [57,62,63]while C2characterizes the pseudo capacitance of the adsorption of a corrosion intermediate(e.g.Mg+[55,59,60,64]or MgH+[58])or a corrosion product(i.e.Mg(OH)2).High C2values were justified and discussed for pseudo capacitance in previous investigations [57,65]and the variation in the capacitance is proportional to the surface of the working electrode.Ball milling for 5h at 250rpm reduces the particles size of AZ91 to 33 μm(Table 2).Note that electrochemical tests are performed using condensed powder.Consequently,milled AZ91 electrode shows more grain boundaries than unmilled AZ91 which act as reaction(i.e.corrosion and hydrolysis)initiator sites and increase the reaction kinetics.Moreover,when corrosion rate increases,the surface of the pellets(i.e.workingelectrode)becomes rougher(consequently the surface area increases)due to the formation of soluble MgCl2in NaCl solution[21].C1increases from 5 μF/cm2for AZ91 to 47 μF/cm2for AZ91 5h and higher than 100 μF/cm2for AZ91 milled with additives(Table 4).This variation is expected since the capacitance is proportional to the surface area of the working electrode(i.e.AZ91 pellets). Table 4 Electrochemical parameters calculated from best fit of impedance data of AZ91,AZ91 5h,AZ91+G 5h,AZ91+AlCl3 5h and AZ91+(G+AlCl3)5h milled at 250rpm. The charge transfer resistance Rtis determined from the EIS Nyquist plot where Zim→0Ω/cm2(atf>0Hz)and the polarization resistance Rpis estimated by(where Zim→0Ω/cm2at f→0 Hz)[57].For both resistances,the rise in their value is attributed to lower corrosion resistance.In the literature,Rtis considered when evaluating the corrosion rate by EIS without considering the inductive loop.Hence,the corrosion rate is underestimated by a factor of 2 [57].Table 4 shows that Rtis higher than Rpas stated by Kinget al.[57]due to the contribution of the inductive loop(by the resistance R3)in the calculation of the polarization resistance.Rpdecreases from 700Ω/cm2for AZ91 to 27Ω/cm2after ball milling mainly for the reason that Rpis inversely proportional to the surface area(while the surface area varies and the corrosion rate vary proportionally previously indicated)[61].The calculated Rpfor milled AZ91 shows that AZ91+G 5h is less corrosion resistant than AZ91 5h as stated by the hydrolysis test.This dissimilarity between Jcorrdetermined by anodic polarization technic and Rpis probably attributed to the protective graphite layer as previously mentioned.The increase of C1values and the diminution of Rpand Rtfor milled AZ91 is attributed to the destabilization of Mg(OH)2induced by the presence of additives.In fact,the exothermic dissolution of AlCl3during the immersion of AZ91+AlCl35h in NaCl solution increase the temperature at the interface material-solution and generates locally Lewis acid cations Al3+et Brønsted acid cations H+[48,54]which increase the solubility of Mg(OH)2at the interface.While the presence of graphite during the mechanical treatment forms a protective layer which prevents the adhesion of Mg(OH)2on the surface. The inclusion of inductor was justified in previous reports[57,63]and is attributed to the adsorption of corrosion intermediate(e.g.Mg+[55,59,60,64]or MgH+[58]).However,the inductance values(L)shown in Supplementary materials Table 1 are only considered as qualitative indication of the formation of reaction intermediate. Ball milling of Mg alloy used in sacrificial anode industry at high speed under H2[32]and under Ar [33]ameliorates its hydrolysis performance.We showed that milling under H2is less efficient than that under Ar,in term of hydrolysis performances,due to the formation of less reactive MgH2[32].Extending milling time to more than 2h without additives or with AlCl3deteriorated the hydrolysis performance while Mg alloy milled with graphite for 5h was optimal and generates 95% of its total H2production capacity in 5 min [33].The best hydrolysis performance was obtained by milling with graphite for 2h followed by milling for additional 2h with AlCl3.Finally,we showed that milling using the“highenergy”Fristch Pulverisette(the same mill device used in this investigation)was more beneficial than the“low-energy”Australian Uni-Ball-II. Higher milling speed was proved beneficial to ameliorate the hydrolysis reactivity of Mg-based alloy,so that AZ91 was milled at 350rpm and the milling time was chosen based on these previous studies.Therefore,AZ91 2h,AZ91+AlCl32h,AZ91+G 5h and AZ91+G 2h+AlCl32h milled at 350rpm were analyzed in this work.The structural and morphological properties and the hydrogen production performances(i.e.hydrolysis,anodic polarization and EIS)of AZ91 2h,AZ91+AlCl32h,AZ91+G 5h and AZ91+G 2h+AlCl32h milled at 350rpm are reported below. 3.3.1.Structural and morphological modifications Milling AZ91 at higher energy(i.e.350rpm)decreases Mg crystallite size from 22 to 17 nm and particles size from 20 to 9 μm for AZ91 2h and AZ91+G 2h+AlCl32h respectively(CfXRD patterns are shown in Supplementary material figure 3).Milling with AlCl3further decreases particles size to 12 μm for AZ91+AlCl32h.In fact,chlorides are known as excellent milling agents because they improve the efficiency of the milling process by creating microstructural defects and enabling the reduction of particles size thus increasing the surface area [66]. SEM micrographs display particles size reduction after ball milling for 2h(Fig.7.a),the formation of relative flat surface in AZ91+G 5h(Fig.7.b)and microstructural defects(i.e.cracks)in AZ91+AlCl32h(Fig.7.c).The surface roughness of AZ91+G 2h+AlCl32h is justified by the effect of both additives(flat surface during the first milling with G and cracks formation when AlCl3is added,CfFig.7.d). Fig.7.SEM images of a)AZ91 2h,b)AZ91+G 5h,c)AZ91+AlCl3 2h and d)AZ91+G 2h+AlCl3 2h milled at 350rpm.Red circles indicate surface defects formed during ball milling with AlCl3. 3.3.2.Hydrogen production Ball milling AZ91 for 2h at 350rpm enhances its hydrolysis performance with a production of 15% of the theoretical H2volume in 60 min(Fig.3.b).AZ91+G 5h generates 65% of its theoretical generation capacity in 35 min while AZ91+AlCl32h produces the same H2volume in only 8 min.Fig.3.b shows that the best hydrolysis performances were obtained for AZ91+G 2h+AlCl32h with 75%of its theoretical H2generation capacity reached in less than 5 min.The reactivity,in term of hydrolysis performances,of the AZ91 milled in this study varies in the same order as the milled AZ91 sacrificial anodes [32].The slightly lower hydrolysis performance obtained with this present AZ91 alloy is attributed to the presence of less corrosive Mg17Al12[35]since only Al(no Mg17Al12)was detected in the sacrificial anode AZ91 [33]. Open circuit potential of AZ91 milled at 350rpm increases from -1.63V/SCE for un-milled AZ91 to an average of -1.52V/SCE.This variation is attributed,as mentioned in Section 3.2.2,to the formation of Mg(OH)2on the surface during the immersion in the“model”seawater solution while the increase of the OCP for AZ91 milled with graphite is attributed to a galvanic coupling between Mg–G and Mg17Al12–G.On the other hand,anodic polarization measurements(Fig.3.b and last 4 rows of Table 3)confirm the hydrolysis results mentioned above.Indeed,the calculated current density(Jcorr)increases from 0.03 mA/cm2for AZ91 alloy to 4.9 mA/cm2for AZ91 2h,8.3 mA/cm2for AZ91+G 5h,10.4 mA/cm2for AZ91+AlCl32h and 11.3 mA/cm2for AZ91+G 2h+AlCl32h(Table 3).The effect of each milling additive was reported in Section 3.2. 3.3.3.Electrochemical impedance spectroscopy EIS spectra(Nyquist plot)of AZ91 milled at 350rpm display the presence of a capacitive and an inductive loop(Fig.8).AZ91 2h and AZ91+AlCl32h spectra display the same trend as AZ91+G 5h and AZ91+G 2h+AlCl32h(Supplementary material Fig.4).Electrochemical parameters calculated from the fit of the experimental data are represented in Table 5.The electrochemical parameters determined using ZView software are summarized in supplementary material Table 2. The double layer capacitance C1increases to 20 μF/cm2for AZ91 2h and 130 μF/cm2for AZ91+G 5h(Table 5).The decrease in C1for AZ91 milled with AlCl3may be attributed to the dissolution of AlCl3which changes the composition of the electrolyte at the interface material-electrolyte.As observed previously(Section 3.2.3),Rtare higher than Rpdue to the consideration of the inductive loop in the calculations.Moreover,both Rpand Rtdecrease in the following order:AZ91 2h Table 5 Electrochemical parameters calculated from best fit of impedance data of AZ91,AZ91 2h,AZ91+G 5h,AZ91+AlCl3 2h and AZ91+G 2h+AlCl3 2h milled at 350rpm. Fig.8.Nyquist(left)and Bode(right)plots for:a)AZ91+G 5h and b)AZ91+G 2h+AlCl3 2h milled at 350rpm. EIS results are in accordance with the results obtained by the hydrolysis tests and the anodic polarization measurements.In fact,ball milling with the chronological addition of graphite and AlCl3is beneficial to improve the reactivity of AZ91 alloy.On the other hand,milling for shorter duration(e.g.2h)with AlCl3is better than milling with graphite for 5h(Jcorr(AZ91+AlCl32h)=10.4 mA/cm2>Jcorr(AZ91+G 5h)=8.3mA/cm2). The crystallinity of AZ91 is affected by the milling strategy:Mg crystallite size is approximately constant for AZ91 milled at 250rpm whileτMgdecreases more for AZ91 milled at 350rpm(Table 2).As for particles size d90,milling at 350rpm without additives further decreases the particles size(33μm for AZ91 5hvs.20 μm for AZ91 2h,CfTable 2).For AZ91+G 5h milled at 250 and 350rpm,the approximately same d90values can be attributed to the lubricant effect ofGwhich minimizes the effect of ball-powder collisions. AZ91 milled at 350rpm are more reactive with the“model”seawater solution than those milled at 250rpm.Indeed,AZ91+G 5h milled at 350rpm generates 65% of its theoretical H2generation capacity in 35 min while the same material milled at 250rpm generates only 45% of its theoretical H2generation capacity in 60 min.Extending milling time with graphite promotes the homogeneous repartition of graphite on the surface of AZ91 hence forming a“continuous”protective layer.On the other hand,the addition of AlCl3improves the hydrolysis performance of AZ91 more than graphite.In fact,AlCl3enhanced the hydrolysis performance of Mg17Al12[35]which constitutes 21wt.% of AZ91 alloy.The synergetic effect of G and AlCl3is beneficial whatever the milling rotation speed.Nevertheless,the chronological addition of G followed by AlCl3(i.e.AZ91+G 2h+AlCl32h)leads to the highest reactivity in term of hydrolysis performances with a generation of 75% of its theoretical capacity in few minutes [32,33]. OCP of milled materials is only affected by the milling additives.For example,each of the following couple of materials have the same potential:AZ91 milled without additives,AZ91 milled with graphite,AZ91 milled with AlCl3and AZ91 milled with graphite and AlCl3(Table 3).The corrosion current densities were higher for AZ91 milled at 350rpm(except for AZ91 milled without additives)in agreement with the hydrolysis tests results. EIS measurements are an efficient tool to evaluate the effect of milling strategies.In this study,we are interested in evaluating the variation of Rpand Rtsince it permits the estimation of the corrosion rate(i.e.Rpand Rtare inversely proportional to Jcorr).The double layer capacitance C1is higher for AZ91 milled at 250rpm suggesting higher thickness of the passivation layer formed on the surface during the immersion in 3.5wt.% NaCl solution.Conversely,Rtand Rpare lower for AZ91 milled at 350rpm indicating higher corrosion rate for these materials.Note that AZ91+G 5h in both cases(i.e.250 and 350rpm)reveals approximately the same electrochemical parameters calculated from EIS fitting.Moreover,AZ91+AlCl32h and AZ91+AlCl35h shows approximately the same Rpand Rt.This suggests that the electrical properties of the surface in combination with physico-chemical processes are only affected by the milling additive and not by the global milling strategy(i.e.milling rotational speed,powder to ball mass ratio and milling sequence). In brief,both milling strategies lead to activate a“standard”AZ91 alloy(i.e.contains Mg,Mg17Al12and Mg0.97Zn0.03)and enhance its hydrolysis performance.However,milling at 350rpm is more energetic and improves the most the reactivity of AZ91.The best material(yield of 75%reached in less than 5 min)was obtained by chronological addition of G and AlCl3where AZ91 was milled at 350rpm for 2h with graphite followed by further milling for 2h with AlCl3.By this process,the hydrolysis yield and consequently the corrosion rate increased by more than 400% compared to AZ91 ball milled 2h at 350rpm and almost 1000% compared to AZ91 alloy.H2production was also faster:this ball milling strategy leads to the multiplication of the hydrogen generation rate by 60 and 128 times compared to AZ91 ball milled 2h at 350rpm and AZ91 alloy respectively. The evaluation of the reactivity of the milled materials is more complex.The rapid Mg dissolution rates complicates the process of determining long-term corrosion rates via anodic polarization.Furthermore,EIS measurements elucidate the effect of the presence of milling additives without distinguishing between 2 materials milled with the same additives but with different strategies.Additionally,the dissimilarity between electrochemical tests and hydrolysis tests can be attributed to the morphological properties of the material.In fact,powders are used for the hydrolysis test while pellets from condensed powder are used in the electrochemical tests.We believe that,in order to investigate the hydrogen production from a material(hence its corrosion),the three technics(i.e.hydrolysis,anodic polarization and EIS)should be considered.The corrosion rate estimated by EIS measurements(i.e.Rpand Rt)is only relevant on the milling additive effect and not on the milling strategy effect. To conclude,starting from a“standard”AZ91,one of the most corrosion-resistant Mg-based alloys,we improved the hydrogen production by its hydrolysis in a“model”seawater solution with a generation of 75% in a few minutes.Replacing pure Mg with Mg-based waste will reduce the cost of hydrogen production by this technology.Therefore,by using this“green”hydrogen generator with a proton-exchange membrane fuel cell,a clean energy generator can be established for light and/or short distance mobility. Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Acknowledgments This work was financially supported by the AZM &SAADE Association,the Lebanese University(Scientific research support program),the Lebanese Council of Scientific Research(CNRSL)and Bordeaux foundation.The authors thank Prof.Olivier Devos for valuable discussion on the EIS results. Supplementary materials Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2020.12.007.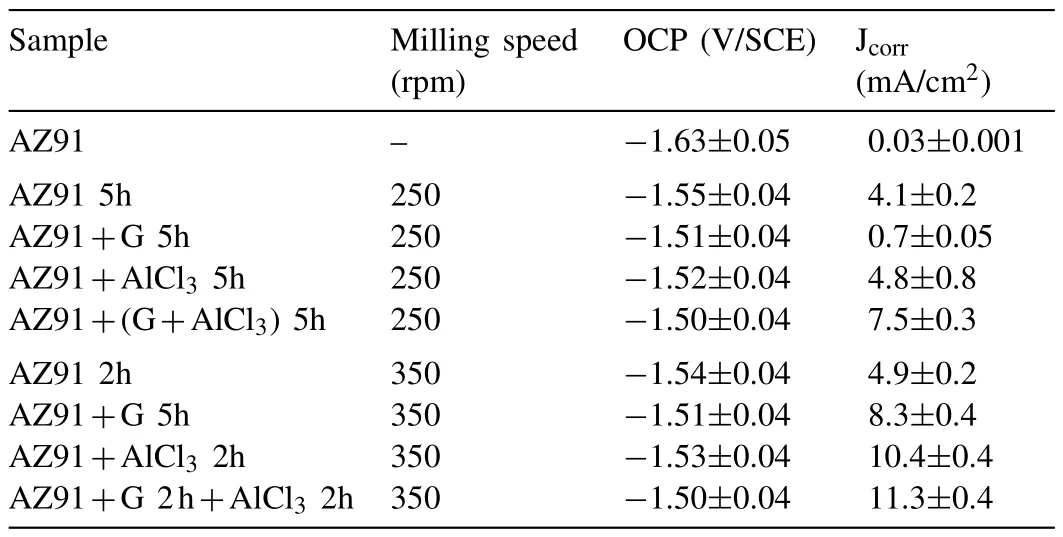

3.3.Ball milling at 350rpm
4.Comparison between both milling strategies
5.Conclusion
杂志排行
Journal of Magnesium and Alloys的其它文章
- Mg-based materials for hydrogen storage
- Magnesium alloys as anodes for neutral aqueous magnesium-air batteries
- Biodegradable Mg alloys for orthopedic implants–A review
- A review on electromagnetic shielding magnesium alloys
- Thermodynamics and kinetics of hydriding and dehydriding reactions in Mg-based hydrogen storage materials
- Exploring the interconnectivity of biomimetic hierarchical porous Mg scaffolds for bone tissue engineering:Effects of pore size distribution on mechanical properties,degradation behavior and cell migration ability
