Magnesium alloys as anodes for neutral aqueous magnesium-air batteries
2021-02-24FangleiTongShanghaiWeiXizeChenWeiGao
Fanglei Tong,Shanghai Wei,Xize Chen,Wei Gao
Department of Chemical and Materials Engineering,Faculty of Engineering,The University of Auckland,Auckland 1010,New Zealand
Abstract Magnesium(Mg)is abundant,green and low-cost element.Magnesium-air(Mg-air)battery has been used as disposable lighting power supply,emergency and reserve batteries.It is also one of the potential electrical energy storage devices for future electric vehicles(EVs)and portable electronic devices,because of its high theoretical energy density(6.8 kWh•kg−1)and environmental-friendliness.However,the practical application of Mg-air batteries is limited due to the low anodic efficiency of Mg metal anode and sluggish oxygen reduction reaction of air cathode.Mg metal as an anode material is facing two main challenges:high self-corrosion rate and formation of a passivation layer Mg(OH)2 which reduces the active surface area.In last decades,a number of Mg alloys,including Mg-Ca,Mg-Zn,commercial Mg-Al-Zn,Mg-Al-Mn,and Mg-Al-Pb alloys,have been studied as anode materials for Mg-air batteries.This article reviews the effect of alloying elements on the battery discharge properties of Mg alloy anodes.The challenges of Mg-air batteries are also discussed,aiming to provide a depth understanding for the theoretical and practical development of high-performance Mg-air batteries.
Keywords: Metal-air battery;Mg anode;Mg alloy;Battery performance.
1.Introduction
Lithium-ion batteries(LIBs)have been successfully used for small,medium,and large portable devices,such as mobile phones,laptops,and electric vehicles(EVs).However,current LIBs are still facing severe challenges,including high cost,low energy density,safety and environmental issues,for future electrical energy storage demand.Metal-air batteries have long been explored since the 1960s for communication transmitters in space applications and EVs [1].They have been considered as one of the promising electricity storage systems for future energy demand because of their low cost and very high specific density [2].
Metal-air batteries use oxygen from the air as one of the battery’s main reactants,allowing battery weight to be reduced and freeing up valuable space for storing energy.Table 1 summarises the theoretical and practical cell voltage and specific energy density of five different types of metalair batteries with the costs of their metal anode materials.Among metal-air batteries,Li-air battery shows the highest theoretical energy densities and high cell voltage.However,commercial application of rechargeable Li-air batteries is facing critical challenges in dendrite formation,poor cycling efficiency,safety issues,and finding a suitable electrolyte with high stability and performance.Furthermore,Li metal anode are nearly as ten times expensive as Mg,Al and Zn metals(Table 1).Another metal-air battery,Zn-air rechargeable batteries,have recently attracted enormous attention because of high practical specific energy density at 350–500 Wh·kg−1.However,their commercial scale deployment is limited due to corrosion and dendrite growth of Zn metal anode,expensive and low efficient electrocatalysts for oxygen evolution reaction(OER)and oxygen reduction reaction(ORR).With considering resource availability,crustal abundance of Mg,Al and Fe elements are at least 100 times more than Li and Zn.Fe-air battery has low operating voltage(0.8 V)and specific energy density(50–75 Wh·kg−1),but it is an attractive candidate for grid-scale energy storage because of excellent resource-efficient and electrochemical stability [3–7].Mg-air and Al-air batteries have very high theoretical energy density and cell voltage,but as mainly primary batteries their applications are facing similar challenges of high polarization and low coulombic efficiency [8,9].This review focuses on the recent research and development in Mg-air batteries.
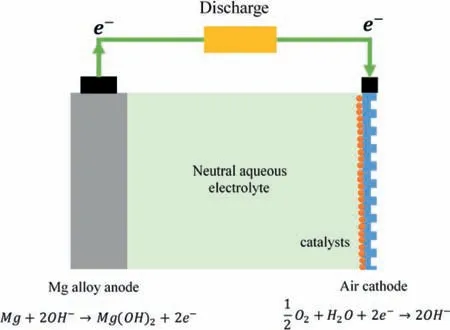
Fig.1.Schematic of Mg-air battery and the electrochemical reactions at anode and air cathode.
1.1.Principles of Mg-air batteries
Mg-air battery is a type of metal-air batteries,or metal-fuel cells(MFC)that consists of Mg metal anode,air cathode and aqueous electrolyte,as shown in Fig.1.Aqueous electrolytes,mainly NaCl solutions have been used for Mg-air batteries.The theoretical electrochemical reaction in Mg-air battery are as follows:
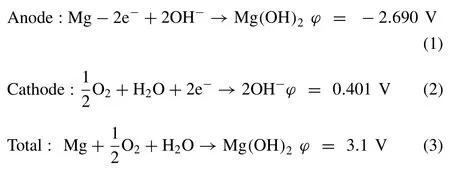
In whichϕis the voltage vs standard hydrogen electrode(SHE).From Eqs.(1)to(3),the theoretical voltage of Mg-air battery is 3.1 V.However,due to the parasitic corrosion of Mg anode together with the sluggish kinetics of oxygen reduction reaction(ORR)in the air cathode,the working voltage of Mgair battery is normally below 1.5 V [21].Furthermore,the performance of Mg-air batteries depends on the Mg metal anode,electrolyte and air cathode,which will be discussed in following sections.
1.2.Current used electrolyte for Mg-air batteries
The anodic performance of Mg relates to pH value,anion,and concentration of aqueous electrolyte [10].Table 2 summarises the different types of aqueous electrolytes and their main properties.It indicates NO3−,NO2−and HPO4−have higher Faradaic efficiency than Cl−and ClO4−.Cl−are more likely to attack the protective film of Mg,leading to high corrosion rates [22].Addition of a small amount of HNa2PO4has been shown as an effective method to decrease the OCV H2evolution and Mg corrosion.But 0.5 M HNa2PO4electrolyte introduces extraordinarily large overpotentials during discharge.Electrode potential of +2.3 V vs Ag/AgCl during oxidation at 3.2 mA•cm−2,whereas OCV was−1.52vsAg/AgCl[19].Thus,Mg(NO3)2,NaNO3,NaNO2,and a small amount of HNa2PO4solutions have the potential for developing high performance Mg-air batteries.
Over past decades,electrochemical reaction mechanism of Mg in aqueous electrolyte has been extensively studied [7,22-28].Addition of corrosion inhibitors such as stannates,quaternary ammonium salts,dithiobiuret and their mixtures has been demonstrated as an effective method to suppress the HER,improving the performance of Mg-air batteries [10,29-32].Deyab has introduced decyl glucoside as a new corrosion inhibitor in NaCl solution.His research has shown that the anodic efficiency(%)and discharge capacity of pure Mg anode increased dramatically from 39.4% to 89.7%,and from 887 mA•h g−1to 2054 mA•h g−1,respectively,at the current density of 2 mA cm−2[29].Wang et al.have added Mg2+complexing agents,such as citric acid(CIT),salicylic acid(SAL),2,6-dihydroxybenzoic acid(2,6-DHB),5-sulfosalicylic acid(5-sulfoSAL)and 3,4-dihydroxybenzoic acid(3,4-DHB),into 3.5 wt.% NaCl electrolyte to enhance the discharge performance of(Mg-Ca)-air battery [30].They have found that these additives can efficiently increase the discharge voltage and specific energy of respective Mg-air batteries[30].Zhang et al.reported that 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)with 50 mol.% butyl acetate electrolyte exhibits minimum fluctuations during discharge,and butyl acetate electrolyte has an outstanding performance over 72 h galvanostatic discharge [31].
Double liquid electrolyte is a very effective method to improve Mg-air battery performance.It consists of an aqueous phase and organic phase,in which the organic phase is immiscible with the aqueous phase,separating Mg anode from the corrosive aqueous electrolyte.In addition,organic phase should have low saturation of water,be stable with Mg,and able to conduct current and transport Mg2+into the aqueous phase [32].Du et al.selected 10 wt.% Mg(TFSI)2solution as the aqueous phase and the butyl butyratee[1-butyl-3-methylimidazolium][bis(trifluoromethanesulfonyl)imide]([BMIM][TFSI])solution containing 20 mass%[BMIM][TFSI]as the organic phase.The double liquid electrolyte separates Mg anode from the corrosive aqueous electrolyte,improving the anodic efficiency of pure Mg from 20% to 91.2% [32].However,the battery with double liquid electrolyte showed inferior discharge voltage,and further research are needed to improve the discharge voltage.
In summary,NaCl solution as a neutral electrolyte has the lowest discharge potential with pure Mg as shown in Table 2,and Cl−has lower Faradaic efficiency than NO3−,NO2−and HPO4−.Therefore,NaCl solution has been widely used as the aqueous electrolyte in Mg-air batteries for scientific research and commercial application.Developing suitable inhibitors for aqueous electrolyte could significantly enhance discharge performance of Mg-air batteries.Introduction of organic phase with aqueous phase as double liquid electrolyte could also significantly improve the anodic efficiency.However,further studies on the ionic conductivity and diffusion coefficient of Mg in organic phase are needed to improve the discharge performance of Mg-air batteries.
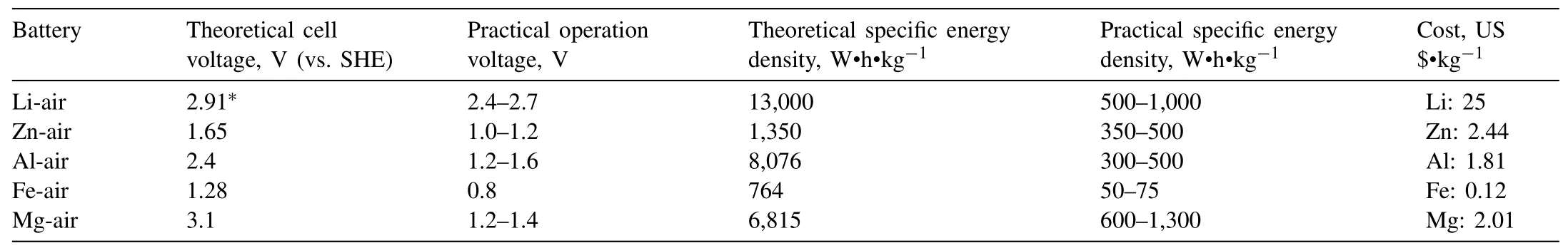
Table 1 Theoretical and practical cell voltage and specific energy density of metal-air batteries,and the price of their anode materials [3–5,10–18,33].
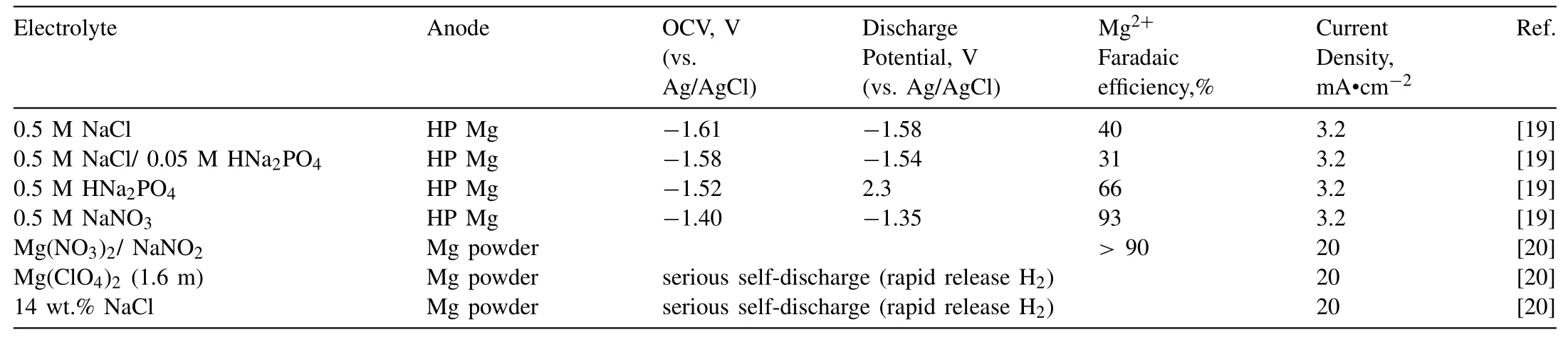
Table 2 Different types of aqueous electrolytes and their properties in Mg-air batteries.

Table 3 Electrochemical and battery properties of pure Mg and Mg-Al alloys.
1.3.Current used air cathode for Mg-air batteries
The discharge performance of Mg-air battery also relies on air cathode,which consists of a waterproof breathable layer,a gas diffusion layer,a catalyst layer,and a current collector[10,33].Fig.2 shows the structure of a typical air cathode.The waterproof breathable layer is used to separate the electrolyte and air,such as paraffin or Teflon.The gas diffusion layer comprises carbon material and hydrophobic binder(e.g.,polytetrafluoroethylene(PTFE))to allow air penetration and seepage water [34].The oxygen reduction reaction(ORR)takes place on the catalyst layer [35].The current collector is typically made of a Ni metal mesh with good electron conductivity [36].
Like Zn-air and Al-air battery systems,the air cathode of Mg-air battery is also facing three critical problems:intrinsic sluggish kinetics,high overpotentials,and poor reversibility[37].The catalyst layer is a critical factor for these issues.The ORR process involves complex physical and chemical reactions,including the formation of hydroperoxide ions.Catalysts which typically speed up a reaction are required to accelerate the decomposition of these hydroperoxide ions[35].Two reactions occur at air cathode in a neutral solution:
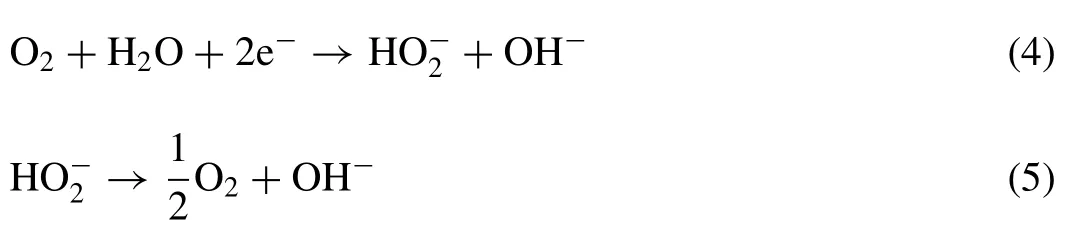
The common catalysts of air cathode are noble metals,carbon materials,and transitional metal oxides [10].Noble metal catalysts(e.g.,Pt,Pd,Au,and Ag)have high catalytic activity,low overpotential,and large limited current density,but high cost [38–42].In the last decades,numerous investigations have been working on developing high performance and low-cost non-noble metal catalysts for air cathodes.Recent several research groups made good progress on catalysts and air cathodes [42-44].
1.4.Issues of anode materials for Mg-air batteries
Mg metal anode is another critical factor influencing Mgair battery performance.Mg is prone to dissolution over a wide pH range from 0 to 10.5 solution.As shown in Eq.(1),Mg anode dissolves to produce two electrons and Mg2+during the discharge process.The schematic in Fig.3 representation of the main activities of Mg metal anode in Mg-air batteries and the main issues of Mg anode are also included.The standard electrode potential of this reaction is−2.37 V.However,due to the side reaction in the Mg anode,i.e.the self-corrosion reaction(Mg+2H2O →Mg(OH)2+H2),Mg metal anode suffers the low anodic efficiency and low discharge voltage in Mg-air batteries.In addition,the discharge product Mg(OH)2accumulates on the surface of Mg anodes,leading to anode polarisation and disrupting the anode performance [45].
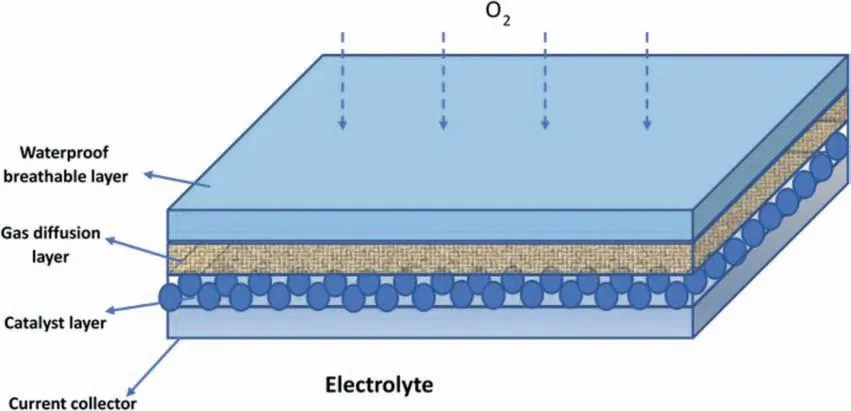
Fig.2.Schematic of the typical structure of air cathode [10].
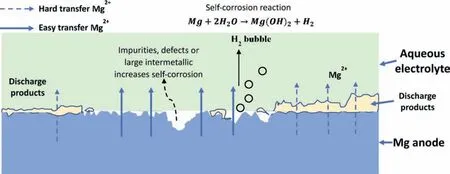
Fig.3.The main activities of Mg metal anode in Mg-air batteries.
Mg-air batteries suffer the low anodic efficiency and high polarisation.Anodic efficiency is also known as Faradaic efficiency,coulombic efficiency or anode corrosion efficiency,defined as the ratio of the energy converted to the energy consumed.Compared to nearly 100% coulombic efficiency of LIBs,the anodic efficiency in Mg-air batteries is about 30–60% depending on the discharge current density.Current density is the amount of charge per unit time that flows through a unit cross-section area,which is measured in amperes per square meter or centimetre.The value of anodic efficiency is determined by the weight loss of anode materials during discharge,which can be calculated by following equations.
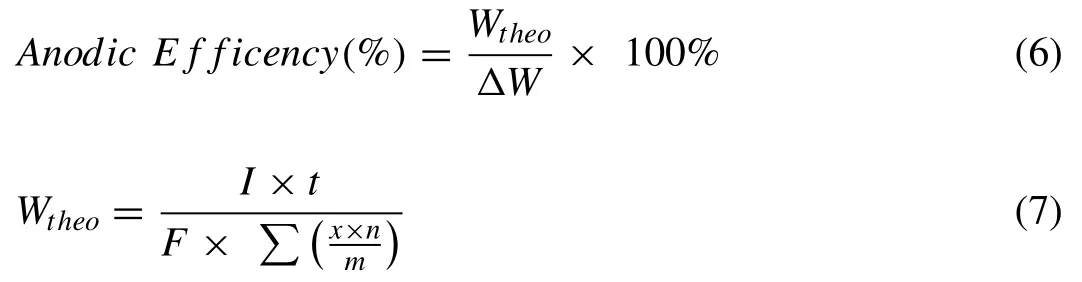
Where∆W(g)is the weight loss during the discharge,Wtheo(g)is the theoretical weight decrease,Iis the discharge current(A),tis the discharge time(h),Fis the Faraday constant(26.8 A•h•mol−1),andx,n,mrepresent the mass fraction,number of exchanged electrons and atomic weight,respectively.
Corrosion behaviour of Mg is affected by many factors,such as hydrogen evolution reaction(HER),negative difference effect(NDE),and impurities.The NDE describes the electrochemical phenomenon.More hydrogen is evolved from an Mg electrode at a more positive potential which does not occur in conventional metals.Since Bussey first conducted the corrosion experiments in 1831,numerous studies have been conducted to understand the corrosion mechanism of Mg and to improve its corrosion resistance[46].The introduction of alloying elements into Mg is one of the most widelyused method to enhance its corrosion resistance,which improves the discharge performance of Mg-air batteries.Since the 1960s,commercial Mg alloys such as AZ31,AZ61,AZ91,and AP65 alloys have been studied as anode materials for Mg-air battery [51-55,59,63,67].The anodic efficiency and discharge capacity of Mg alloys could be significantly enhanced by adding appropriate concentration of alloying elements.These alloys have also been further studied as anode materials after heat treatment and plastic deformation.
The corrosion mechanisms,the effect of alloying elements and corrosion properties of Mg alloys have been very well reviewed [7,22-28].There are also review articles focusing on the basic principles,electrolytes and cathodes materials for Mg-air batteries [9,10].However,rare reports summarised the battery discharge performance of Mg alloys.This review focuses on the recent research on Mg alloy anode.The anodic efficiency and discharge properties of Mg alloys are summarised at different current density levels,which could be used as a data source for Mg-air batteries.
2.Pure Mg anode
The discharge performances of pure Mg are included in Table 3.Pure Mg has low anodic efficiency 34.4% and discharge capacity 759 mA•h•g−1at low current density 0.5 mA•cm−2.Anodic efficiencies of Mg increase to 64%and 62%,with increasing the current density to 180 and 300 mA•cm−2.However,the average voltage exhibits a totally different trend.Pure Mg has the highest discharge voltage of 1.36 V at a low current density of 0.5 mA•cm−2.When the current density increases,the discharge voltage declines gradually to 0.75 V at current density 40 mA•cm−2.The relationship between discharge capacity of pure Mg and current density are more complex.Discharge capacity of Mg grows up with increasing current density.It reaches the peak value 1210 mA•h g−1at the current density of 10 mA•cm−2,then goes down.Nanostructured Mg,such as nanospheres,nanoplates,nanorods,and urchin-like nano/mesoscale structure(Fig.4),have also been studied as anode materials for Mg-air battery[20].These Mg nanomaterials displayed higher current density in linear sweep voltammograms(LSV)and better discharge performance than commercial Mg [20].Mg films of 100 nm thick exhibit a flat discharge plateau with an open-circuit voltage at 1.41 V,and a small discharge capacity at 821 mA•h·g−1[47].However,nano/micro-structured Mg,as same as the bulk Mg,suffers high self-corrosion rate and large NDE.

Fig.4.SEM image of nanospheres,nanoplates,nanorods,urchin-like magnesium [20].
3.Mg alloy anode
3.1.Mg-Al alloys
Aluminium(Al)is one of the most commonly used alloying elements in Mg alloys.Addition of Al significantly increases the mechanical properties of Mg alloys and also enhances their corrosion resistance [48].It was reported that Mg-6Al alloy has corrosion currentjcorr=9.73 μA•cm−2,much lower than that of pure Mg(˜50–90 μA•cm−2)[49–56],indicating that the Al may improve the corrosion resistance of Mg alloys.Song et al.reported that the finely and continuously distributed Mg17Al12phase could act as a corrosion barrier,while the coarse and discontinuous phase accelerates corrosion as a galvanic cathode in AZ91 alloys [26,57].
3.1.1.Mg-Al binary alloys
Table 3 summarises the discharge properties of two Mg-Al binary alloys.Mg-3Al alloy shows higher anodic efficiency and specific capacity than Mg-6Al alloy at current densities of 5 and 10 mA•cm−2.However,comparing with pure Mg,the anodic efficiency and specific capacity reduced from 54.9% and 1210 mA•h·g−1at 10 mA•cm−2to 46.7% and 1,054 mA•h·g−1with the addition of 3 wt.% Al.With adding 6 wt.% Al,the anodic efficiency and specific capacity further decrease to 34% and 761 mA•h·g−1.The maximum solubility of Al in Mg is 12.7 wt.% at eutectic temperature 437 °C.Additions of Al below the solubility limit tend to reduce the anodic kinetics of Mg;and Mg-Al alloys nominally have high corrosion resistance[24].It is interested to see that the anodic efficiency and discharge capacity of Mg are deteriorated with the addition of 3 wt.% and 6 wt.% Al,although discharge voltage slightly increases.
Wang et al.have reported that the addition of Al forms discharge product Al(OH)3that can affect the battery performance,which peels Mg(OH)2to form 2Mg(OH)2•Al(OH)3film [58].The formation of Mg17Al12phase can also prevent further self-peeling of discharging products,and the high concentration Al(8–9 wt.%)much decreases the corrosion resistance and increase the discharge activation due to the break-off of Mg17Al12and damage Mg(OH)2film [59].Although these investigations have reported that Al alloying elements affect the discharge products and performance,the mechanisms of deteriorating of anodic efficiency and specific capacity are not fully understand,especially,Mg-3Al alloys.
3.1.2.Mg-Al-Zn(AZ)alloys
Mg-Al-Zn(AZ)alloy system has been studied intentionally,especially AZ31,AZ61,and AZ91 commercial alloys.This alloy system has been adopted as the anode of Mgair battery since 1969 [63].AZ series alloys have excellent mechanical properties and relatively good corrosion resistance[64].For example,AZ31 is a wrought alloy with good formability,and AZ91 is mainly used for die-cast products.The microstructure of AZ61 and AZ91 alloys consists ofα-Mg matrix and Mg17Al12secondary phase,which is mainly distributed along the grain boundaries.A continuous network Mg17Al12can act as a corrosion barrier and slow down matrix corrosion [65].
Table 4 summarises the discharge properties of AZ alloys.Like Mg-Al binary alloy in Table 3,AZ31,AZ61 and AZ91 alloys have low anodic efficiency and discharge capacity,but slightly improved discharge voltage.Addition of 1 wt.% Zn does not improve the discharge performance of Mg-Al alloys much,although it has been reported that a small amount of Zn can reduce the self-corrosion rate of Mg alloy and improve the anodic efficiency in Mg-Zn based alloy system [66].
In the last decade,a number of alloying elements,such as Ca,Ce,In and Gd,have been added into AZ series alloy for improving its discharge performance.Li et al.made an AZ63–1In alloy,which has a relatively low hydrogen evolution rate and more negative corrosion potentialEcorr,resulting in better corrosion resistance and higher discharge activity than pure Mg [67].The fine and dispersive Mg17Al12phase in AZ91 are refined by alloying Ca,Sm and La to prevent the corrosion progress in the matrix [68].Electrochemical impedance spectra(EIS)results have shown that modified AZ91 alloys have larger inductive reactance than as-cast alloy,suggesting that the surface products are difficult to fall off [68].Zhu et al.have reported that AZ61–0.5Ce alloy shows a high and stable discharge capacity ∼1370 mA•h•g−1and the anodic efficiency increases from 55% to 63% when current density ranges from 10 to 20 mA•cm−2.The addition of 0.5 wt.%Ce reduces the content of impurities and refines the grains of AZ61,resulting in improvement of anodic performance [54].
Furthermore,external field treatment during fabrication,post-heat treatment and plastic deformation have been introduced to enhance the discharge performance of AZ alloys.It can be seen from Table 4 that annealing treatment slightly improves the anodic efficiency and discharge capacity.The external field treatment can refine theα-Mg grains andβ-phases of AZ80 billet,improving the electrochemical activity and corrosion resistance of AZ80 anode.

Table 4 Electrochemical and battery properties of AZ alloys.

Table 4 (continued)
3.1.3.Mg-Al-Pb(AP)alloys
Mg-Al-Pb alloys have been studied as anode materials in seawater batteries [49,73-77].Both alloying elements Al and Pb can activate the Mg matrix.As the electrode/electrolyte interfacial reaction is activation-controlled[49],the precipitated Pb oxides on the surface of alloy accelerate the precipitation of Al(OH)3and peel the Mg(OH)2film[58].
Comparing to pure Mg and AZ31,the corrosion currentjcorrof AP alloys are lower than Mg,and the corrosion potentialEcorris slightly more negative than AZ31,implying that these alloys have better corrosion resistance and higher electrochemical activity [50,55,78].Table 5 summaries the electrochemical and battery discharge properties of AP alloys.Anodic efficiency of AP alloy reaches 81.7% at the high current density of 300 mA•cm−2.La,Ce,Y,and In were added in the AP alloys to further improve their corrosion and electrochemical properties.The electrode potential of Al2Ce and Al2Y compounds(γphase)is more negative than that ofβphase,indicating that AP65-RE alloys have a better electrochemical performance [55].The average discharge potentials of AP-RE alloy are more negative than that of AP alloys.Additions of La,Ce and Y can increase the anodic efficiency at low current densities,while In improves the discharge properties at higher current densities.
Because of the high solubility of Pb(41.7 wt.%)in Mg matrix [24],the main phases in AP alloys are the Mg matrix and Mg17Al12phase [50].Additions of Ce,La,and Y form Al2Ce,Al11La3,and Al2Y phases,which restrain the formation of Mg17Al12and decrease micro-galvanic corrosion [78].Addition of In reduces the grain size of AP alloys and improve corrosion resistance,because the grain boundaries act as corrosion barriers to reduce the corrosion rate [50,53,80].The discharge products of AP65–1In alloy have many cracks,whereas there are much fewer cracks on the pure Mg and AP65 discharge products [50].Therefore,the electrolyte can penetrate and fully contact with the electrode,improving the discharge property.
3.1.4.Mg-Al-Mn(AM)alloys
The addition of Mn reduces the corrosion rate of Mg-Al alloy by incorporation of low-soluble metals in Al-Mn phase[24].It also effectively mitigates Fe impurity when the Fe/Mn ratio is lower than 0.032 [25].Table 6 summarises the battery discharge properties of Mg-Al-Mn and Mg-Al-Mn-Ca(AMX)alloy anodes.The AMX alloy contains Mg matrix,Al-Mn phase,and Al2Ca particles,which induce galvanic cathode effect [81,82].The corrosion rate of AMX602 alloy is twice higher than the AM60 alloy [81].On the other hand,the addition of Ca enhances the anodic efficiency and discharge capacity.The reason is that the discharge products are often cracked by the dissolution of Al2Ca phase;and the cracks promoted the penetration of electrolyte to the anode,keeping a good discharge activity [81].
3.1.5.Mg-Al-Sn(AT)alloys
Addition of Sn enhances the battery performance of Al metal anodes in Al-air batteries [83,84].Researchers expect the same positive effect on Mg-air batteries [23,85-87].AT61 alloy has been used as anode materials for seawater batteries,which showed negative potential and high electric current density of 100 mA•cm−2during the galvanostatic discharge process [88].It can also promote the fall off of passivation film and accelerate the dissolution of Mg anode as an activation point [85].Xiong et al.studied AT alloys as anode materials for Mg-air batteries,and found that solution-treated,extruded and annealed anode present better battery performance[45,86].
Table 7 summarises the discharge performance of AT alloys under different treatment conditions including solution treatment,extrusion,and annealing treatment,showing that the discharge voltage,anodic efficiency and discharge capacity of AT alloys vary due to the microstructure variation.The main phases in AT alloy are Mg matrix,Mg17Al12,and Mg2Sn.The Mg17Al12phases can act as the cathode to promote the corrosion of Mg alloys,and the Mg2Sn phase can increase the H2evolution rate [89].The discharge products cannot be easily peeled off from the surface of the as-cast alloy due to the Mg17Al12phases acting as barriers[86].The accumulated discharge products increase the internal resistance of Mg-air batteries,reducing the output voltage[45].After the solution treatment,extrude,and anneal treatment,corrosion resistance and battery performance of AT61 anode are improved.
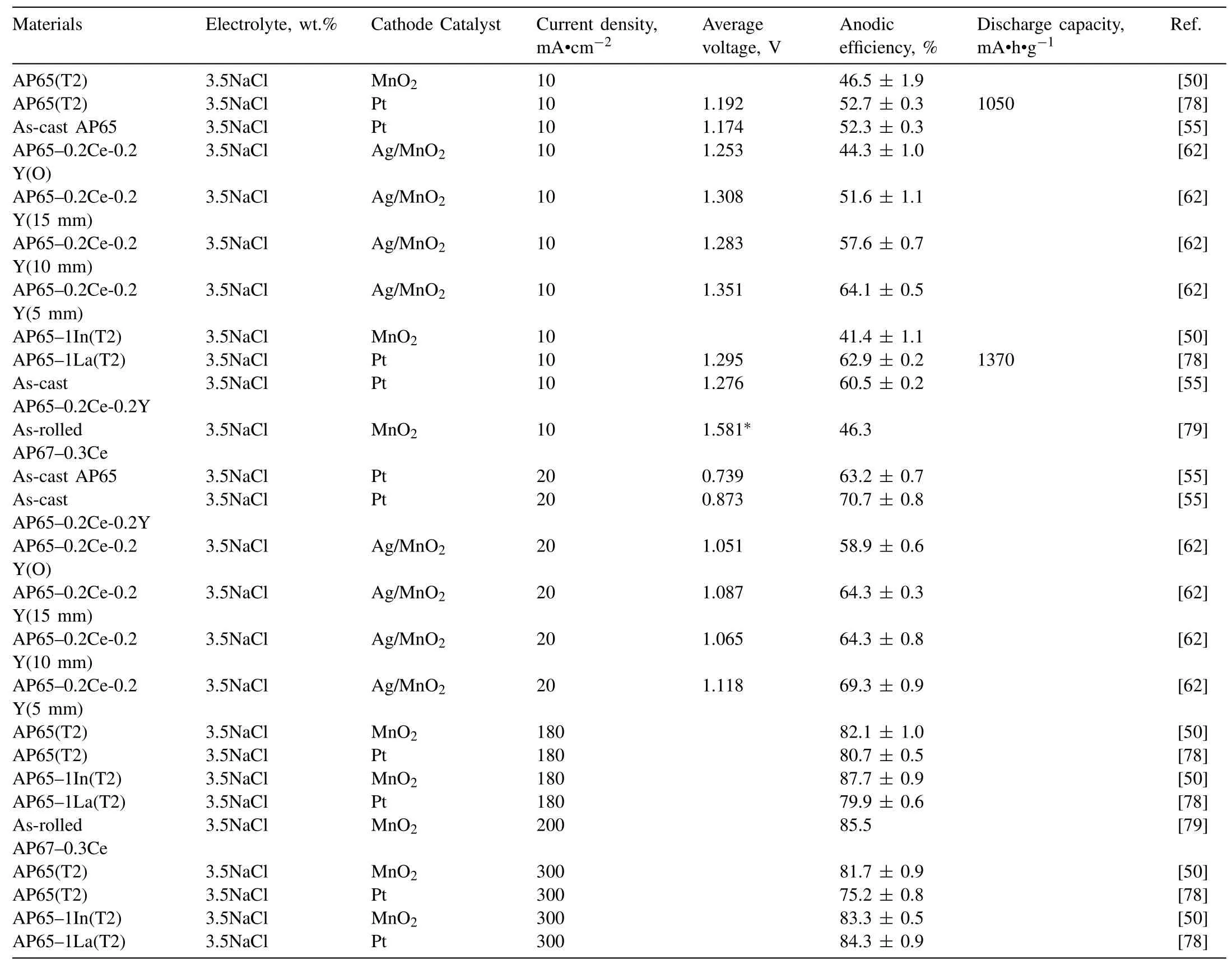
Table 5 Electrochemical and battery properties of AP alloys.
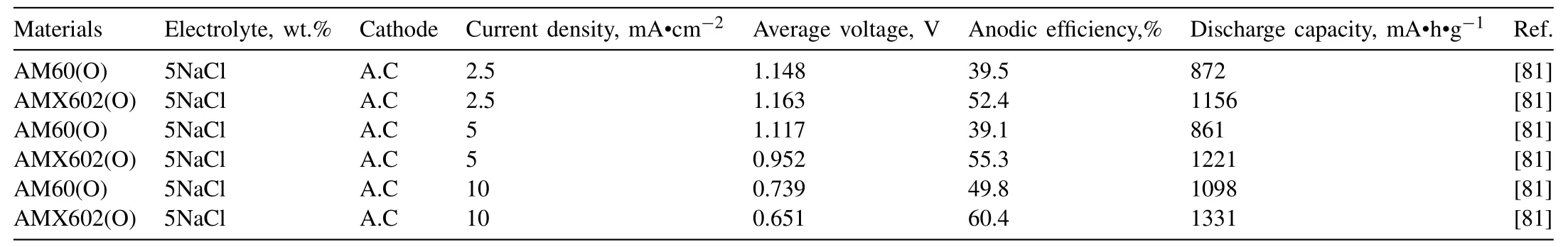
Table 6 Electrochemical and battery properties of AM alloys.

Table 7 Electrochemical and battery properties of AT alloys.
Compared to the as-cast alloys,the secondary phase of the solution treatment AT alloy are fine,which can prevent corrosion extend.The extruding and annealing can refine grains.In addition,the refined grains and re-distributed second phases can decrease self-corrosion [86].The annealed sample has some cracks,which increase the surface area of the anode and accelerate the self-peeling of discharge products,enhancing the discharge performances.Xiong et al.reported that the second phase(Mg17Al12and Mg2Sn)dissolved into the Mg matrix after solution treatment and refined the second phase in the grain boundary areas [45].Sn promotes peeling offthe passivation film by the combined action of the corrosion products and high-valence cations [60].
3.1.6.Others Mg-Al alloys
Rare-earth element,In,and Ga have also been added as alloying elements in Mg-Al alloy to improve its electrochemical properties and battery performance.Ce can refine grain size and forms Al11Ce3phase,which acts as a corrosion barrier and makes the discharge products thinner and more porous [54,56].Indium mainly plays a role in activating Mg-3Al alloy [60],and facilitates Cl−adsorption on the anode surface to break the surface passivation film [90].Ga improves the corrosion resistance in Mg-Hg seawater batteries [91].
Table 8 summarises the electrochemical properties and battery performance with Mg-Al-In,Mg-Al-Ce and Mg-Al-Ga alloy anodes.Mg-3Al-1Ga shows the highest anodic efficiency of 61.6% and discharge capacity of 1376 mA•h•g−1at 10 mA•cm−2.The addition of Ga and In can improve the anodic efficiency and discharge capacity.Mg-3Al-1Ga has passive films with homogeneous cracks,which slow down the transfer rate of Mg2+and decrease self-corrosion [60].Mg-Al-In alloy has a high electrochemical activity due to its more negative corrosion potential and lower charge transfer resistance(Rt)[56,60].Indium increases the voltage significantly at a low current density and discharge capacity,it also promotes the combination of discharge products and NaCl to accelerate Mg(OH)2film fall-off and keep further discharge[50,92].Mg-6Al-1In-1Ce alloy has large cracks on the surface [56]due to the corrosion barriers formed by its large amount of secondary phase.These cracks or porous discharge products allow electrolyte to penetrate the passive layer and react with the Mg matrix more effectively [50,52,81,92,93].However,discharge products of Mg-6Al-1Ce alloy are compact and flat.This conflicts with Zhu et al’s work in which Al11Ce3phase makes the discharge products thinner and more porous [54].
3.2.Mg-Li alloys
Mg-Li alloys are the lightest structural metal and have also been studied as anode materials owing to their extremely negative potentials and large Faradic capacities [51,94-97].Addition of nearly 11 wt.% Li converts HCP structure of pure Mg to a BCC structure alloy,while Mg-Li alloys with a low Li content(<5.5 wt.%)are composed ofα-Mg phases.H.Yang et al.have recently reported that LAZ531 shows high discharge voltage [98,99].On the other hand,as Mg-Li alloys contain mainlyα-Mg matrix andβ-Li phase [52,70,96,100],they have a good corrosion resistance due to the dense and fine phase(AlLi and MgLiAl2)in theβ-Li matrix that can inhibit the micro-galvanic coupling [101].Li enhances electrochemical activities of Mg due to the formation of an activeβ-Li phase when Li concentration is higher than 5 wt.%[52].Table 9 lists the electrochemical and battery properties of Mg-Li-Al alloys.

Table 8 Electrochemical and battery properties of other Mg-Al alloys.
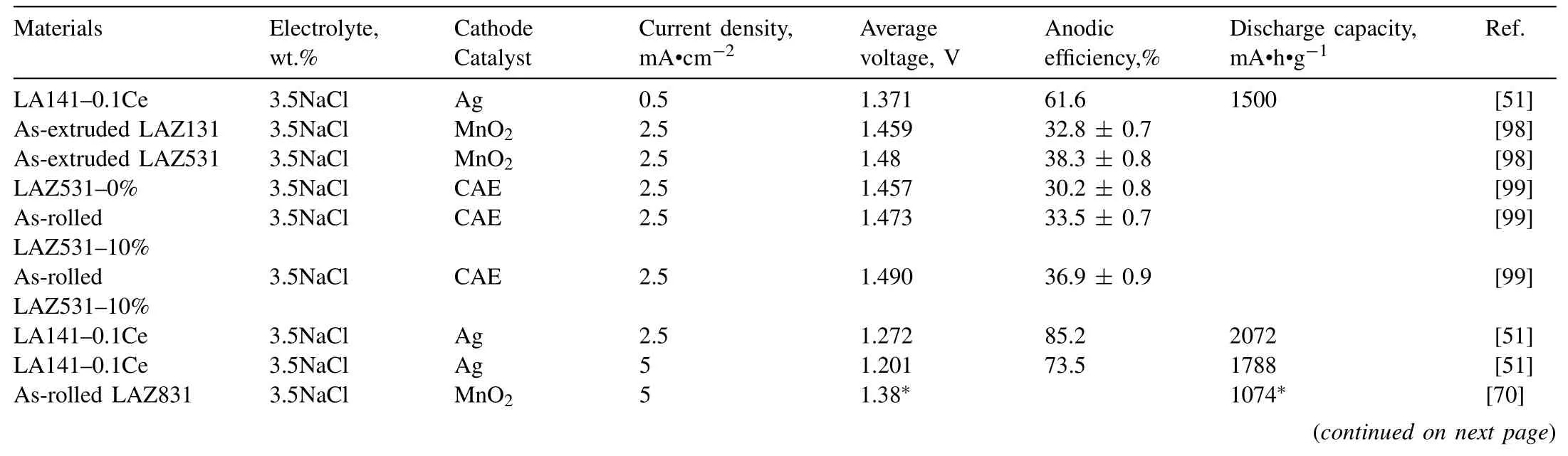
Table 9 Electrochemical and battery properties of Mg-Li-Al alloys.

Table 9 (continued)

Table 10 Electrochemical and battery properties of Mg-Ca alloys.
The discharge capacities and anodic efficiency of Mg-Li-Al are obviously higher than other Mg alloy anode materials.Lin et al.found that the AlLi phase enhances the discharge voltage and capacity of the anode in MgCl2electrolyte because that the potential of AlLi phase is more negative than that of Mg matrix[102].Adding Ce and Y,the battery performances have been improved because Ce enhances both discharge activitiesand anodic efficiency [93],while Y can change the structure of alloys or assist in forming an easy peel-off layer on the alloys in NaCl solution [97,103].
LAZ1131 anode is reported to have better corrosion resistance than others because of AlLi and MgLiAl2phases [70].Besides,β-Li phase is corroded preferentially and provides a high and stable working voltage.LAZ831–1Ce-1Y has better corrosion property and battery performance than LA83–1Ce-1Y.Because the standard electrode potential of Zn is more positive than Mg and Li,which might decrease the microgalvanic reaction rate [52,104].The combination of Zn and AlLi phase accelerates the self-peeling of the discharge products and hence improves the discharge activity of Mg-Li-Al-Ce-Y-Zn alloy.For LA141–0.1Ce alloy,the discharge products have many pores and cracks,which allows continuous electrochemical reactions [51].
3.3.Mg-Ca alloys
Ca has a more negative standard potential than Mg [53].Table 10 summaries the battery discharge properties of Mg-Ca alloys.Deng et al.investigated Mg-Ca binary alloy as the anode for Mg-air battery,and reported that Mg-0.1Ca alloy has the best self-corrosion behaviour and discharge performance [53].For Mg alloys with high Ca content,the weak effect of Mg2Ca phase on Mg-Ca alloy corrosion in NaCl solution exists but the higher charge transfer resistance(Rt)may result in the prior oxidation of the active Mg2Ca phase at the initial stage [105].The Mg matrixes fall off(Chunk effect)due to the dissolution of Mg2Ca phase at the grain boundaries during discharge,which significantly reduces the electrode efficiency [53,106].In addition,water cooled(WC)Mg-0.5Ca also receives high discharge capacity 1530 mA•h•g−1at 10 mA•cm−2.
Fig.5 shows the discharge cracks of Mg-0.5Ca(WC)tend to penetrate along grain boundaries,the efficiency and specific energy density are improved,and the battery has a stable discharge voltage [107].Nevertheless,in Mg-Ca(AC)alloy,the second phase Mg2Ca is located in the grain boundary areas,causing metal matrix cracking severely during the discharge,and decreasing the anodic efficiency and discharge capacity[107].
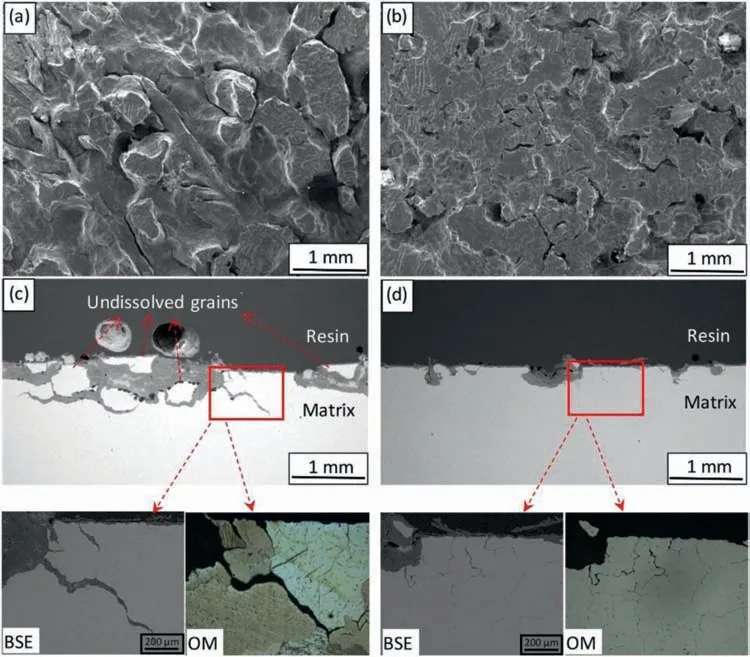
Fig.5.Surface morphologies and cross-sections of Mg-0.5Ca alloys:(a,c)AC,and(b,d)WC after discharge in 3.5 wt.% NaCl solution at 2 m mA•cm−2 for 16 h.(a,b)surface after removing discharge products;(c,d)cross-section with discharge products [107].
Adding 0.2 wt.% In in Mg-0.1Ca alloy can significantly improve the anodic efficiency to 80.2% at a low current density of 5 mA•cm−2.The combination of In3+ions with Mg dissolution can redeposit metallic In on Mg anode surface,which can promote the dissolution of anode,and ruptures the film structure to decrease the transport overpotential [108].
3.4.Mg-Zn alloys
A small amount of Zn can improve the corrosion resistance of Mg alloys [66,110,111].The hydrolysation of dissolved Zn2+can decrease the pH value of the electrolyte near the anode surface,accelerating the dissolution of discharge product Mg(OH)2in seawater battery [112].Chen et al.have reported that the icosahedral quasicrystal phase(Iphase)in Mg-Zn-Y alloy can provide a good interface and atomic bonding between quasicrystal and hexagonal structure [113-115],and enhance mechanical properties and corrosion resistance[116,117].
Table 11 indicates that the discharge performance of ZW61 is significantly better than that of ZK60.Y element in Mg-Zn can improve the discharge voltage,anodic efficiency,and capacity.In ZK60 alloy,the MgZn2phase has a higher corrosion potential to increase Mg matrix corrosion [118].Large and deep discharge holes have been observed in ZK60 anode after discharge,while ZW anodes show relative regular and even discharge holes.ZK60 anode has poor desorption ability,which means that discharge products can be accumulated.However,the morphologies of ZW anodes imply a stable discharge process and homogeneous discharge products,which can fall off because of the relatively smooth surface [117].
Recently,authors have studied the self-corrosion and battery discharge behaviour of Mg-Zn alloys [119].The results show that 2 wt.% Zn significantly improves the anodic efficiency and specific capacity of Mg,while addition of 6 wt.%and 15 wt.% Zn reduces its discharge properties.This is due to the large volume fraction of Mg7Zn3and Mg4Zn7intermetallic phases act as cathode accelerating the self-corrosion of alloy anode.Mg-2 wt.% Zn alloy anode has the best battery performance with 54.4% utilization efficiency and 1186 mA•h•g−1specific capacity at 10 mA•cm−2(Table 11).Mg-2 wt.%Zn anode also has the highest specific energy density of 1140 mW•h•g−1at 2 mA•cm−2[119].Addition of Sn significantly improves the anodic efficiency and discharge capacity of Mg-Zn alloy.It is due to low content of Zn and Sn addition enhancing the corrosion resistance of Mg in NaCl solution.ZT41 alloy shows the best utilization efficiency and discharge capacity among different current density.

Table 11 Electrochemical and battery properties of Mg-Zn series alloys.
3.5.Mg-Bi alloys
Bi element in Mg alloys can restrain the hydrogen evolution reaction and enhance the corrosion resistance of Mg alloys [121,122].However,the intermetallic compound Mg3Bi2has the effect of refining Mg17Al12,which can influence thecorrosion resistance [26,57,123].Mg3Bi2phase increases the corrosion current density of AZ91E alloy and accelerates both anodic and cathodic reaction rates [122,124].Cheng et al.reported that BX10(Mg-1Bi-0.5Ca)alloy with coarse-grains exhibited superior electrochemical activity due to Mg2Bi2Ca acted strongly at the cathodic sites during the corrosion process.BX10 alloy shows a highCPEvalue,implying that a large active area could be provided during the discharge process [125].
Table 12 listed the properties of Mg-Bi anodes,showing that Ca can improve the discharge performance.Mg2Bi2Ca phase could cause local galvanic corrosion and thus enhance the spalling of the discharge products and a negative potential.Besides,the number of crystallographic planes with non-basal orientation in BX10(Mg-1Bi-0.5Ca)alloy is less than that of B1(Mg-1Bi)alloy.This orientation easily reacts with the aqueous electrolyte and exhibits a good electrochemical activity,which could improve the discharge performance[75,126].In addition,at a low current density,B1 anode has thick discharge products film with few cracks,whereas the discharge products of BX10 become thinner with numerous cracks occur,promoting electrolyte penetration [125].
3.6.Mg-Sn alloys
Sn has been used as a practicable element for highperformance Mg-ion rechargeable battery due to its high the-oretical specific capacity(903 mA•h•g−1)and low theoretical potential(0.15 V vs.Mg2+/Mg)[127,128].Mg-Sn alloy anode contains secondary phase Mg2Sn,which accelerate the dissolution of Mg anode and break the passive film,significantly improving the discharge performance in Mg-air battery[86].However,excessive Mg2Sn phase will result in severe self-corrosion and reduce the anodic efficiency.Table 13 summarized the properties of Mg-Sn alloys.

Table 12 Electrochemical and battery properties of Mg-Bi series alloys.
It was reported that the surface of the TZQ411(Mg-2Sn-1Zn-1Ag)alloy consisted of a relatively high fraction of crystallographic planes with non-basal orientation [129].Due to the low binding energy and high surface energy of the non-basal plane,the Mg alloys could expect an excellent electrochemical activity [130].As can be seen from Table 13,the discharge performance of the extruded Mg-0.5Sn-0.5Mn-0.5Ca alloy was slightly improved by homogenization treatment.

Table 13 Electrochemical and battery properties of Mg-Sn series alloys.
3.7.Mg-RE alloys
Shrestha et al.have reported a series of Mg-Re alloy as Mg-air battery anode materials.These alloys have better corrosion resistance than pure Mg in 0.6 M of NaCl,0.5 M of NaNO3and 0.1 M of Na2SO4solution [133,134].The surface enrichment of Zr and MgxREysecondary phases enhanced the negative difference effect(NDE)of alloy.EV31A has higher efficiency than pure Mg due to its thinner surface layer,which attributed to the higher hydrogen evolution rate[133].
4.Challenges and future outlooks
Mg-air batteries/fuel cells have been attracted much attention from both research communities and industry sectors in the last decades.As shown in previous sections,a wide range of Mg alloys have been investigated as potential anodes for primary Mg-air battery.Compared to pure Mg anode,Mg alloying anodes have significantly improved the performance of Mg-air battery.Figs.6 and 7 illustrate the main performances of specific aqueous Mg-air batteries with different anode materials.Mg-Li alloy has outstanding voltage and capacity,which can be one of the potential anode materials for Mg-air battery.However,the mechanism of Li enhancing battery performance are not very clear.Al is an element that improves the corrosion resistance.Addition of Al improves the discharge voltage apparently and form discharge product Al(OH)3,while the capacity is still lower than pure Mg.Addition of Zn decreases the discharge voltage but increases the corrosion resistance and battery capacity.Ca has almost no solid solubility(1.35 wt.%)in Mg and its main influence is the formation of intermetallic compounds.Alloying elements Pb,Sn,In and Ce not only improve the specific capacities but also increase cell voltage.Solute Sn can form SnO2on the surface to protect Mg matrix [135].Rare earth elements have been added as alloying elements for high performance Mg alloys.They also play an interesting role in enhancing the battery performance of Mg air battery.
Furthermore,a wide range of heat treatment and wrought process technologies,including rolling and extrusion,have also been demonstrated as a useful method to enhance thebattery discharge properties of Mg alloy anodes.For example,rolling process changes the texture(grain orientation)and twinning structure of Mg alloy.Yang et al.[99]recently report that rolling deformation increases the corrosion resistance and discharge properties of LAZ531 alloy,which is due to the change of texture and the increased density of twins.Grain boundaries and twins play the same roles on the corrosion and discharge behaviour of LAZ531 alloy [98,99].
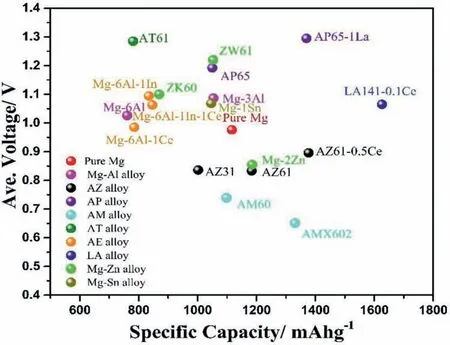
Fig.6.The discharge voltage -specific capacity plots of Mg-air battery with selected alloy anodes at current density 10 mA•cm−2 in NaCl electrolyte[45,51,54,56,60,78,81,117,119,131].
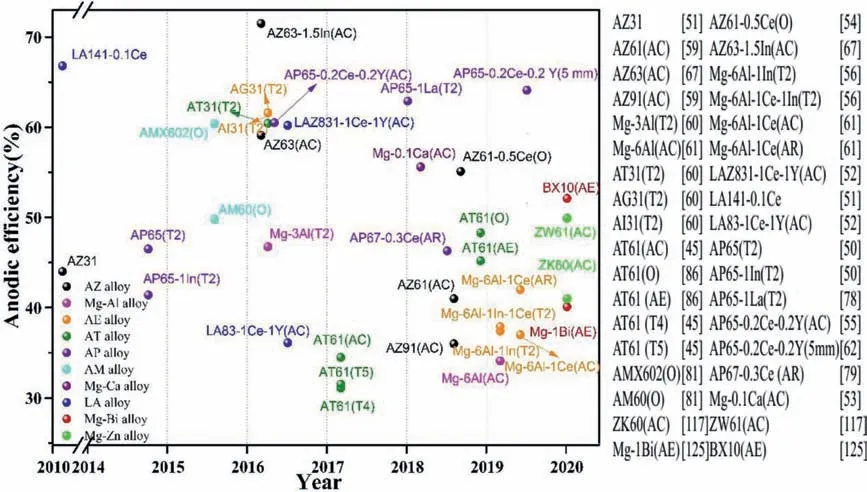
Fig.7.The anodic efficiency of alloy anode for Mg-air batteries at the current density 10 mA•cm−2 in NaCl electrolyte in recent years.[136]
With addition of alloying elements,a wide range of intermetallic compounds can form.As shown in Section 3,intermetallic compound is one of the critical factors to influence battery performance.Table 14 summarizes the second phases that normally identified in Mg alloys and their influence on the anode performance.In general,the intermetallic compounds with more positive potentials than Mg matrix,such as Mg2Ca and Mg4Zn7,act as galvanic cathode to accelerate Mg matrix dissolve.However,fine and dispersive ones can reduce corrosion extend.In addition,the amount of intermetallics should be very low,otherwise,it will cause chunk effect.

Table 14 Intermetallic compounds in Mg alloys and their influence on Mg alloy anode battery performance.
Although discharge performances of Mg metal anode materials are improved via adding alloy elements or post-treatment processes,some key factors need to be considered whendesigning and developing high performance anode materials for Mg-air batteries.
(1)Low-cost and environmental-friendly anode materials
Current alloy anode development mainly bases on Mg-Al alloys,particularly commercial AZ alloy anodes,i.e.,AZ31,AZ61,AP65 and AM60.A wide range of alloying elements,such as In,Y,La,Ga,Ce have been added to modify the microstructure,reduce the self-corrosion and improve electrode efficiency.Recent investigations have shown that these alloying elements have positive effects in enhancing battery performance.However,many of these developments may not be practicable for industry applications.
Firstly,some alloying elements are not environmentalfriendly and harmful for operators,for example,Mg-Al-Pb alloy and AP alloys for Mg-air batteries.Pb is a highly poisonous metal that can cause severe damages to the human body.The extraction,production,use and disposal of Pb and its related alloys and batteries will cause significant pollution to Earth’s soils,water and endanger the users.The requirements of sustainable development limits the application of AP alloys as anode materials for Mg-air batteries.
Secondly,Mg-air battery is a primary battery,although it can be reused by replacing Mg anode and electrolyte.The price of Mg-air battery needs to be cheap,otherwise there will be a limited market for these batteries.Therefore,the new alloy anode has to be low cost.As listed in Table 1,the price of Mg raw material(2.01 USD•kg-1)is higher than Al(1.71 USD•kg−1).The costs of manufacturing Mg products are also higher than Al because of its low ductility and poor formability.In order to broaden the applications of Mgair battery,it is necessary to control the costs from alloying design and manufacture.The composition of high costing elements such as Y,Ga and In should be as low as possible.Furthermore,other materials processing technologies,such as plastic deformation and heat treatment,could also be explored to enhance battery performance.
(2)High performance Mg-air battery with high discharge voltage
Mg-air battery has a very high theoretical voltage at 3.1 V.However,the practical discharge voltages of Mg-air batteries are rather low.As listed in the Tables of Section 3,the practice discharge voltages are about 1.3 V at very low current density of<5 mA•cm−2.When the current density increases to 30 mA•cm−2,the discharge voltage declines to∼0.8 V.This is mainly due to the self-corrosion behaviour of Mg anodes–anI-Rdrop across the layer of corrosion products.
Recent studies on Mg alloy anodes have shown significant improvement in specific capacity and anodic efficiency.However,the discharge voltages are still very low.Further investigations are needed to understand the formation of corrosion products and electrochemical reaction interface between Mg alloy anode and aqueous electrolyte,in order to improve the discharge potentials of Mg-air batteries.Furthermore,like other metal-air batteries,developments of low-cost,stable and high-performance air cathodes and electrolytes are crucial for the application of Mg-air batteries.
5.Summary
In this review,we have summarised the recent research and development of Mg alloy anode in Mg-air batteries.The main problems of Mg anode in Mg-air battery are the selfcorrosion of Mg and discharge products accumulation on the electrode surface.Self-corrosion of Mg reduces the efficiency and capacity of Mg-air batteries,and the accumulation of discharge products affects the normal operation of the batteries.In addition,the operating voltages of Mg-air batteries are much lower than their theoretical voltage of 3.1 V.
A number of Mg alloy systems and a wide range of alloying elements have been added into Mg to improve its discharge properties.High anodic efficiency and specific capacity have been achieved in Mg-Li-Al and Mg-Ca-In alloy anodes.Mg-Al-Pb alloy anodes show a high anodic efficiency.The addition of Rare Earth elements can also improve the anode performance of Mg-air battery.However,the practical applications of these high-cost alloy anodes in Mg-air batteries may be limited.In addition,the low discharge voltages of Mg-air batteries need to be improved.Future research on the electrochemical reaction interface between Mg alloy anode and aqueous electrolyte,especially the formation mechanisms of discharge products,could provide insight to develop new Mg alloy anodes for Mg-air batteries with high operational voltage.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is partially supported by the Marsden Fund Council from Government funding,managed by Royal Society of New Zealand Te Apārangi(Fast-Start Marsden Grant project No.UOA1817).Fanglei Tong acknowledges the scholarship from China Scholarship Council(No.201808060410).
杂志排行
Journal of Magnesium and Alloys的其它文章
- Mg-based materials for hydrogen storage
- Biodegradable Mg alloys for orthopedic implants–A review
- A review on electromagnetic shielding magnesium alloys
- Thermodynamics and kinetics of hydriding and dehydriding reactions in Mg-based hydrogen storage materials
- Valorization of AZ91 by the hydrolysis reaction for hydrogen production(Electrochemical approach)
- Exploring the interconnectivity of biomimetic hierarchical porous Mg scaffolds for bone tissue engineering:Effects of pore size distribution on mechanical properties,degradation behavior and cell migration ability
