Mg-based materials for hydrogen storage
2021-02-24YunyunShngCludioPistiddkhnGizerThomsKlssenMrtinDornheim
Yunyun Shng ,Cludio Pistidd,∗ ,Gökhn Gizer ,Thoms Klssen,b ,Mrtin Dornheim,∗
a Department of Materials Design,Institute of Hydrogen Technology,Helmholtz-Zentrum hereon GmbH,Max-Planck-Straße 1,21502,Geesthacht,Germany
b Department of Mechanical Engineering,Helmut-Schmidt-University,Holstenhofweg 85,22043,Hamburg,Germany
Abstract Over the last decade’s magnesium and magnesium based compounds have been intensively investigated as potential hydrogen storage as well as thermal energy storage materials due to their abundance and availability as well as their extraordinary high gravimetric and volumetric storage densities.This review work provides a broad overview of the most appealing systems and of their hydrogenation/dehydrogenation properties.Special emphasis is placed on reviewing the efforts made by the scientific community in improving the material’s thermodynamic and kinetic properties while maintaining a high hydrogen storage capacity.
Keywords: Hydrogen storage materials;Magnesium-based hydrides;Metal hydrides;Nanostructures;Catalysts;Hydrogenation and dehydrogenation;Kinetics;Thermodynamics;Activation energy.
1.Introduction
1.1.Environmental concerns -hydrogen -magnesium
The access to abundant and cheap energy has been mankind’s most essential foundation of economic prosperity.Fossil fuels,which have been the main energy resources in the last two centuries,have supported the progress of human civilization and economic development [1].However,the finite nature and the excessive consumption are leading to a fast depletion of these energy sources.Moreover,the emission of greenhouse gasses from the combustion of fossil fuels led to threatening environmental changes [2].Therefore,it is urgent to find sustainable energy supply solutions.Although,renewable energy sources such as wind,solaretc.could cover the actual energy demand,due to their intermittent nature and unevenly distribution on Earth,their exploitation is challenging unless a suitable energy media is found [3].
In this regard,hydrogen is considered as a potential energy vector [4–6]due to its high gravimetric energy density,e.g.lower heating value(LHV)of 33.3 kWh·kg−1(gasoline 12.4 kWh·kg−1and natural gas 13.9 kWh·kg−1)[7,8].However,although highly appealing,the employment of hydrogen as energy carrier is partially hindered by the lack of appropriate storage solutions [8].The ideal hydrogen storage method should possess the following characteristics[9]:high volumetric and gravimetric hydrogen density,complete reversibility,adequate safety,and possibility to be operated under ambient conditions.
Nowadays hydrogen is mainly stored in three different forms:compressed gas storage [10],liquid storage [11],and solid-state storage in form of hydrides(e.g.metal hydrides and complex metal hydrides)[12].Although,currently,compressed hydrogen technology is the mostly implemented storage method [13],it suffers from several major drawbacks.Firstly,lightweight carbon-fiber tanks are difficult to produce and are expensive.Secondly,the achieved volumetric energy density remains poor if compared with gasoline.For example [14],when the hydrogen is compressed to 700 bar,the energy density value is 5.6 MJ·L−1only,while for gasoline it is 32.0 MJ·L−1and thus 6 times higher.Thirdly,a large portion of the stored energy is consumed for the compression work,about 13 to 18% of the LHV is needed when hydrogen is compressed to 700 bar [15].Hydrogen storage in liquid form implies an energetically unfavorable deep cooling to−253 °C.Theoretically,the energy required for liquefaction is about 10%of the total hydrogen energy content however,in real applications this value increases to 20–30% [16].Moreover,due to the boiling off phenomenon,a daily hydrogen loss of 1 to 2% must be taken into account [17].
In comparison,due to its high achievable volumetric hydrogen density and high safety,solid-state storage can be considered as an alternative method to store hydrogen.This type of hydrogen storage in metal-based systems is known since the 1866 when Graham discovered the high affinity of hydrogen for Pd [18].However,it was just in the 1960s that metal hydrides have been started to be investigated for hydrogen storage purposes.In this regard,among several high potential hydride systems,magnesium hydride has been one of the most investigated materials due to its high volumetric and gravimetric hydrogen density(i.e.110 kg H·m−3and 7.6 wt.%,respectively).It must be noted that these density values are much higher than those of compressed hydrogeni.e.23 kg H·m−3at 350 bar and 38 kg H·m−3at 700 bar,and of the 71 kg H·m−3of liquid hydrogen [19–21].
It was Wiberg et al.that as the first synthesized MgH2directly by heating Mg at 570 °C and 200 bar H2(using MgI2as a catalyst)in 1951 [22].Once MgH2is formed,the reversible reaction between magnesium and hydrogen can be described by the following equation [23]:
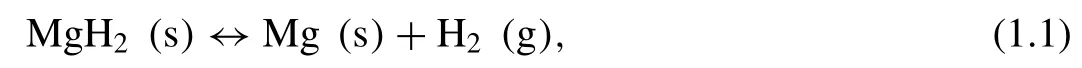
for this reaction the measured changes of enthalpy(∆H)and entropy(∆S)are 74.1±0.4 kJ·mol−1H2and 133.4±0.7 J·K−1·mol−1H2,that entail an equilibrium pressure of 1 bar at 283 °C [24].The crystal structure of solid Mg is hexagonal close-packed(hcp),whereas MgH2can exist in different polymorphic crystal structures depending on applied conditions [25].Under ambient conditions,MgH2is calledα-MgH2,with a tetragonal TiO2-rutile-type structure.At pressures higher than 3.9 kbar,α-MgH2converts intoγ-MgH2,which has an orthorhombicα-PbO2-type structure.This phase is metastable under ambient pressure conditions.However,small amounts of it can be formedviaintensive mechanical treatment ofα-MgH2.As an example,α-MgH2partially convert intoγ-MgH2during high energy ball milling [26].For applied pressures higher than 38.4 kbar at RT,the stable MgH2phase is theβ-MgH2,with cubic modified CaF2structure.Experimental results show that at room temperature for applied pressures higher than 102.6 kbar,independently from the starting polymorph(i.e.α,γ,β),MgH2will adopt an AuSn2-type orthorhombic structure.This particular structure is indicated asδ’-MgH2.The range of pressure stabilities of the different MgH2polymorphs theoretically calculated and experimental determined are reported in Fig.1(a)and(b),respectively.
Table 1.1 summarizes the crystal features(i.e.composition,space group,structure type,unit cell,positional parameters and Mg-H interatomic distance)for Mg and of the MgH2polymorphs.Both theα-MgH2and theγ-MgH2are built from MgH6octahedra.There are two types of Mg-H bonds in the octahedra ofα-MgH2,including equatorial(1.961 ˚A)and axial(1.944 ˚A)Mg-H bonds.While for theγ-MgH2,the MgH6octahedra are increasingly deformed,and contains multiple Mg-H bonds with lengths between 1.920 and 2.011 ˚A.The shortest Mg-H bond inγ-MgH2,which is 1.920 ˚A,is shorter than that ofα-MgH2(1.944 ˚A)due to the cell contraction.The cubicβ-MgH2has one type Mg-H bond of 1.906 ˚A,which is shorter than the Mg-H bonds inγ-MgH2.For theδ’-MgH2,the Mg-H bonds range from 1.796 to 2.085 ˚A.Except for the bond of 2.085 ˚A,the rest of the Mg-H bonds inδ’-MgH2are shorter than those inβ-MgH2.
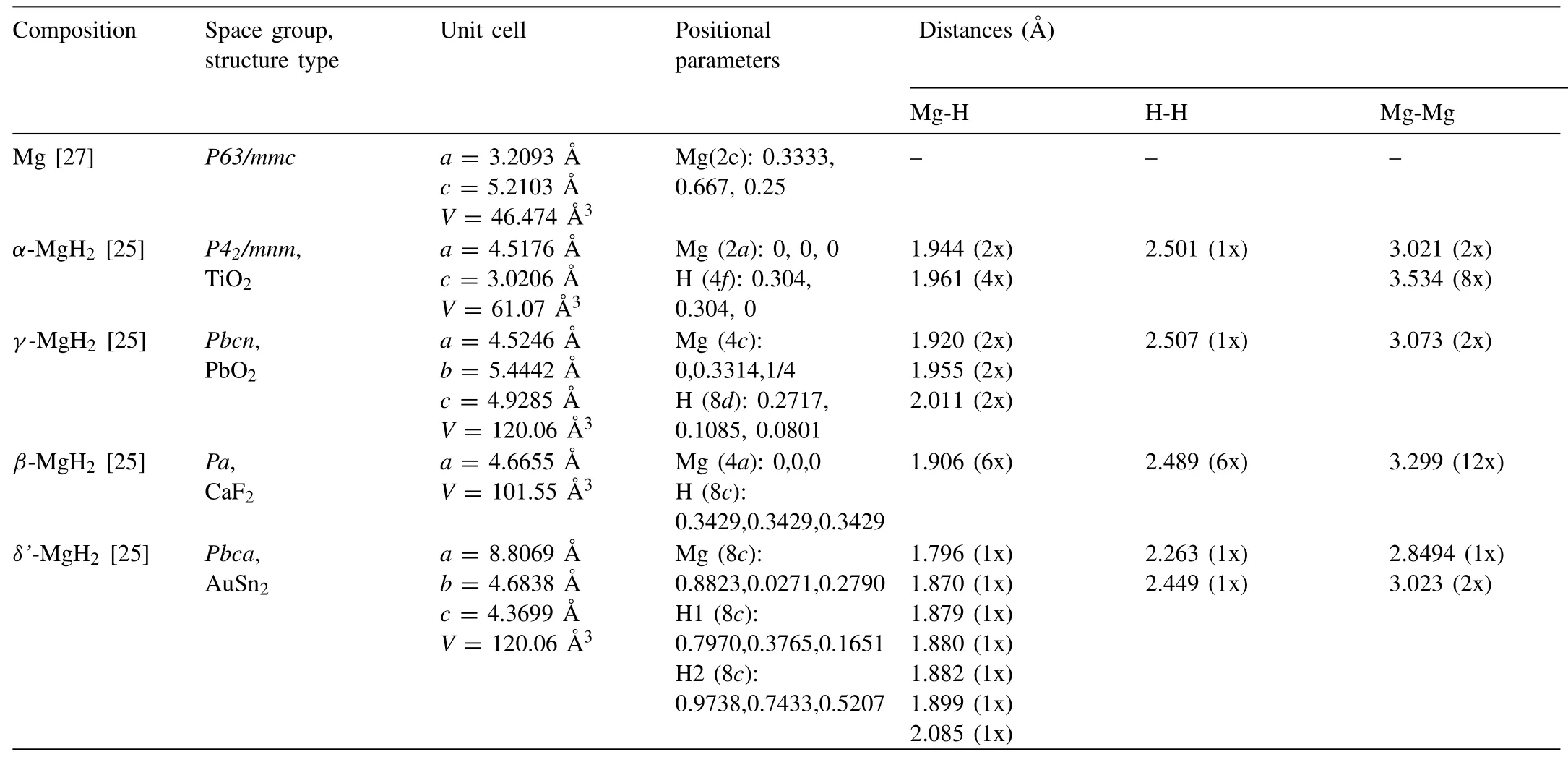
Table 1.1 Crystal structure data of Mg, α-MgH2, γ-MgH2, β-MgH2,and δ’-MgH2.
1.2.Thermodynamic properties of MgH2
When forming a metal hydride,a series of steps would occur:(1)physisorption,i.e.the attraction of Van der Waals forces between the metal surface and hydrogen molecules traps the hydrogen molecules in a volume close to the metal;(2)chemisorption,i.e.the formation of the metal-hydrogen bond after the dissociation of the hydrogen molecules at the metal surface;(3)the formation of anα-phase,i.e.the hydrogen diffuses to the metal’s interstitial sites and randomly occupies them;(4)the formation ofβ-phase,i.e.the concentration of the hydrogen atoms in the crystal lattice reaches a critical value that lead to the formation of a new phase with peculiar physicochemical properties;(5)the growth of theβ-phase and the gradual disappearance ofα-phase.As shown in Fig.2,the thermodynamics of hydrides formation can be described by pressure-concentration-temperature(PCT)plots [28].At the beginning of the hydrogenation process,some hydrogen atoms dissolve into the host metal to form a solid solution,e.g.αphase.With the increase of H2pressure and H concentration within theα-phase,the local interactions between hydrogen atoms and the atoms of hosting lattice lead to the nucleation and growth of hydride(β-phase).The isotherms exhibit a flat plateau when the two phases coexist,and the length of the plateau indicates that the amount of H2that can be stored.With the increasing of the temperature under which the PCT measurement is performed,the plateau length decreases.The transition fromα-phase toβ-phase is continuous above the critical pointTC.
Some of the most important parameters in the selection of hydrogen storage materials are the∆Hand the∆Sof hydrogenation and dehydrogenation.Such information can be easily derived from the PCT plots using the so called van’t Hoff plot.This plot is obtained by plotting the logarithm of the plateau pressure(ln)versus T−1(Fig.2).
According to the van’t Hoff equation:

wherePis the equilibrium pressure,Tis temperature andRis the gas constant.The enthalpy and the entropy values can be obtained from the slope and the y-intercept,respectively.

Fig.1.(a)Calculated and(b)experimental cell volume vs.pressure relation for MgH2 with different structures [25].
It was Stampfer et al.[23]that first reported on the thermodynamic properties of MgH2.Subsequently,several other attempts to measure the MgH2thermodynamic properties were made [29–39].The results of these investigations are summarized in Table 1.2.Note that there are large discrepancies in the reported∆Hand∆Svalues probably due to inaccuracies in the followed experimental procedures and/or presence of contaminants in the investigated specimens.
For example,Paskevicius et al.[24]determined the thermodynamic properties of bulk and nano-sized MgH2viavan’t Hoff method,the resulting plots can be seen in Fig.3.In this graph the fugacity values of H2were plotted instead of the values of the pressure of H2.The calculated values are reported in Table 1.3.The∆Hand∆Svalues for the bulk MgH2are 74.1±0.4 kJ·mol−1H2and 133.4±0.7 J·K−1mol−1H2,respectively.While the∆Hvalues for the nanosized MgH2range from 71.2±0.5 to 74.8±0.4 kJ·mol−1H2,and the∆Svalues fall into a range from 129.6±0.8 to 134.7±0.7 J·K−1mol−1H2.Based on density of states(DOS)analysis,the formation∆Hfor MgH2varies with different crystal structures in the order[41]:β-MgH2<γ-MgH2<α-MgH2.
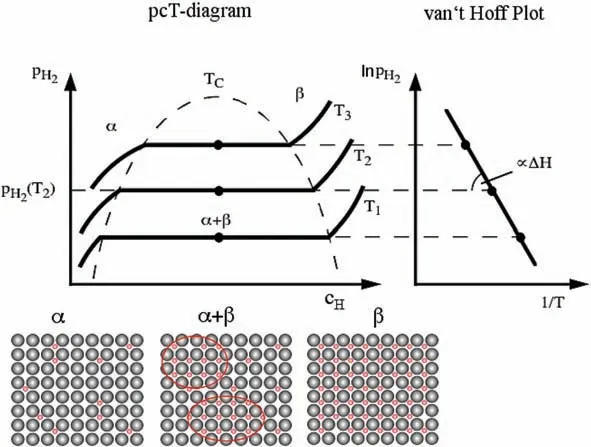
Fig.2.Schematic PCT diagram and corresponding van’t Hoff plot [28].
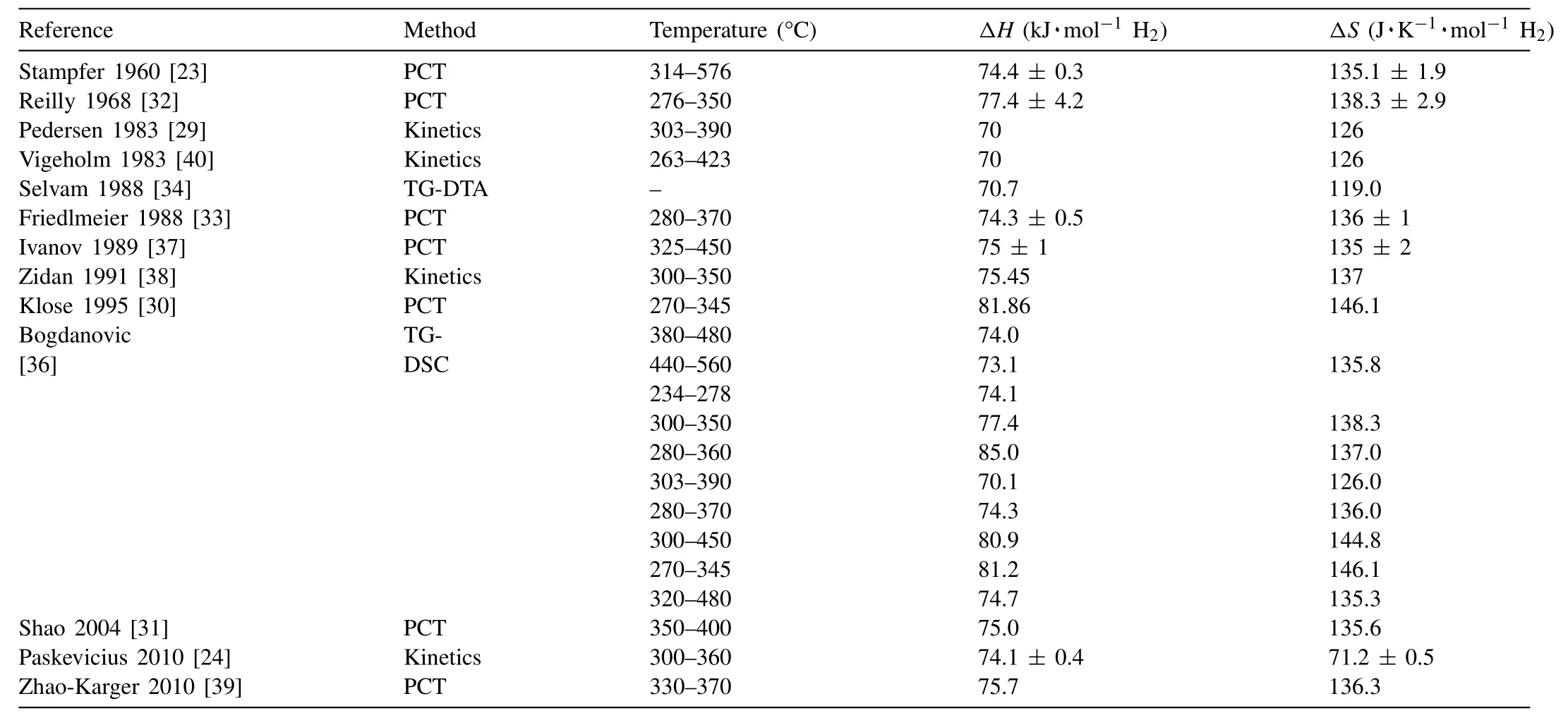
Table 1.2 Reported thermodynamic data of desorption/absorption of MgH2/Mg.
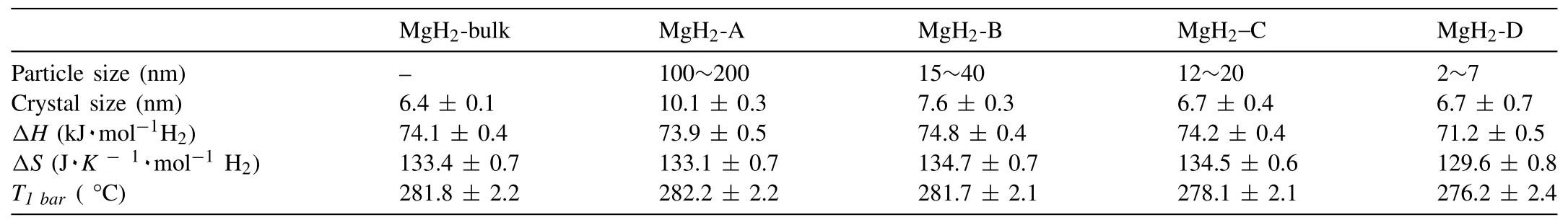
Table 1.3 The equilibrium pressures at different temperatures and thermodynamic properties of decompositions of bulk and nano-sized magnesium hydrides [24].
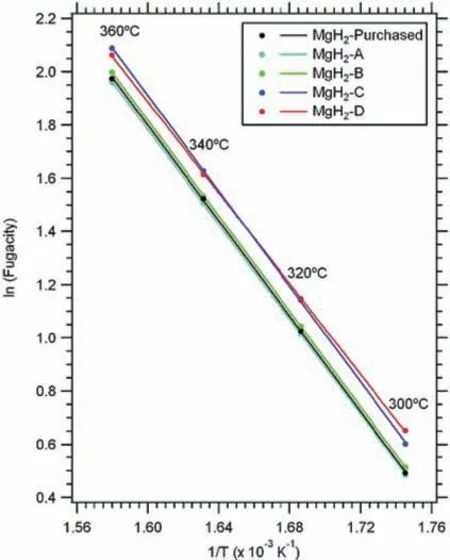
Fig.3.The decomposition van’t Hoff plots of bulk(MgH2-as-purchased)and nano-sized MgH2(MgH2-A,-B,-C,and–D),where A,B,C,and D refer to different particle sizes,with an order of:A > B > C > D [24].
1.3.Hydrogenation and dehydrogenation in Mg/MgH2
The kinetic properties of a hydrogen storage material must be taken into account when selecting a potential hydrogen storage system.In fact,obtaining fast hydrogenation/dehydrogenation kinetics is a key issue for many hydrogen storage materials among with MgH2.Pedersen et al.[29]found that 6.4 wt.% of hydrogen can be absorbed in Mg at 363 °C under 22.7 bar H2in 1500s,while about 6.0 wt.%of H2can be desorbed at 390 °C under 1.5 bar H2in 500 s.The reversible hydrogen storage capacity of MgH2strongly depends on the material preparation methods,applied conditions and use of additives [42-44].The measured reversible hydrogen storage capacity of pure Mg [45]is about 75% of the theoretical capacity,when performing hydrogenation at 375 °C and 40 bar H2,and dehydrogenation at 350 °C under vacuum.However,after 70 cycles,the hydrogen capacity is reduced by to 70% of the theoretical value.
In the following,the activation energy and limiting steps of the hydrogenation/dehydrogenation process of Mg/MgH2will be discussed.For a reaction to occur,an excess energy should be initially provided to the atoms and molecules composing the system.This excess energy is called activation energy and it is indicated asEa.There are two widely used methods for the determination ofEa.One is the Kissinger method[46],which by determining the peak maximum in calorimetric measurements performed at several heating rates allows calculating the processEausing the following equation [46]:
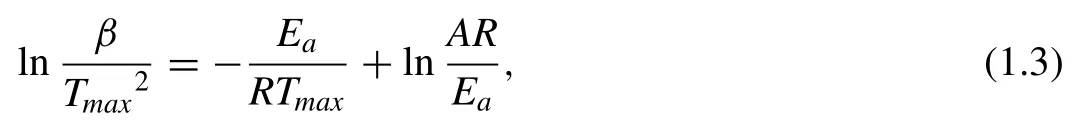
whereβis the heating rates,Ais the pre-exponential factor,andTmaxis the temperature at the peak of the calorimetric curve.The activation energy value can be identified by the slope of the linear fit of the ln(β•Tmax−2)versusT−1plot.
The other way is ascertaining the rate constant for different sorption measurements at different temperatures.Considering the pressure variation caused by temperature in an activated kinetic process,the rate constant,k,can be described by an expended Arrhenius equation [47–49]:

whereAis the pre-exponential factor.ThenEacan be determined by the Arrhenius plot of lnkversus1·T−1.
Many attempts for determining theEavalue for desorption and absorption of MgH2/Mg have been done,providing a broad range of activation energies for the absorption/desorption processes(90–130 kJ·mol−1H2for absorption,120–195.3 kJ·mol−1H2for desorption)[39,50-58].For example,Fernandez et al.[50]calculated the activation energy for desorption and absorption of MgH2/Mg.As shown in Fig.4,theEafor desorption is 160±10 kJ·mol−1H2,while theEafor absorption is 90±10 kJ·mol−1H2,which is 1.8 times smaller than that of desorption.It is known that the activation energy changes with the MgH2/Mg particle size dimension [58–60].For instance,Huot et al.[58]found that for the nano-sized(10–20 nm)MgH2,Ea=120 kJ·mol−1H2,which is lower thanEa=156 kJ·mol−1H2of the MgH2with a particle size of 20μm.However,the low activation energy of the nano-sized MgH2could not be preserved upon cycling due to the coarsening of Mg/ MgH2particles.
As previously mentioned,hydrogen absorption or desorption reactions occur through several distinct steps including the surface reaction,dissociation and chemisorption,hydrogen diffusion,as well as the new-phase formation [61].It is generally accepted that the overall hydrogen sorption kinetics is determined by the slowest step in the process of absorption/desorption [62].For the absorption of Mg,the ratedetermining step is the diffusion process [50],e.g.hydrogen diffusion through the MgO/Mg(OH)2layer on the topmost surface at the beginning of the hydrogenation and through the MgH2layer once the hydrogenation progress.Wu et al.[63]studied the penetration of H2through the Mg with a top layer of MgO by density functional theory(DFT).Hydrogen can pass through the top layer to the sublayers of MgO,and then diffuse into the Mg structure.During the hydrogen diffusion process,the most favorable positions for hydrogen dissociation are the interstitial octahedral sites of MgO.As shown in Fig.5,the hydrogen diffusion barrier between surface and subsurface is 1.52 eV,while that for subsurface 1(SB1)to inner subsurface 2(SB2)is 1.02 eV,and from SB2 to inner subsurface 3(SB3)is 1.2 eV,which indicating that when the hydrogen penetrates the surface,the further diffusion will be easier.
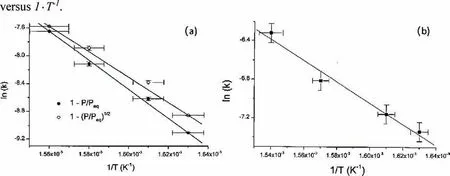
Fig.4.Arrhenius plots of(a)desorption rate and(b)absorption rate of MgH2/Mg [50].
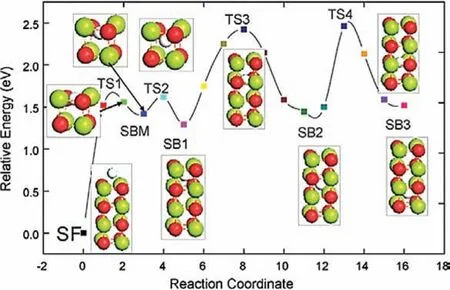
Fig.5.The H diffusion minimum-energy path from surface to subsurface,where SB means subsurface,SF means surface,TS and SBM are some intermediate images during the H diffusion process [63].
Thus,at the beginning of the hydrogenation reaction,the presence of an oxide layer presents a barrier which is difficult to overcome.The hydrogenation kinetics can be improved by breaking the oxide layer in several ways [61,64],such as tailoring the microstructures,controlling the outer dimensions of the material,and using selected additives.Once a MgH2layer is formed,this will constitute an additional barrier to the diffusion of hydrogen into the bulk Mg,since the hydrogen diffusion coefficient in MgH2is 1.1×10−20m2·s−1at 32°C,which is much lower than that in Mg(7 × 10−11m2·s−1at 27 °C)[65,66].
For better understanding the hydrogen absorption/desorption kinetic mechanism in hydrogen storage materials the determination of the rate limiting step is of crucial importance.In order to gains such a piece of information the modeling of the hydrogenation/dehydrogenation curves can be done,which is of great importance to further improve the hydrogen storage properties regarding to the rate that the material can absorb/desorb hydrogen and keep a desired capacity.Furthermore,kinetic models are convincing strategies to understand the in-depth kinetic mechanism.There are many kinetic models and analysis methods used for practical application,as shown in Table 1.4.These models and corresponding analysis methods have provided some foundations for enhancing the kinetics of solid-state hydrogen storage materials.
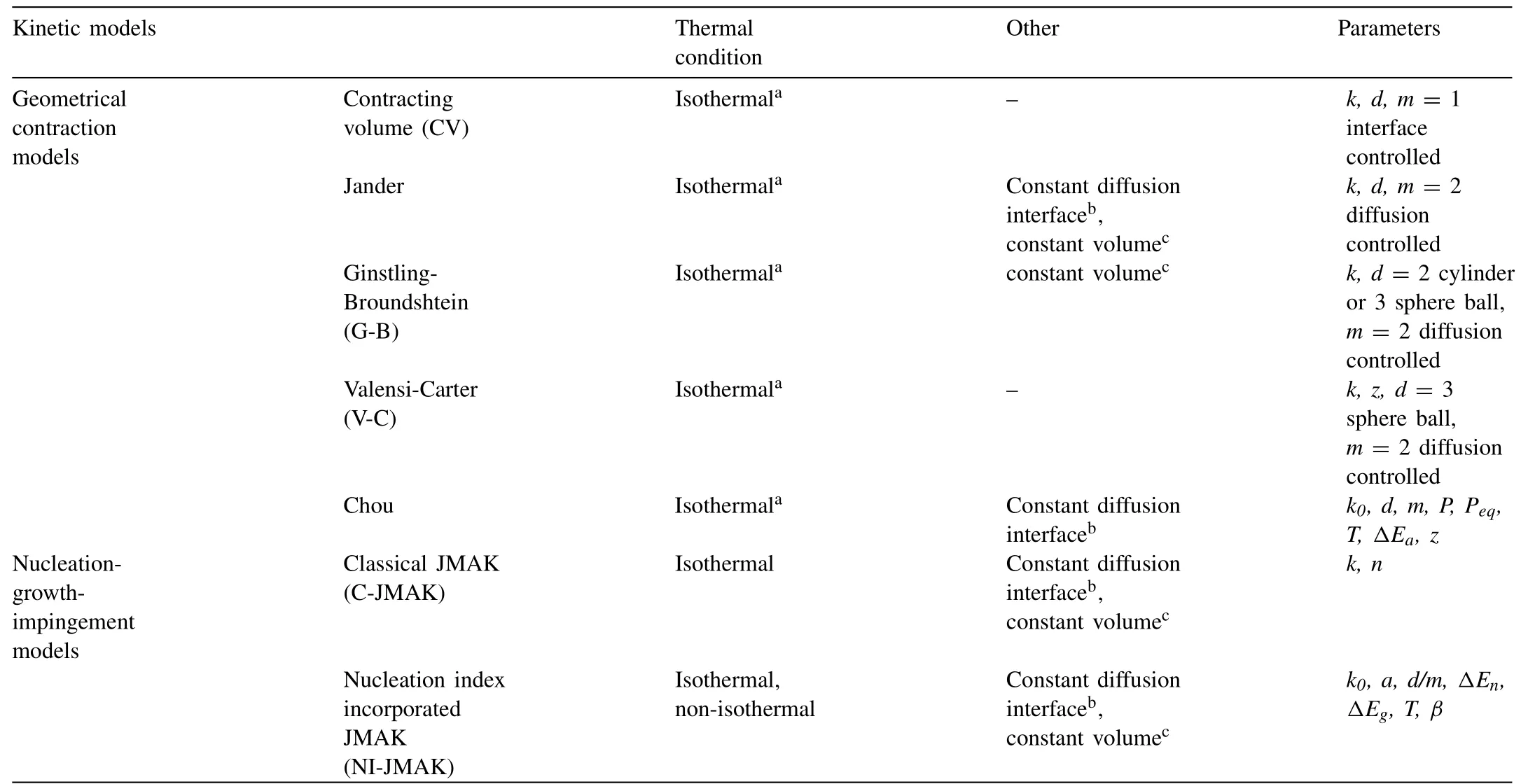
Table 1.4 The kinetic models used in the hydrogen storage materials [67].
For MgH2,Jensen et al.[68]studied the dehydrogenation kinetics by JMAK model as shown following:

wheretis the time,α(t)is the reaction fraction,ηis the Avrami exponent.To elucidate the effect of temperature variations,the Avrami exponent can be fixed toη=4.By fitting the experimental data with the JMAK model,the rate constant can be obtained,indicating that the rate-limiting stepfor dehydrogenation of MgH2is the nucleation and growth of Mg.
Shao et al.[69]investigated the hydrogenation kinetic behaviors of MgH2.The Jander model,as shown in the following equation [67]is used to simulate the absorption curve for the commercial MgH2:
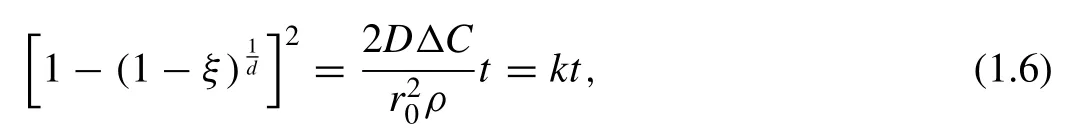
wheretis the time,kis the rate constant,ρis the material density,Dis the diffusion coefficient,∆Cis the concentration difference,ξis the fraction of reaction,r0is the radius of the particle,dis the dimensionality.
Under 100 bar hydrogen,at 300 °C,325 °C,and 350 °C,the hydrogenation behaviors fit the Jander model well,indicating that the rate-limiting step for hydrogenation is the diffusion of hydrogen through the MgH2.Thus the dehydrogenation/hydrogenation kinetics can be simulated by JMAK and Jander models,respectively,as summarized in Table.1.5.
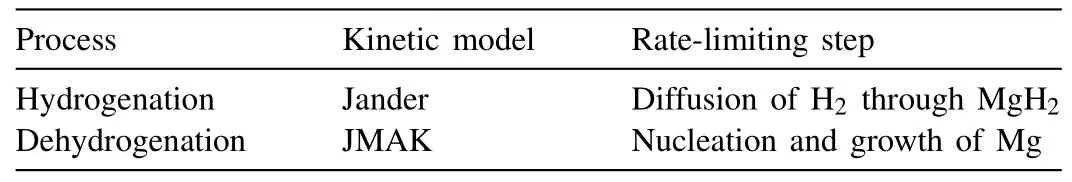
Table.1.5 Kinetic models of hydrogenation/dehydrogenation of MgH2 [58,68,69].
2.Effects of O2 and H2O on the hydrogen storage properties of Mg-based materials
Mg-based materials,are known to readily react with atmospheric O2and H2O to form a dense MgO/Mg(OH)2layer onthe outmost exposed surface.This layer,usually has a thickness of few nanometers and once formed will prevent the material from further reacting with O2and H2O [70,71].Moreover,rate of formation for the oxide layer is much faster than that of hydroxide layer [72].Splinter et al.found that the O2adsorption rate on Mg surface grains with open-packed and high index crystallographic orientations is faster than other grains [73].Based on different grades of exposure to water vapor and humid laboratory air,the oxidation kinetics of Mg can be divided into three stages:the original dissociative adsorption of water on magnesium surfaces;fast nucleation of oxide and growth by islands;and the oxide layer continues to be thickened [74].
Previously,hydrogen storage properties of Mg-based materials exposed to O2and H2O have been extensively studied[63,75–80].Friedrichs et al.[81]studied the oxygen passivation behavior of nanocrystalline Mg.Formation of a 3–4 nm thick oxide layer on top of the Mg particles was observedviahigh resolution transmission electron microscopy(HRTEM).On one hand,the presence of O2can deteriorate the hydrogen capacity [82,83].Pedersen et al.[82]found that the Mg powder exposed to 0.5%O2has about 20%permanent loss of the initial capacity.On the other hand,small amount of MgO can enhance the absorption and desorption kinetics due to the catalytic effects [84,85].Hjort et al.[84]found that the Mg films that are exposed to small amount of O2(150 ng·cm−2)have higher H2absorption rate,but when the amount of oxygen is larger,the absorption rate is slower,due to the formed thick oxide layer.
However,it should be noted that the positive effects of O2and H2O on the hydrogen storage properties of Mg-based materials are only possible when the oxygen or water contents are very small.When a certain critical value is reached,the samples will be no more interesting for hydrogen storage[84].
3.Achievements on MgH2 in the last 30 years
MgH2has been considered during the past decades as a potential lightweight and low-cost hydrogen storage material[86–89].However,due to its high thermodynamic stability(∆Hof 74.1±0.4 kJ·mol−1for MgH2)[24]and poor kinetic properties,the hydrogen stored in magnesium hydride is only accessible at high temperatures(e.g.≥300 °C)thorough lengthy reactions [19].Thus,in order to allow a widespread use of magnesium hydride in hydrogen storage applications,tuning its thermodynamics and kinetics is mandatory [69,90–94].Several approaches have been pursued for this purpose.
One of the most widely applied approaches to tune the kinetic properties of Mg-based hydrogen storage materials is the use of additives.The first report on this topic appeared in the early 1960s [95,96].In the period 1970s–2000s [55,97-103],great achievements were obtained on enhancing the kinetic properties of MgH2viathe use of selected additives such as Nb2O5[104],symbiotic CeH2.73/CeO2[105],ZrF4[106],TiCl3[106],carbon scaffolds [107,108]etc.At the same time,the kinetics of MgH2can be enhanced by modifying its microstructuree.g.viahigh energy ball milling.In 1961,Dymova et al.[109]milled Mg powder in a hydrogen atmosphere(i.e.200 bar).The yield of formation for MgH2after a ball milling time of about 6 h is 75%.Under the same experimental conditions,the yield is increased to 97% when small amounts of additives(i.e.0.5–3 wt.% I2,CCl4and Mg2Cu)are used.By breaking the layer of formed MgH2during ball milling,fresh Mg surfaces can be created continuously,which enhances the reaction rate for the formation of MgH2[110,111].Nowadays,ball milling is one of the most widely used method in the preparation of Mg-based hydrogen materials [19,112–114].Alternative synthesis methods are also implementede.g.hydrogen plasma-based technology[115],severe plastic deformation [116,117],or chemical vapor deposition[118].Using these methods,MgH2can be synthesized in the form of nanopowders [58],thin films [119],nanowires or nanofibers [118],and nanocrystals [120].Although these methods allow high purity MgH2synthesis in different forms,they are not suitable for large scale production[86].Recently[111,121-124],several improvements were made on the development of synthesis methods of MgH2.A brief description of the most prominent approaches and achieved results are given in the following section.
3.1.Effect of the preparation methods
3.1.1.Ball milling
Ball milling is a technique extensively used in the preparation of hydrogen storage materials,which can reduce the material particle and crystallite sizes,finely disperse additives,as well as provide fresh and highly reactive surfaces [125,126].Ball milling of Mg particles alone or in the presence of additives can lead to a particle sizes in the submicron scale[127–129].As an example,in Fig.6,the scanning electron microscope(SEM)micrographs of MgH2particles before and after 1 h of ball milling can be seen.
Hanada et al.[130]studied the correlation between hydrogen desorption performance and microstructure of MgH2during the ball-milling process.After 2 h of ball milling,the hydrogen desorption temperature was lowered,but the hydrogen storage capacity decreased from 7.3 wt.% to 6.1 wt.%.This behavior probably results from the increased grain-boundary regions,since the bulk Mg can absorb more hydrogen than the Mg located at the grain boundary.[130].Varin et al.[131]studied the effects of particle size,grain size and presence of theγ-MgH2phase on the desorption properties of nanocrystalline MgH2produced by controlled mechanical milling(Table 3.1).In this study,dehydrogenation peak temperatures decrease slowly with the decreasing MgH2particle size.For example,onset and peak temperature of desorption for the as received MgH2are 406 and 415 °C,respectively.For the MgH2possessing a particle size distribution between 350 and 600 nm,the desorption temperatures decrease of about 50 °C.It must be mentioned that for the MgH2ball milled for 10 h or longer,the formedγ-MgH2appears to act as a catalyst,which helps to reduce the desorption temperature about 30–60 °C.However,upon cycling,the benefits gained by ball milling slowly disappear due to particle coarsening [132].
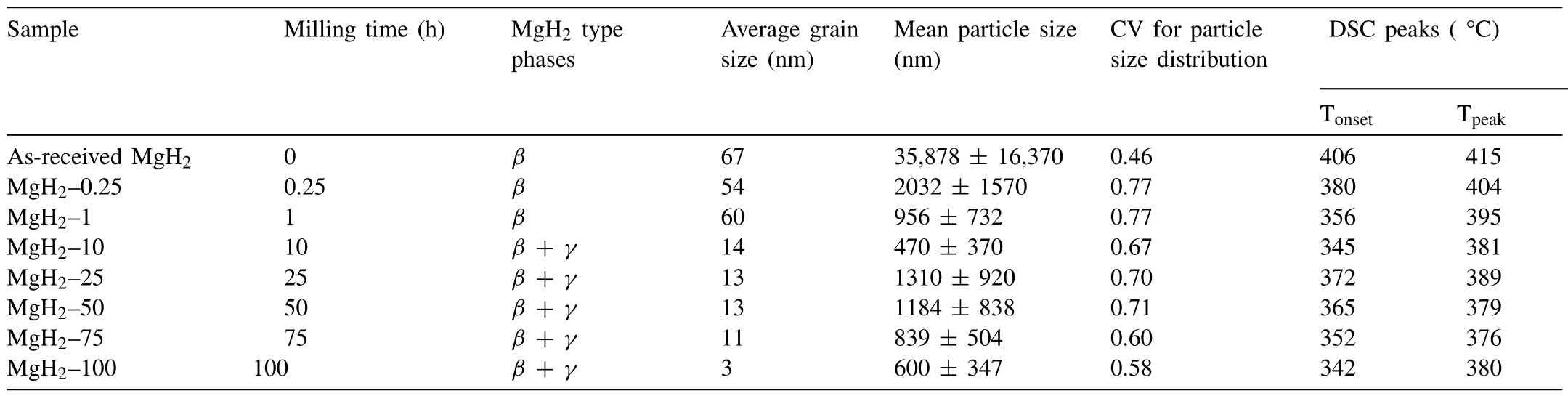
Table 3.1 Desorption onset and peak temperatures of ball milled MgH2 with different time [131].
3.1.2.Thin films
The manufacturing of pure Mg thin films and Mg multilayered films can provide large surface areas,leading to fast hydrogenation/dehydrogenation rates [133].The Mg-based thin films can be synthesized through electron beam evaporation [134,135],plasma sputter deposition [136],direct current magnetron sputtering deposition [137],radio frequency magnetron sputtering deposition[138],and pulsed laser deposition[139],with a broad range of thicknesses.
In a recent work,Hadjixenophontos et al.[140]studied the H2diffusion process in Mg thin films deposited on Si substrates.In order to prevent detrimental oxidation reactions,Mg was covered with a Pd layer [65,141].Fig.7 shows the transmission electron microscopy(TEM)results which provide some insights into the hydrogenation process of the Mg film.The samples were fully hydrogenated after 1 h at 150°C and 5 bar H2pressure.The starting Mg film as well as the forming MgH2were both crystalline.The formed MgH2layer had a thickness of 400 nm.The MgH2was nucleated at the interface between Pd and Mg,then it grew uniformly towards the substrate.The kinetic study [142]performed at 200 °C shows that the limiting factor for the hydrogenation of Mg is the diffusion of H2through the Pd/Mg or MgH2/Mg interface.
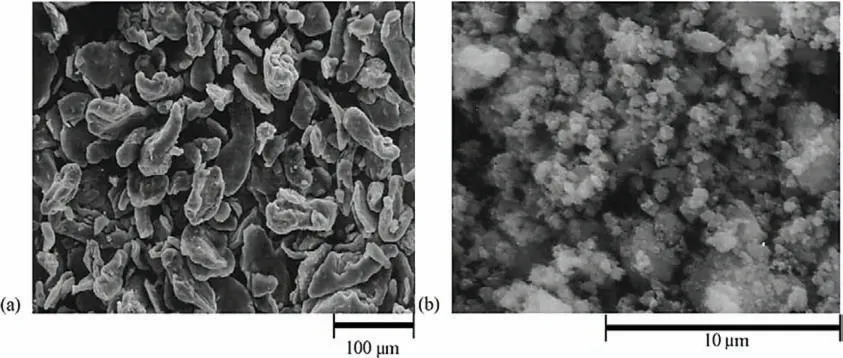
Fig.6.SEM images of MgH2 powder(a)before ball milling and(b)after ball milling for 1 h [130].

Fig.7.TEM images of cross sections for(a)as deposited;(b)half hydrogenated;and(c)fully hydrogenated Mg thin films [140].
Qu et al.[143]investigated the hydrogen absorption kinetics of Mg-Pd thin films possessing Mg layer thicknesses in the range of 20 to 100 nm and temperatures between 25 and 65 °C.With the decrease of thickness,the hydrogen diffusion coefficient and hydrogen absorption flux of Mg thin films increase.The absorption kinetics are improved significantly especially when the thickness is lower than 60 nm.For example,the Mg film with a thickness of 20 nm can store up to 5.5 wt.% of hydrogen at 25 °C at a pressure of 0.7 bar H2.The formation of MgH2at Pd/Mg interface and the hydrogen diffusion into the Mg layer are the critical factors determining the kinetics of the system.Baldi et al.[144]observed that the plateau width of the PCT curves(which represents the re-versible hydrogen storage capacity)of Mg capped with a Pd layer deteriorates significantly after 4 cycles at 60 °C due to the formation of Mg-Pd alloys.However,the hydrogen storage capacity of the Pd-capped MgH2with a 2 nm Ti-buffer layer in between decreases only slightly during cycling,indicating that the addition of a buffer layer can prevent the alloying of Mg and Pd at interfaces.The addition of a Tibuffer layer decreases the equilibrium hydrogen pressure of the Pd-capped MgH2significantly at 60°C.These results suggest that the cycling stability and thermodynamics of MgH2thin film can be tuned by modifying the architecture of the films.
Due to the existence of abundant and continuous interfaces,the impact of strain on hydrogen storage properties in thin films is more visible than in nanoparticles [145].Baldi et al.[141]destabilized the Mg-H system by elastic constraints.By means of elastic clamping,Mg films that covered by Pd,Ni and other Mg-alloy-forming elements exhibit 1 order of magnitude higher hydrogen plateau pressures of around 5 × 10−3bar than the bulk Mg(about 1.2 × 10−4bar)[146],indicating that the thermodynamics can be tuned by elastic stress/strain.
3.1.3.Nanowires and nanofibers
One strategy to reduce the particle size of MgH2is to fabricate it in the shape of nanowires or nanofibers [119].Li et al.[147]prepared Mg nanowires by a vapor-transport approach using commercial Mg powders under Ar flow.The SEM and TEM micrographs of the obtained nanowires are shown in Fig.8.Fig.8(a)and(b)show the morphology of the as-synthesized Mg nanowires prepared with an Ar gas flow of 200 cm3·min−1.The nanowires have average size of 40 nm in diameter and several micrometers in length.Fig.8(c)and(d)show Mg nanowires prepared with an Ar gas flow of 300 cm3·min−1.These nanowires have a diameter of 80–100 nm,which begin to be tangled together,showing a zigzag morphology.The Mg nanowires prepared with an Ar gas flow of 200 cm3·min−1are shown in Fig.8(e)and(f).These nanowires have a diameter of 150–170 nm,exhibiting short rod-like structure.The TEM and HRTEM images of the Mg nanowires prepared with an Ar gas flow of 200 cm3·min−1(Fig.9(g-i))indicate that the nanowires grow along the[001]direction.The hydrogenation/dehydrogenation kinetics performed at 200 and 300 °C indicate a clear correlation between the enthalpies,activation energies and thickness.The dehydrogenation ∆Hof nanowires with diameters of 30–50 nm,80–100 nm,and 150–170 nm are 65.3,65.9,and 67.2 kJ·mol−1H2,respectively,while the dehydrogenation activation energies are 38.8,46.5,and 81.1 kJ·mol−1H2,respectively.
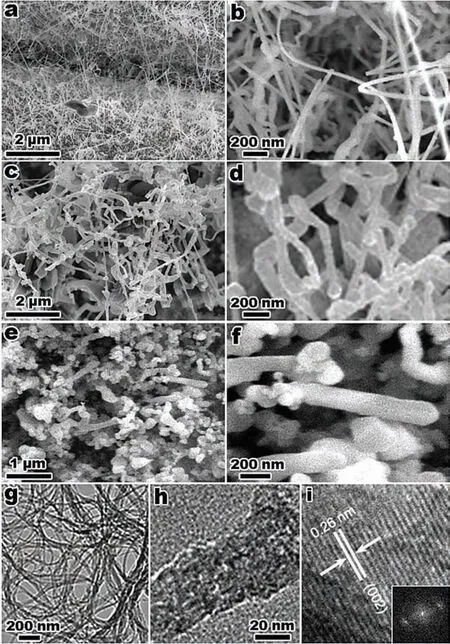
Fig.8.SEM images of(a)and(b)Mg nanowires prepared with an Ar gas flow of 200 cm3·min−1;(c)and(d):Mg nanowires prepared with an Ar gas flow of 300 cm3·min−1;(e)and(f):Mg nanowires prepared with an Ar gas flow of 400 cm3·min−1.corresponding(g),(h)TEM images and(i)HRTEM pattern of the Mg nanowires prepared with an Ar gas flow of 200 cm3·min−1,the inset in(i)is the corresponding pattern [147].
Sun et al.[148]synthesized Mg nanofibers with a width of 40 nm by direct reduction of MgBu2by Ca.The typical microstructure of the nanofibers can be seen in Fig.9.Fig.9(a)and(b)show the nanofibers with an average diameter of 40 nm,and lengths between 0.4 to 4 μm.Fig.9(c),(d)and(f)show that besides Mg phase,the nanofibers contain also small amounts of MgO and Ca.The structures of Mg nanofibers remain stable upon cycling.The Mg nanofibers show fast absorption kinetics at 300 °C and 350 °C(90%overall H2capacity in 20 min),as well as fast desorption kinetics at 350 °C(90% overall H2capacity in 20 min).The measured hydrogenation/dehydrogenation ∆Hand ∆Sare 67.6±5.3/103.1±2.7 kJ·mol−1,123.3±9.3/123.3±4.6 J·K−1·mol−1H2,respectively.However,these data might be affected by the presence of Ca particles that could lead to the formation of CaH2during hydrogenation and/or to the formation of Mg8Ca19H54(a compound more stable than MgH2).
3.1.4.Hydrogen plasma-based technology
Hydrogen plasma-based technology is a nanomaterialfabrication method.Besides achieving extremely high purity,this method also allows to control the particle sizes [149].Shao et al.[31]synthesized Mg particles by hydrogen plasmabased technology.The typical morphology of the synthesized Mg particles is shown in Fig.10(a).The Mg is hexangular shaped,and has a particle size distribution ranging from 50 to 500 nm,with an average particle size of 300 nm.After hydrogenation under 40 bar H2at 350 °C,formed MgH2(Fig.10(b))has particle size distribution between 20 and 600 nm,with an average size value of 300 nm.Fig.10(c)shows that after dehydrogenation,the surface of the newly formed Mg particles present several cracks compared to the starting Mg particles.These particles absorb 7.51 wt.% hydrogen under 40 bar H2in 65 min at 400 °C in the first cycle,and then absorb 7.55 wt.% hydrogen in 8 min at 400 °C,in 20 min at 350 °C,and in 30 min at 300 °C.The small particle size and large surface area are considered to be the main reasons for the excellent kinetic properties displayed by these specimens.
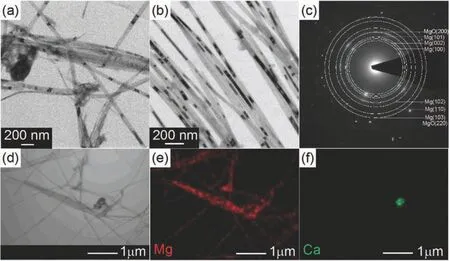
Fig.9.(a),(b)Typical TEM images;(c)corresponding SAED pattern;(d)scanning transmission electron microscopy(STEM)image;and(e),(f)associated elemental mapping of the synthesized Mg nanofibers [148].

Fig.10.Bright field TEM images of(a)Mg ultrafine particles before hydrogen absorption;(b)formed MgH2 ultrafine particles after one hydrogenation/dehydrogenation at 400 °C,then absorption under 40 bar H2 at 350 °C,the inset is the corresponding SAED pattern;(c)Mg ultrafine particles after desorption,the inset is the corresponding SAED pattern [31].
3.1.5.Severe plastic deformation
Severe plastic deformation(SPD)method has been also investigated as a potential method to process cost effective Mg-based hydrogen storage materials in a relatively short time [150,151].There are four different SPD approaches to synthesize hydrogen storage materials,e.g.high-pressure torsion(HPT)[152],extensive cold rolling(CR)[153],extensive cold forging(CF)[116],and equal channel angular pressing(ECAP)[154].HPT refers to the processing method that occurs under simultaneous compressive and torsional forces[155].CR refers to the processing method where metal sheets are shed between rollers and then compressed at temperatures lower than the recrystallization temperature of the metal[156].CF refers to the manufacturing method where a metal rod is fed into a die and extruded at room temperature,changing its original shape until it fits the shape of the die [157].
Leiva et al.[120]studied the hydrogen absorption behavior of commercial Mg processed by HPT,CR,and CF.At 350°C and in a hydrogen atmosphere of 20 bar the sample prepared by CR absorbed 5.5 wt.% hydrogen in 20 h.The sample prepared by HPT absorbed 3.9 wt.% H2in 45 h,and the sample produced by CF absorbed 1.0 wt.% H2in 45 h.The results indicate that,compared to the samples prepared by HPT and CF,the samples prepared by CR possessed superior hydrogen storage properties.As shown in Fig.11(a),the asreceived Mg has an average grain size in milimeter range.The TEM images of Fig.11(b-d)show the Mg after CF,HPT,and CR treatments,respectively.By applying these methods,the grains were refined in the order of CF>HPT>CR.As the consequence of the applied forces,dislocation tangles and deformation bands are detected in the refined grains of Mg.Moreover,all materials after deformation show a fiber type(002)texture.
ECAP [158,159]is an effective method for producing nanostructured Mg,through extrusion.Some microstructural features of Mg after ECAP processing can be seen in Fig.12 [160].Fig.12(a)shows that the Mg billets pressed at 300 °C at a ram speed of 3 mm·min−1have some recrystallized grains with an average size of 50 nm,and a substructure formed based on the old grain boundaries.The Mg billets pressed at 300 °C at a ram speed of 25 mm·min−1exhibits a strong texture in the(101)direction,as can be seen in Fig.12(b).To enhance the hydrogen storage properties of the obtained materials,a subsequent CR treatment was carried out.The obtained materials show strong(002)textures,where the Mg billets pressed at 300 °C at a ram speed of 3 mm·min−1with combination of subsequent CR can absorb 5.7 wt.% hydrogen,and the billets prepared at a ram speed of 25 mm·min−1with combination of subsequent CR can absorb 4.1 wt.% hydrogen.The results indicate that the(101)texture is unfavorable for hydrogen storage properties.These findings indicate that the hydrogen storage properties of Mg can be modified by altering its microstructure.
3.2.Additives
A widely applied method to enhance the of hydrogen storage properties is to use a suitable additive.While choosing suitable additives for enhancing the hydrogenation/dehydrogenation properties of MgH2,three important factors must be taken into accounti.e.the nature of additives,their size and distribution,and their stability.In the following,the research related to the utilization of non-metal additives[161–168],metals and their oxides [169–173],metal halides and fluorides are briefly discussed [174–176].
Among the non-metal-based additives,carbon-based system [161–165]are the most utilized materials.This class of additives includes graphite,carbon nanofibers,carbon nanotubes,and graphene.As an example,the addition of graphene plates to Mgviaball milling at the presence of organic solvents sensibly enhances the hydrogenation kinetics and reversibility of Mg by reducing agglomeration phenomena [177].Ruse et al.[166]demonstrated the possibility to tune the hydrogenation/dehydrogenation kinetic behavior of Mg by mixing it with different nanocarbon structures,e.g.the 1D carbon nanotubes,2D graphene nanoplates and 3D activated carbon,thus suggesting that the microstructural features of carbon based additives should be taken into account when selecting and/or designing high performance hydrogen storage materials.Furthermore,such carbon based additives offer the additional advantage to increase the thermal conductivity of Mg and MgH2powders or powder pellets,which is important for the construction of hydrogen storage systems.
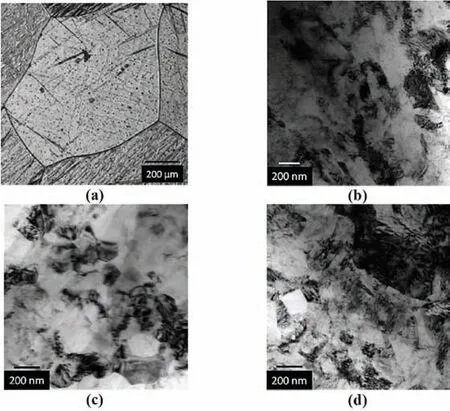
Fig.11.(a)Optical micrograph of as received Mg.TEM images of Mg after(a)CF;(b)HPT;and(c)CR [120].
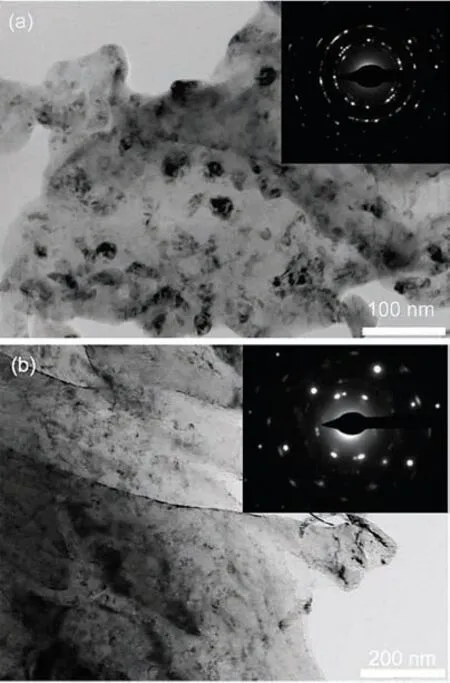
Fig.12.Bright field TEM images of the Mg billets pressed at 300 °C at ram speeds of(a)3 mm·min−1 and(b)25 mm·min−1 prepared by ECAP,respectively.The insets are the corresponding SAED patterns [160].
Transition metals and their oxides are known to be excellent catalysts for Mg-based materials [169–173].Hanada et al.[178]studied the catalytic effect of 3d-transition metal particles of Fe,Co,Ni,and Cu on the hydrogen storage properties of MgH2.All investigated additives improved the dehydrogenation properties of MgH2,especially Ni.2 mol%Ni-doped MgH2is able to desorb 6.5 wt.% of H2in the temperature range of 150–250 °C.Among metal oxides,Nb2O5is one of the most effective catalysts for MgH2.The first report on the use of Nb2O5for improving the kinetic properties of MgH2was made by Barkhordarian et al.[179]in 2003.Compared with other metal oxide-based catalysts,e.g.Fe3O4,and Cr2O3,Nb2O5allows achieving relatively faster reaction kinetics [180].0.2 mol% of Nb2O5containing MgH2can absorb 7 wt.% of hydrogen in 60 s and desorb the same capacity in 130 s at 300 °C.As a comparison,0.2 mol%Cr2O3added MgH2can absorb only 5.9 wt.% of hydrogen in 60 s and desorb in 370 s.The positive effects of Nb2O5on the hydrogen storage properties of MgH2can be attributed to the following factors:(1)during milling Nb2O5can serve as dispersive agent,leading to the particle refinement of MgH2[181];(2)Nb2O5is relatively stable [182];(3)Nb2O5enhances the splitting of the H2molecules at the Mg/MgH2surface and the transportations of atomic hydrogen into the Mg/MgH2lattice [183];(4)the niobium has high interaction energy with hydrogen,leading to the improvement of hydrogen sorption properties [182].The effect of Nb2O5content(0.05,0.1,0.2,0.5,and 1 mol%)on hydrogen sorption properties shows that the reaction rates increase with the Nb2O5content and reach maximum with 1 mol% additive.Meanwhile,the desorption activation energy decreases with the increasing of Nb2O5content and reaches a plateau of 62 kJ·mol−1with 0.2 mol% additive,as shown in Fig.13.Moreover,the rate limiting steps in dehydrogenation are different depending on the Nb2O5content.At 250 °C,the desorption rate limiting step for the 0.05 and 0.1 mol% Nb2O5containing samples is three dimensional growth controlled,for the 0.2,0.5 and 1 mol% Nb2O5containing MgH2is interface controlled.Comparably,at 300 °C,the rate limiting step for 0.05 and 0.1 mol% Nb2O5added MgH2is three dimensional growth controlled,but for the 0.2,0.5 and 1 mol% Nb2O5doped MgH2is interface controlled.
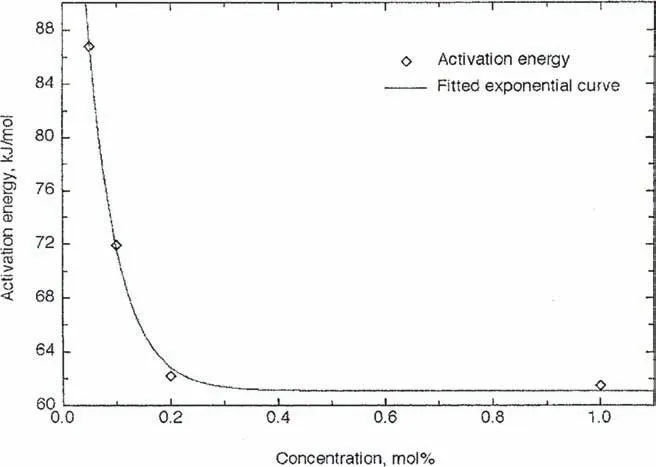
Fig.13.Activation energy versus Nb2O5 concentration for the desorption of doped MgH2 [180].
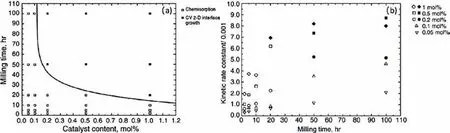
Fig.14.(a)The dependence of desorption kinetic rate limiting step on ball milling time and Nb2O5 catalyst content;(b)the relationship between the desorption kinetic rate constant and milling time with different Nb2O5 content [171].
Note that the hydrogenation/dehydrogenation kinetics of Nb2O5-doped MgH2can be also affected by the milling time[171].By increasing the milling time,the kinetics can be improved to a certain extent.The absorption kinetics were found to be controlled by diffusion,whereas chemisorption and CV 2-D interface growth models were found to control the desorption process,as shown in Fig.14.The results show that not only Nb2O5content,but also ball milling time have positive effects on the desorption kinetics.
Metal halides and fluorides can be also used as catalysts for improving kinetics of Mg-based hydrogen storage materials [174–176,184–192].Malka et al.[106]ball milled the MgH2with metal chlorides and fluorides,including ZrCl4,NbCl5,VCl3,TiCl3,CrCl3,BaCl2,ZrF4,NbF5,TaF5,TiF3,FeF2,FeF3,CdF3,CrF2,CeF3,NaF,and BaF2.Among these additives,the catalytic effects of ZrF4,TaF5,NbF5,VCl3and TiCl3were found to be the most relevant.As an example,the calculated desorption activation energies for ZrF4,TaF5,NbF5,VCl3and TiCl3are 77,97,83,97,and 85 kJ·mol−1H2,respectively,whereas the calculated value for the noncatalyzed MgH2is 152 kJ·mol−1H2[106].
A further example of fluorides utilized as additives for MgH2is CeF4.Lin et al.[187]found that by doping MgH2with 2 mol% of CeF4,the dehydrogenation temperature and activation energy are decreased from 393 °C to 273 °C(heating rate of 2 °C ·min−1)and from 162 kJ·mol−1H2to 111 kJ·mol−1H2,respectively.The observed kinetic enhancement was attributed to the presence of high valence Ce-cation and of Ce-F-Mg which should enhance the electron mobility at the MgH2/CeF4.
4.Hydrogen storage in magnesium alloys
Since 1960s,it is known that the thermodynamics of Mg can be modified by alloying it with several metal elements.This approach was firstly pursued by Mikheeva et al.[96]that introduced Ce and Al into the Mg matrix in 1963.Subsequently,Reilly et al.[32,193]studied the hydrogenation behavior of Mg-Cu and Mg-Ni alloys in 1967 and 1968.This method can be effectively applied to modify the thermodynamic properties and the storage capacity.
4.1.Mg alloys with p-elements(X=Si,Ge,Sn,and Al)
4.1.1.Si,Ge,and Sn
In the group IV,elements such as Si,Ge,and Sn can form intermetallic compounds with Mg [194–197].The reactions can be described by the following equations:
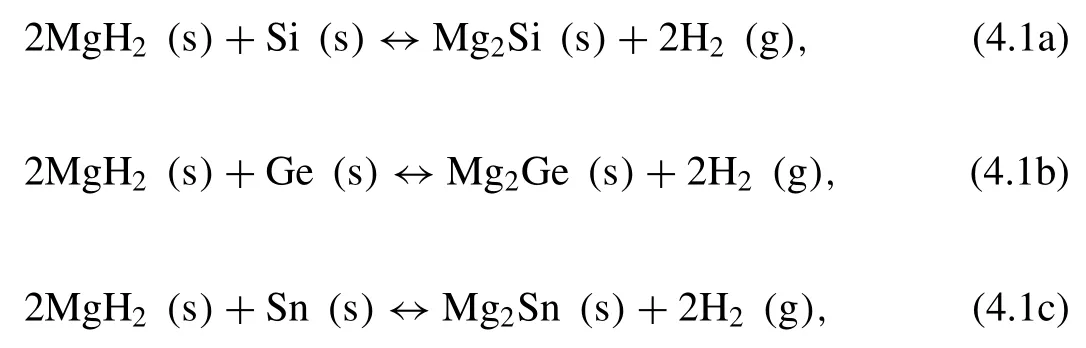
where the∆Hare 36.4,14,and 44.5 kJ·mol−1H2,respectively [194,196,198,199].Chaudhary et al.[200]studied the thermodynamic destabilization behaviors of MgH2by Si,Ge and Sn with mechanical alloying.The obtained results show that Si,Ge,and Sn successfully decrease the hydrogen release temperatures in the following order:Ge>Sn>Si.Moreover,the hydrogen capacities of 2MgH2-Si,2MgH2-Ge,and 2MgH2-Sn are 5,3.2,and 2.4 wt.%,respectively at temperatures up to 350 °C.
Based on tabulated thermodynamic data [201],Vajo et al.[196]calculated that the predicted equilibrium pressures of 2MgH2-Si are 1 bar at 20 °C,and 100 bar at 150 °C,which is 1000 times better than MgH2.Imamura et al.[199]found that 17 mol% addition of Sn to MgH2lowers the desorption peak temperature from 432 °C to 217 °C.Gennari et al.[202]reported that depending on the amount of Ge added to MgH2,the desorption temperature can be sensibly decreasedi.e.by 50 °C for 5 mol% Ge and 150 °C for 50 mol% Ge.Additionally,the alloying of Si,Ge and Sn with Mg leads to the formation of catalytic sites which enhance the H mobility into the alloy [202].
4.1.2.Al
By combining Al and MgH2,upon dehydrogenation the following reversible reaction will occur:
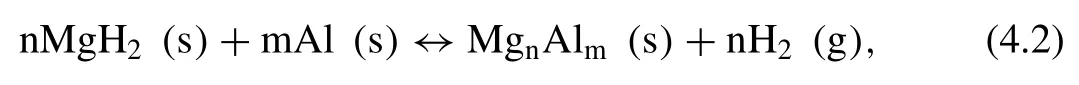
Depending on the amount of added Al,different MgAl alloys might form,e.g.Mg2Al3[203],Mg17Al12[203–205].Shang et al.[206]calculated the formation enthalpy of MgH2+8 mol% Al to be 28.4 kJ·mol−1H2,which is 47.6 kJ·mol−1H2smaller than the calculated value of the MgH2.This sample with Al additive has a hydrogen capacity of 5.4 wt.%.Based on the DOS analysis,Al was found to cause a reduction in the band gaps of the MgH2,while the Mg-H bond is easier to dissociate,leading to the decrease of formation enthalpy [41].
Andreasen et al.[207]found that in Mg17Al12alloys,Al improves the oxygen-resistance ability.The activation energy measured for the dehydrogenation of Mg17Al12alloy is 160 kJ·mol−1H2,and the alloy is less sensitive to oxygen when compared with pure Mg,suggesting that alloying with Al will simplify the pretreatment to activate the sample.
Zhong et al.[203]observed that by adding 10 mol% Al to Mg,6 wt.% of hydrogen can be stored at 344 °C and under 20 bar hydrogen pressure in 2000s,while 5.5 wt.%of hydrogen can be absorbed in 600 s.The addition of Al triggers a decrement of the desorption activation energy from 100 kJ·mol−1of the pure Mg [50]to 56 kJ·mol−1of the Mg90Al10alloy.The improved kinetics can be attributed to the following reasons:(1)the intermetallic Mg-Al formation can provide energy for the destabilization of MgH2[203];(2)the addition of Al will generate some interfaces,which can accelerate the diffusion of H2and also the nucleation of Mg [208–210];(3)due to the existence of Mg17Al12and Al3Mg2alloy,Mg particle agglomeration can be inhibited,the diffusion distance can be shortened [203].
4.2.Mg alloys with d-elements(Ni,Cu,Co,Ti,Pd,Fe,and Zn)
It is well known that Mg forms alloys with several elements of the d-block of the periodic table(e.g.Ni,Cu,Co,Ti,Pd,Fe,and Zn).In the following,the hydrogen storage properties of some of the most investigated systems are discussed.
The first example of Mg alloys with d-elements that are going to be discussed is Mg2Ni.The hydrogenation of Mg2Ni alloy can be described as following:
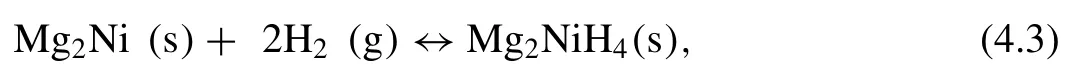
The dehydrogenation enthalpy[32]measured for Mg2NiH4is 64.4±4.2 kJ·mol−1H2,while the value [24]for MgH2is 74.1 kJ·mol−1H2.The addition of Ni to Mg leads to multiple beneficial effects.In fact,from one side,Ni catalytically influences the hydrogen sorption properties of Mg/MgH2[211,212],and from the other side the interaction between Mg and Ni and the consequent formation of the alloy Mg2Ni sensibly influences the thermodynamic properties[213].Thus,owing to a low dehydrogenation∆H,an equilibrium temperature of about 240 °C at 1 bar of hydrogen pressure is expected [87].Nevertheless,the addition of Ni entails a considerable reduction of the hydrogen storage capacity(i.e.gravimetric hydrogen storage capacity of Mg2NiH4=3.53 wt.%)[32,214].
Another example of an alloy formation between Mg and delements is Mg2Cu.The hydrogenation reaction of the Mg2Cu alloy can be seen in Eq.(4.4)[193]:
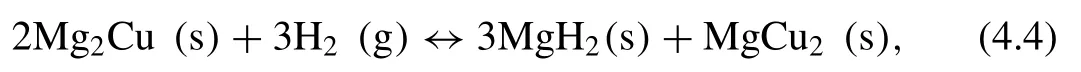
for which the∆Hand∆Svalues are 72.8±4.2 kJ·mol−1H2and 142.3±2.9 J·K−1·mol−1H2,respectively,with a hydrogen capacity of only 2.53 wt.% [193].
Another example is Mg-Co-H system.Zolliker et al.first synthesized Mg2CoH5hydride in 1985 [215].The reactions can be described as following [215,216]:
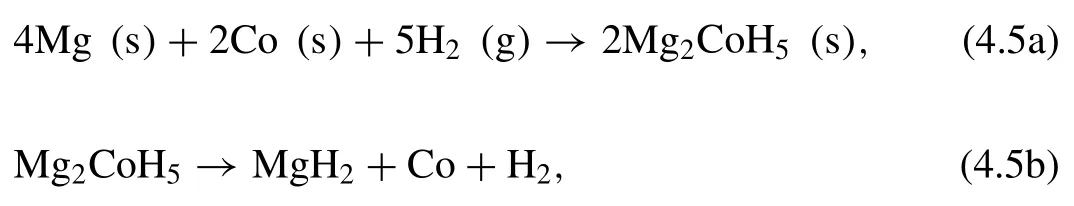
The∆Hvalues for desorption and absorption of Mg2CoH5are 86 and−60 kJ·mol−1H2,respectively [215].
Kyoi et al.[217]first synthesized the ternary Mg7TiHxhydrides by ball milling.The dehydrogenation process can be described by the following equation:
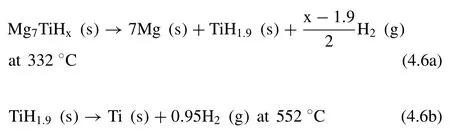
The total hydrogen storage capacity is 5.5 wt.%(x≈12.7).For the Mg-Ti-H system,some works [218–220]have shown that the addition of Ti notably enhances the hydrogen sorption kinetics.As an example,Shao et al.[219]found that the MgH2–0.1TiH2system can absorb 4 wt.% of hydrogen at 100 °C in 4 h,even can absorb 1.2 wt.% of hydrogen at 40 °C in 5 h.
Mg-Zn alloys and their hydrogenation reaction pathways can be described as following [221]:
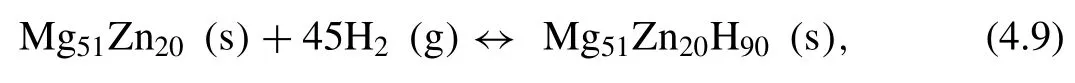
For this system the measured hydrogen storage capacity is 3.44 wt.%,and the∆Hand∆Sare 81.2 kJ·mol−1H2and 125.1 J·K−1·mol−1H2[221].
Kume et al.[222]first investigated the hydrogen storage properties of Mg6Pd intermetallic compound.The hydrogenation reaction of Mg6Pd is shown in Eq.(4.10)[222,223]:
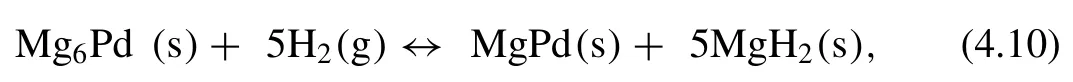
The∆Hand∆Swere calculated to be 80.3 kJ·mol−1H2and 148 J·K−1·mol−1H2[222].Callini et al.[223]found that Mg6Pd compounds can transform reversibly to different types of Mg-Pd compounds,hence the overall hydrogen storage capacity is 3.96 wt.%.
Didisheim et al.[224]found that Mg and Fe can react with H2to form Mg2FeH6according to following equation[224,225]:
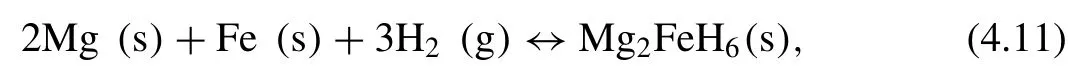
Note that intermetallic compounds of Mg-Fe does not exist due to the positive energy of mixing.The∆Hfor absorption and desorption are 55±3 and 98±3 kJ·mol−1H2,respectively.
The general properties of all systems discussed in this section are summarized in Table 4.1.The values listed in Table 4.1 can not be directly compared due to different preparation and measuring conditions.
4.3.RE-Mg-Ni alloys(RE=La,Pr,and Nd)
Several intermetallic compounds can be formed between the rare-earth(RE)elements and Ni,with the atomic ratio between 1:2 to 1:5(here we denote them as AB2-,AB3-,A2B7-types,and other types).These structures are built from AB2Laves-type layers alternating with AB5layers.Based on the alloying methods for hydrogen storage materials,many researchers have found that when adding a third element to a RE-Ni(RE=La,Pr,and Nd)alloy,the hydrogen capacity,kinetics,as well as the sorption stability can be improved[236,237].Therefore,these intermetallic compounds can be alloyed with Mg,where the lightweight Mg can replace the RE elements in the ABxmodel according to some previous study [238,239],demonstrating the enhancement of hydrogen storage capacity and cycling stability of the intermetallic compounds.Based on different ratios between RE element and Mg with Ni,we divide the RE-Mg-Ni hydrides into the following types.
4.3.1.AB2-type
Oesterreicher et al.[240]first synthesized the singlephased La1-xMgxNi2(x=0.0,0.25,0.50,0.60,0.67,0.70,and 1.0)ternary compounds by induction melting method.The results show that from LaNi2to La0.33Mg0.67Ni2,all compounds possess C15-type(MgCu2type)structure.When the Mg content is higher,the structure transforms to C36-type(MgNi2type).During dehydrogenation,these hydrides tend to decompose to Ni,MgH2and LaH3.With the increase ofxvalue from 0 to 1,the hydrogen capacity at room temperature and 10 bar H2of the compounds decreases from 1.7 wt.% to 0.
4.3.2.AB3-type
Series of hydrogen storage alloys,e.g.REMg2Ni9(RE=La,Pr,and Nd),building from MgNi2blocks with Laves-type structures alternating with Haucke-phased AB5layers,were first synthesized by K.Kadir [241]in 1997.Since then,the AB3-type Mg-based alloys have attracted extensive attention [242–244].By increasing Mg content in La3-xMgxNi9[242]compounds,the unit cell volumes have a linear decrease.As shown in Fig.15(a),dehydrogenation equilibrium pressure changes from 0.011 bar to 18 bar of H2with the increment of Mg content(xvalue)from 0.7 to 2.Meanwhile,the desorption∆Hvalue decreases with the increase of Mg content,which changes from 40.3 kJ·mol−1H2for La2.3Mg0.7Ni9to 24.0 kJ·mol−1H2for La1.0Mg2.0Ni9.Fig.15(b)shows that hydrogenation/dehydrogenation equilibrium pressures depend linearly on the Mg contents,confirming that the crystal structure can greatly affect the thermodynamics.
4.3.3.A2B7-type
Denys et al.[245]found that by replacing La with Mg in La2Ni7to form La1.5Mg0.5Ni7,the crystal structure remains to be Ce2Ni7-type,but unit cell volume decreases.The∆Hand∆Svalues for hydrogenation/hydrogenation of La1.5Mg0.5Ni7compound are 15.7±0.9/16.8±0.4 kJ·mol−1H2,and 46.0±3.7/48.1±1.5 J·K−1·mol−1H2,respectively.By partially replacing La by Mg,the interstitial hydride was formed,and the hydrogenation mechanism changes from anisotropic hydride to isotropic hydride.

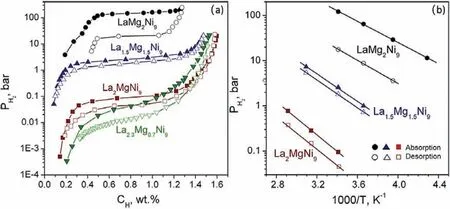
Fig.15.(a)The absorption/desorption isotherms at 20 °C and(b)Van’t Hoff plots of La3−xMgxNi9 compounds [242].
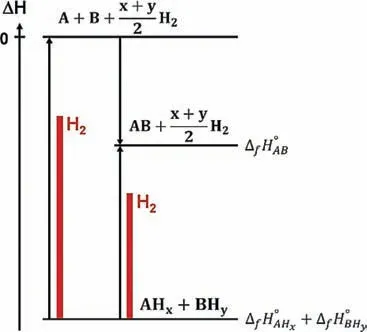
Fig.16.Schematic enthalpy diagram of reactions in RHCs [256].
5.Hydrogen storage in Mg-based RHCs
Due to their high thermodynamic stability,Mg and Mgbased alloys are not considered as suitable candidates for operating in combination with Polymer Electrolyte Membrane Fuel Cells(PEMFCs).One of the most promising routes which allows utilizing Mg-based compounds for hydrogen storage applications is to destabilize them with light-weight complex metal hydrides to form the so-called reactive hydride composites(RHCs)[61,246–249].Differently from the alloying strategy proposed by Reilly et al.[193],the utilization of reacting partners that contain large quantities of hydrogen(in RHCs)allows maintaining a high hydrogen storage capacity with a lower enthalpy,as seen in Fig.16.With this approach,several new hydrogen storage systems based on Mg were developede.g.M(BH4)n−MgH2(M=Li,and Na)[250–252]and M(BH4)n−Mg2NiH4(M=Li,Na,and Ca)[253–255].
5.1.M(BH4)n-MgH2(M= Li,and Na)
Vajo et al.[247]and Barkhordarian et al.[246]found that by mixing MgH2with LiBH4in the stoichiometric ratio 1:2,8–10 wt.% of hydrogen can be reversibly stored with a reaction enthalpy of 40.5 kJ·mol−1H2.The reaction enthalpy of this RHC system lies well below the reaction enthalpies of the single compoundsi.e.67 kJ·mol−1H2for LiBH4and 74.1 kJ·mol−1H2for MgH2.The reaction pathway can be described as following [247]:
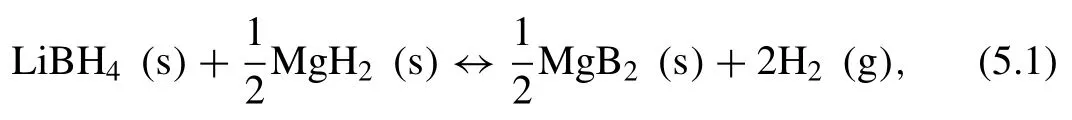
The exothermic formation of MgB2during desorption is the reason for the lower reaction enthalpy.In order to thoroughly understand the desorption mechanism,Bösenberg et al.[257]systematically investigated the influence of pressure and temperature on the desorption mechanism of 2LiBH4−MgH2RHC.It was found that the reaction mechanism and reversibility strongly depend to the applied conditions.Reaction pathways under different hydrogen pressure and temperature conditions are listed in Table 5.1.

Table 5.1 The expected reaction pathways of different 2LiBH4-MgH2 RHC samples and of in-situ PXD measurements at different desorption conditions [257].
Another RHC system is NaBH4−MgH2.Garroni et al.[258]investigated the desorption mechanism of the NaBH4−MgH2RHC.The dehydrogenation reaction occurs in multiple steps as following [258]:
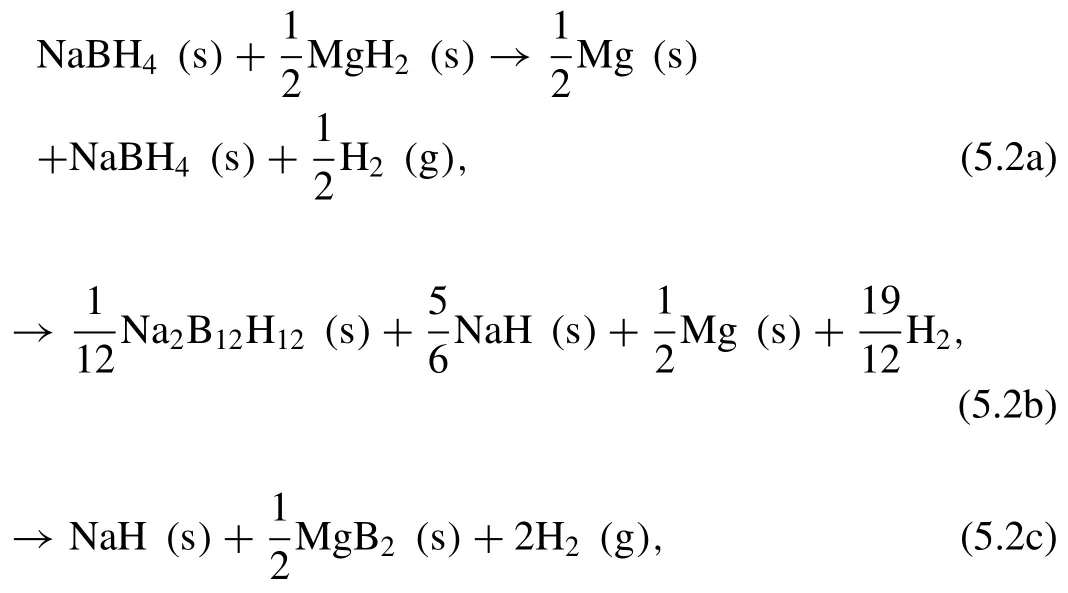
As in the 2LiBH4−MgH2system,the dehydrogenation enthalpy of the NaBH4−MgH2(62 kJ·mol−1H2)is lower than that of NaBH4(88.2 kJ·mol−1H2)and MgH2(74.1 kJ·mol−1H2),due to the exothermic formation of MgB2during dehydrogenation [259].The measured reversible hydrogen capacity(∼6.0 wt.%)is lower than the theoretically expected value(7.8 wt.%).This hydrogen storage capacity mismatch is most likely due to the undesired formation of NaMgH3by the reaction of NaH with MgH2[260].
Pistidda et al.[260]investigated the hydrogen absorption mechanism of the 2NaH-MgB2RHC.Different intermediate phases are forming resulting from different hydrogen pressures.For instance,under 5 bar H2NaBH4is directly formed while heating the starting material from room temperature to 400 °C.However,if the hydrogen pressure is increased to 50 bar,upon heating,an unknown phase is formed,then NaMgH3and finally NaBH4.These results clearly indicate that by modifying the hydrogen pressure,the reaction paths of NaH-MgB2RHCs can be tuned.
5.2.M(BH4)n-Mg2NiH4(M=Li,Na,and Ca)
Recently,the M(BH4)n-Mg2NiH4(M=Li,Na,and Ca)systems have been studied.Dehydrogenation reaction of 4LiBH4–5Mg2NiH4is according to Eq.(2.11)[253,261]:
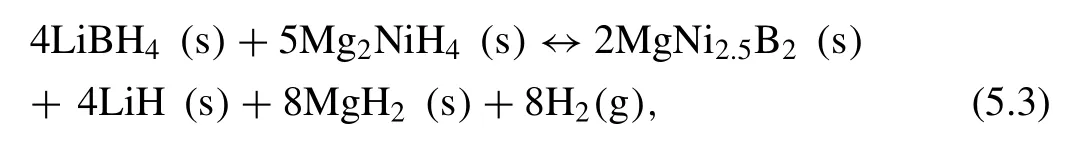
where the enthalpy∆H=15.4±2 kJ·mol−1H2)and entropy(∆S=62.2±3 J·K−1mol−1H2)are relatively lower compared to 2LiBH4−MgH2,and 4LiBH4–10MgH2–5Ni RHCs,as shown in Fig.17.This system is reversible,however,the reversible hydrogen capacity is only 1.7 wt.%,while the theoretical capacity is 2.5 wt.%.Moreover,the capacity will deteriorate about 30% to 50% of the initial capacity after the first cycle [253,262].
Afonso et al.[254]investigated the destabilized 4NaBH4–5Mg2NiH4RHC system,in which the reactions(Fig.18)can be described by the followings [254]:
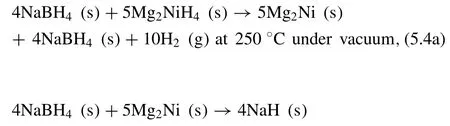

As shown in Fig.18,the dehydrogenation∆Hof the reactions in Eqs.5.4(a),5.4(b),and 5.4(c)are 67±4,76±5,and 95±7 kJ·mol−1H2,respectively.Although the initial hydrogen capacity is 4.8 wt.%,after several cycles the hydrogen storage capacity decreases about half(to 2.5 wt.%).due to the irreversible formation of MgNi2.The activation energy is calculated to be 131±24 kJ·mol−1H2,which is higher than 90.1±4.3 kJ·mol−1H2of the pure Mg2NiH4[228],indicating that the diffusion of H2is more sluggish in the 4NaBH4–5Mg2NiH4RHC.The free Ni could act as catalysts for hydrogen diffusion,and slow kinetics may be related to less free Ni presence.
Bergemann et al.[263]proposed dehydrogenation reaction pathway for the Ca(BH4)2–2.5Mg2NiH4system under 1 bar H2at 450 °C as following:
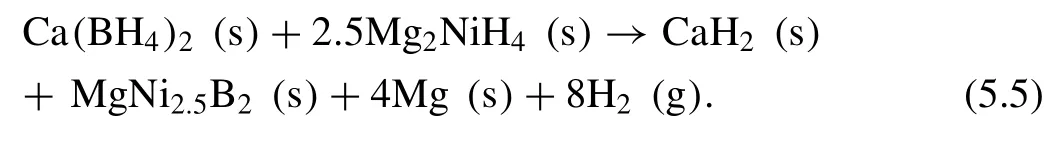
The hydrogen desorption capacity was measured to be 4.6 wt.%.MgNi2.5B2is formed during dehydrogenation,which suppresses the formation of CaB12H12or amorphous boron.Therefore,degradation of the hydrogen capacity caused by the irreversible formation of CaB12H12and amorphous boron [264–268]can be eliminated.And the presence of Mg2NiH4lowers the desorption temperature of Ca(BH4)2by 55 °C,which paves the way for the subsequent researches.
In summary,for the 2LiBH4-Mg2NiH4and 5NaBH4–4Mg2NiH4systems,although their reversible capacities and kinetics do not meet the requirements for practical applications,they still shed light on combining the borohydrides with the Mg-based alloys as complex hydrides.For the Ca(BH4)2–2.5Mg2NiH4system,due to the absence of some inactive boron-containing products,the reversible capacity could probably be improved.In addition,the Mg2NiH4plays an important role on destabilizing the M(BH4)n(M=Li,Na,and Ca)RHCs.There are some intermediate phases during dehydrogenation,e.g.Mg2Ni,MgNi2.5B,which decrease the desorption temperature of the RHCs.The most important finding is that it was the first time that the mutual destabilization of the reactants was obtained,thus was the first time that the RHC approach was fully proven [261].
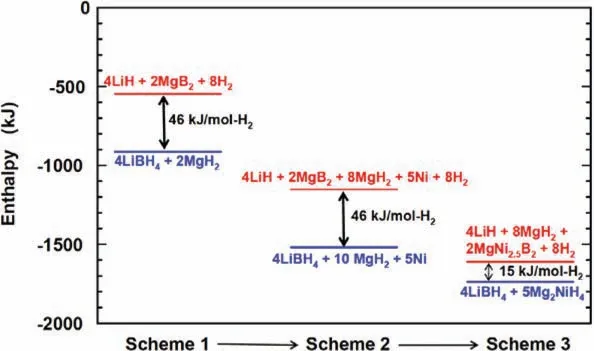
Fig.17.The enthalpy diagram of 2LiBH4-MgH2,4LiBH4–10MgH2–5Ni,and 4LiBH4–5Mg2NiH4 RHCs [253].
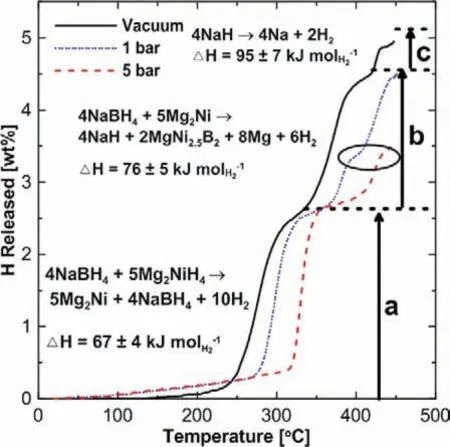
Fig.18.The temperature-programmed desorption(TPD)profiles of 4NaBH4–5Mg2NiH4 under three different H2 pressures.Where the horizontal dashed lines are the ideal released hydrogen amount of the reactions listed on the left [254].
6.Use of Mg wastes
The production cost of hydrogen storage materials is one of the main obstacles to their employment in large scale energy storage applications.In order to reduce the cost of the production,Mg-based waste materials can be used in preparing MgH2[269,270],RHCs based on magnesium such as Mg(NH2)2-LiH [271],and alkali borohydrides [272].Pistidda et al.[269]and Hardian et al.[275]explored the conversion process and hydrogen sorption properties of the Mg-based industrial wastes,e.g.AZ91 alloy and Mg-10 wt.% Gd alloy.The obtained results indicate that,despite having been stored under atmospheric conditions for a long time,the MgH2obtained after hydrogenation of the milled waste materials can be utilized to reversibly store large quantities of hydrogen(i.e.AZ91 up to ≈6 wt.% Fig.19).Interestingly the obtained MgH2shows fast reaction kinetics and full reversibility even after several tents of dehydrogenation/hydrogenation cycles.These findings were justified by the presence in the starting alloys of several well dispersed transition metal elements that alone or after forming oxide species(during the exposure to the atmosphere)act as dehydrogenation/hydrogenation catalysts.
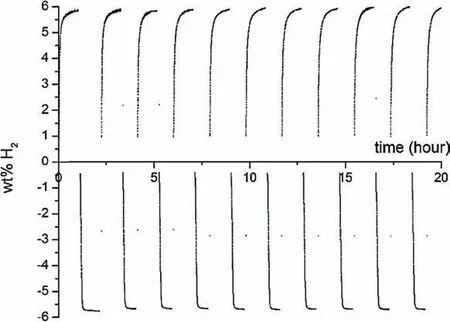
Fig.19.Hydrogen desorption/reabsorption curves measured at 350 °C under a hydrogen pressure of 13 bar for the absorption process and at 350 °C and 0.5 bar hydrogen pressure for the desorption process [270].
Cao et al.[271]synthesized the Mg(NH2)2–2LiH RHC using waste AZ91 alloy as the magnesium source .The AZ91 alloy was ball milled with LiH with a molar ratio of 1:2 under 50 bar of hydrogen for 12 h to form LiH and MgH2.Subsequently,the mixture was ball milled under 7 bar of NH3for 28 h to get the Li2Mg(NH2)2.Then the Li2Mg(NH2)2was desorbed under 1 bar hydrogen from room temperature to 250 °C to obtain the Mg(NH2)2–2LiH composites [273–277].As a comparison,the same system was prepared utilizing pure Mg as the magnesium source.The investigations of hydrogenation/dehydrogenation kinetics show that the composite synthesized from the waste material can reach a hydrogen capacity of 4.6 wt.% in 100 min after 5 cycles,while the one synthesized from the pure Mg has a capacity of 4.4 wt.% in 100 min after 5 cycles.In addition,the hydrogen desorption peak temperature of the Mg(NH2)2–2LiH composites synthesized from waste alloy is decreased to 220°C,which is 15°C lower than that of the material synthesized from pure Mg.The obtained results suggest that recycled magnesium alloys can be used for hydrogen storage,which may reduce the costs significantly.
7.Summary and conclusions
Magnesium hydride and magnesium based systems are considered suitable candidates for hydrogen storage applications as well as due to their relatively high reaction enthalpy for thermal energy storage.Over the last fifty years a large number of scientific achievements were made to modify the hydrogen storage properties of this material family.Thermodynamic tuning has been achieved in several different manners.In the pioneering years of the metal hydrides development the addition of alloying elements to magnesium allowed modifying significantly its stability.However the addition of element such as Cu,Pd,Ti,Ni,etc.,which did not bond hydrogen themselves(under the investigate temperature and hydrogen pressure conditions)led to decreasing the overall system hydrogen storage capacity.Later the possibility to tune the thermodynamic stability of magnesium and magnesium based compounds by nanostructuring was also reported.At the beginning of the 2000s the introduction of the RHC approach for the design of hydrogen storage materials allowed developing multicomponent hydrogen storage systems possessing low thermodynamic stabilities while exhibiting still high hydrogen storage capacities(e.g.Mg(NH2)-LiH,Mg(NH2)-LiH-LiBH4,LiBH4−MgH2,NaBH4−MgH2,Ca(BH4)2−Mg2NiH4,etc.).The possibility to enhance the hydrogenation/dehydrogenation kinetics and reversibility of magnesium and magnesium based systems by the use of selected TM based additives(e.g.Nb2O5)was also proven.In recent years the possibility of utilizing low purity magnesium sources for the synthesis of high-efficiency hydrogen storage materials was demonstrated.
Although the influence of selected additives as well as the impact of the utilized processing routes on the hydrogen storage properties of MgH2have been thoroughly investigated over the last decades,possible new research directions might be identified.In many of the reported studies,the achieving of intimate contact between Mg/MgH2and the utilized additives appears to be crucial for modifying the system properties.However,in most cases,the use of commercially available Mg/MgH2and additives which are then mixed together make it difficult to understand the reasons standing behind the observed enhancements.In our opinion,for such types of studies,it would be recommendable(when possible)to use well designed metallurgical approaches for obtaining Mgbased systems which contain finely dispersed and intimately imbedded additives.The investigations of the hydrogen storage properties of such systems might provide more insightful information on the role of the additives(e.g.importance of the Mg-additives boundaries)in enhancing the system properties.In terms of the commercial application of magnesium based hydrogen storage systems,key parameters that are still waiting to be improved are cost,thermal management and air sensitivity of the storage systems.
Declaration of Competing Interest
There are no conflicts of interest to declare.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Magnesium alloys as anodes for neutral aqueous magnesium-air batteries
- Biodegradable Mg alloys for orthopedic implants–A review
- A review on electromagnetic shielding magnesium alloys
- Thermodynamics and kinetics of hydriding and dehydriding reactions in Mg-based hydrogen storage materials
- Valorization of AZ91 by the hydrolysis reaction for hydrogen production(Electrochemical approach)
- Exploring the interconnectivity of biomimetic hierarchical porous Mg scaffolds for bone tissue engineering:Effects of pore size distribution on mechanical properties,degradation behavior and cell migration ability
