Comparative analysis of constitutes and metabolites for traditional Chinese medicine using IDA and SWATH data acquisition modes on LC-Q-TOF MS
2021-01-21DinKngQingqingDingYngfnXuXioxiYinHuiminGuoTengjieYuHeWngWenshuoXuGungjiWngYnLing
Din Kng,Qingqing Ding,Yngfn Xu,Xioxi Yin,Huimin Guo,Tengjie Yu,He Wng,Wenshuo Xu,Gungji Wng,**,Yn Ling,*
aKey Lab of Drug Metabolism&Pharmacokinetics,State Key Laboratory of Natural Medicines,China Pharmaceutical University,Tongjiaxiang 24,Nanjing,210009,PR China
bDepartment of Geriatric Oncology,First Affiliated Hospital of Nanjing Medical University(Jiangsu People's Hospital),No.300 Guangzhou Road,Nanjing,210029,PR China
ABSTRACT
Keywords:
LC-Q-TOF MS
SWATH-MS
IDA-MS
Sanguisorbin extract
Sanguisorbins
1.Introduction
Prototype constituents and metabolites of traditional Chinese medicines(TCMs)are the promising sources for discovering new drugs since they all may be pharmacologically active substances[1-3].So far,the detection of constituents and metabolites for TCM is still a challenge,due to low exposure of prototype components,complex metabolic pathways,and massive endogenous interference[4-7].High-resolution MS,especially the hybrid quadrupole time-of-flight mass spectrometry(Q-TOF MS),has become the most common analytical tool for TCM study[8,9].In LC-Q-TOF MS analysis,the accurate m/z(mass-to-charge ratio)recorded in MS and MS/MS spectra could provide crucial information for the structure elucidation.The first step of constitute screening for TCM is to acquire single-stage TOF MS information and to preliminarily classify the type of compounds based on the accurate mass of the protonated/deprotonated molecular ion,isotopic pattern,and a priori known retention time[10-12].The second step is to select the precursors and to make them into fragment ions in a collision cell.In general,the user's predefined criteria(information-dependent acquisition,IDA)are used for the selection of the precursor.According to previous reports,there is always a compromise in IDA settings.Larger number of IDA experiments is theoretically conducive to more trigger events,but leads to longer cycle times.Longer exclusion time helps prevent repeated trigger events for the same compound,but may cause the loss of active ingredients of TCMs and reduce the chance of positive identification once the actual peak maximum is excluded[13-15].
To achieve the purpose of comprehensive analysis,several dataindependent acquisition strategies,including sequential window acquisition of all theoretical fragment-ion spectra(SWATH),shotgun-collision induced dissociation,MSE,Multiplexed DIA(MSX),all ion fragmentation(AIF)and others,have been developed over the last few years[16-18].In IDA-MS analysis,product ion(MS2)spectra are continuously acquired over the entire LC run in an unbiased fashion,without requiring detecting precursor ions nor prior knowledge about precursor m/z values.For instance,SWATH is a a data-independent acquisition(DIA)method where all the precursor ions within the predefined m/z range are isolated and subjected to co-fragmentation[19].Compared to the traditional IDA-MS strategy,SWATH-MS could significantly improve the hit rate of low-level ingredients because it could sequentially obtain all MS/MS spectra of all mass windows across the specified mass range[20-22].Thus,SWATH methods are more suitable for the analysis of extremely small sample amounts than IDA method,and have already been used in proteomic and metabolomic research[19,23,24].
In this study,the suitability of SWATH-MS for TCMs' prototype constituent and metabolite identification for TCMs was systematically assessed by comparing the identification efficiency of SWATH-MS and IDA-MS data acquisition modes,and sanguisorbin extract was used as a mode TCM.Sanguisorba officinalis L.(S.officinalis),a TCM belonging to the Rosaceae family,has hemostatic,detoxifying,anti-inflammatory,analgesic,antibacterial,antitumor and neuroprotective activities[25-27].Sanguisorbins are the main active ingredients in Sanguisorba officinalis L.[25,28-30].The results indicated that the qualitative efficiency of SWATH-MS data acquisition on sanguisorbin extract was significantly higher than that of IDA-MS data acquisition.In SWATH-MS mode,rolling CE and variable Q1 isolation windows could be more efficient for sanguisorbin identification than the fixed CE and Q1 isolation window.A total of 47 sanguisorbins were detected when SWATH mode was used.In addition,26 metabolites of sanguisorbins were identified in rat plasma,and their metabolic pathways could be inferred as decarbonylation,oxidization,reduction,methylation,and glucuronidation according to their fragmental ions acquired by LC-Q-TOF MS in SWATH-MS mode.
2.Materials and methods
2.1.Chemicals
Authentic standards of ziyuglycoside I and ziyuglycoside II(purity>98.0%)were purchased from Shanghai Yansheng Technological Development Co.,Ltd(Shanghai,China).The powder of sanguisorbin extract was kindly supplied by Chengdu Di Ao Pharmaceutical Group Co.,Ltd(Chengdu,China).Acetonitrile and methanol were purchased from Merck(Merck,Germany).Deionized water was prepared by the Milli-Q system(Millipore Corporation,Billerica,MA).All other chemicals were purchased from Sigma-Aldrich(St.Louis,MO,USA)or Thermo Fisher Scientific(Waltham,MA,USA).
2.2.Preparation of sanguisorbin extract solution
The powder of sanguisorbin extract was kindly supplied by Chengdu Di Ao Pharmaceutical Group Co.,Ltd.For extract solution preparation,50.00 mg of sanguisorbin extract was poured into a 5 mL volumetric flask,added with methanol to the mark,ultrasonically dissolved,prepared into 10 mg/mL stock solution,and stored in a 4°C refrigerator.It was diluted to the corresponding concentration with the medium step by step before use.
2.3.Animal experiments
Male healthy Sprague-Dawley rats(8-10 weeks,200±20 g)were purchased from the Laboratory Animal Center of Peking University Health Science Center(Beijing,China)and kept in an environmentally controlled breeding room(temperature 22 ± 2°C,relative humidity 50±10%,and 12 h dark-light cycle).The rats were fed with free access to standard laboratory food and water for at least 3 days before experimentation.All animal-related experimental procedures were conducted in accordance with the Guidelines for Animal Experimentation of China Pharmaceutical University(Nanjing,China),and the protocol was approved by the Animal Ethics Committee of this institution.
The crude sanguisorbin extract suspension was prepared with 0.1% sodium carboxymethyl cellulose,and was then administered to rats(n=6)at 200 mg/kg.Hepatic portal vein blood was collected into heparinized tubes at 0.5,1,2,and 4 h after oral administration of sanguisorbin extract.All samples were centrifuged immediately at 10,000 × g for 10 min at 4°C,and then the plasma specimens were stored at-80°C until analysis.
2.4.Sample preparation for rat plasma
Rat plasma samples were purified using a liquid-liquid extraction technique.To each tube containing 100μL of rat plasma,0.75 mL of n-butanol was added.The mixture was then vortex-extracted for 3 min,and then centrifuged for 10 min at 10,000×g.The supernatant(0.50 mL)was evaporated to dryness in a rotary evaporator at 60°C under high vacuum.The residue was reconstituted in 100 μL of acetonitrile,and 5 μL of aliquot was analyzed by LC-triple QTOF MS.
2.5.HPLC and high resolution MS settings
LC separation was performed using a Shimadzu UFLC-30A system(Shimadzu,Kyoto,Japan),configured in binary 30A pumps,SIL-30AC autosampler and a CTO-30AC column oven.All the components were eluted onto a C18analytical column(2.1 mm×150 mm,5μm;Phenomenex Luna)guarded with a C18guard column(2.0 mm I.D.×4.0 mm;Phenomenex Luna).The column oven was set at 40°C,and the autosampler was cooled at 7°C.The flow rate was 0.2 min/mL.The mobile phase A(MPA)was H2O containing 0.02% acetic acid(V/V),and the organic phase B(MPB)was acetonitrile.The gradient elution program was as follows:an isocratic elution of 25% MPB for the initial 1.5 min,followed by a linear gradient elution of 25%-45% MPB from 1.5 to 14 min,and then followed by a linear gradient elution of 45%-90% MPB from 14 to 30 min;after holding the composition of 90% MPB for the next 3 min,the column was returned to its starting conditions till the end of the gradient program at 40 min for column equilibration.
MS analysis was performed using an AB Sciex 5600+Triple TOF mass spectrometer(Concord,Ontario,Canada),which operated in negative ionization mode with a DuoSpray ion source.The source conditions were set as follows:ion-spray voltage floating 5.5 kV,declustering potential 70 V,turbo spray temperature 400°C,nebulizer gas(Gas 1)50 psi,heater gas(Gas 2)50 psi,and curtain gas 30 psi.Continuous recalibration was carried out every 6 h by injecting and analyzing the mixed standards with the aid of the automated calibration delivery system.All the parameters were controlled and run by Analyst TF 1.7 software(Sciex,Concord,Ontario,Canada).Data were processed with PeakView 2.0 and MasterView 2.0 Softwares(Sciex,Concord,Ontario,Canada).
Data acquisition in IDA mode mainly consisted of a full MS1scan and information-dependent trigger MS/MS fragmentation events.The accumulation time for MS1full scan was 100 ms for scanning a mass range from 400 to 1250(m/z).The accumulation time for each IDA experiment was 50 ms,and the CE was set to 15,20,25,30,35,40 and 45 eV with a CE spread of 15 eV in high sensitivity mode.The scanning range of product ions was from 100 to 1250(m/z)with charge state 1.IDA criteria were set as follows:8 most intense ions with an intensity threshold were above 200 cps,dynamic background subtraction was switched on,isotope exclusion was switched off,and the exclusion time(half peak width)was 6 s.
The acquisition using SWATH consisted of a full scan,followed by a Q1 isolation strategy.The full scan covered a mass range of m/z 300-1250 with an accumulation time of 100 ms.Two different CE voltage setting modes,i.e.,fixed(35 eV with a collision energy spread of 15 eV)mode and rolling mode,were adopted to ensure high qualitative efficiency for sanguisorbins.In addition,the two kinds of Q1 isolation window settings(fixed and variable Q1 isolation windows)were used to detect sanguisorbins.In the fixed Q1 isolation windows,the Q1 isolation strategy covered a mass range of m/z 300-1250 with a 65 Da window width for Q1 isolation(overlap 1 Da).The workflow of preparing the SWATH variable window is shown in Fig.1.The first step was to generate the variable window table manually in Excel by defining the varying windows width with 3 Excel columns(column 1:‘Q1 Start m/z',column 2 ‘Q1 Stop m/z',and column 3 ‘collision energy spread,CES').The document was then saved as a*.txt file(Fig.1A).In the second step,the variable window text file just established was imported into the LC-MS acquisition method in the Analyst software(Fig.1B).Finally,the “OK”button was clicked to build the variable window SWATH acquisition method which consisted of the TOF MS scan(experiment 1)and product ion scans(experiments 2-16)(Fig.1C).
Different combinations of CE(fixed and rolling)and Q1 isolation windows(fixed and variable)were used to optimize SWATH conditions:(i)fixed 35 V CE and fixed 65 Da windows spanning the mass range 300-1250 Da,(ii)fixed 35 V CE and variable Q1 isolation windows spanning the mass range 300-1250 Da,(iii)rolling CE with a collision energy spread of 4 eV and fixed 65 Da windows spanning the mass range 300-1250 Da,(iv)rolling CE with a collision energy spread of 4 eV and variable Q1 isolation windows spanning the mass range 300-1250 Da.
3.Results and discussion
3.1.Detection of sanguisorbins in sanguisorbin extract using IDAMS mode
All of the IDA and SWATH analyses were performed using identical LC and Triple TOF 5600+MS settings.Data acquisition was implemented in both positive and negative ion modes.The MS1full scan of the sanguisorbins demonstrated that the intensity of negative profile was much higher than that of positive profile.Hence,negative ionization mode was employed to investigate the analytical performance of sanguisorbins.Besides,CE value was optimized for the MS/MS experiment.After the MS/MS profiles acquired under a series of CE values(15,20,25,30,35,40 and 45 V),35 V was found to produce more abundant fragment ions for most of sanguisorbins than other CE intensities.
The work of measuring chemical components from sanguisorbin extract in vitro was originally implemented in IDA-MS mode.As shown in Table 1,a total of 18 sanguisorbins,mainly including urethane-type triterpenoid saponins and oleanolic-type triterpenoid saponins,were unambiguously or tentatively characterized.Clearly,most sanguisorbins could produce characteristic fragment ions with m/z 603,585,469 and 453.The compounds eluted at 8.25 min(m/z 825.4470)and 14.90 min(m/z 603.3804)were identified as ziyuglycoside I and II by comparing their chromatographic and mass spectrometric behaviors with the corresponding authentic standards.Taking ziyuglycoside I as an example:the precursor ion of ziyuglycoside I at m/z 825.4470 was the base peak in the negative ion ESI conditions,and its formula was then calculated as C41H66O13([M+CH3COO]-).A total of 5 fragment ions with m/z of 765.4284,645.3868,603.3776,601.4069,and 585.3714 were dominated in its MS/MS spectrum.The product ion at m/z 765.4284 was generated by losing adduct ion CH3COO-,which could further generate product ions at m/z 603.3776 and 601.4069 by missing glucosyl residue.The fragment ion that lost glucosyl residue could further generate an ion at m/z 585.3714 by neutral loss of H2O(Fig.2A).For ziyuglycoside II,3 main product ions with m/z 585.3702,543.3625,and 453.3323 appeared in its MS/MS spectrum.The product ions at m/z 585.3702 and 543.3625 were generated by neutral loss of H2O and cleavage of arabinose,respectively.The product ion at m/z 453.3323(C30H46O3)was deduced to be a product of lost arabinosyl group(Fig.2B).
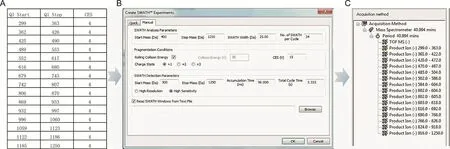
Fig.1.The workflow of preparing the variable windows for SWATH-MS.(A)generating the variable window table manually in Excel,(B)importing the variable window text file into the LC-MS acquisition method,and(C)building the variable window SWATH acquisition method.
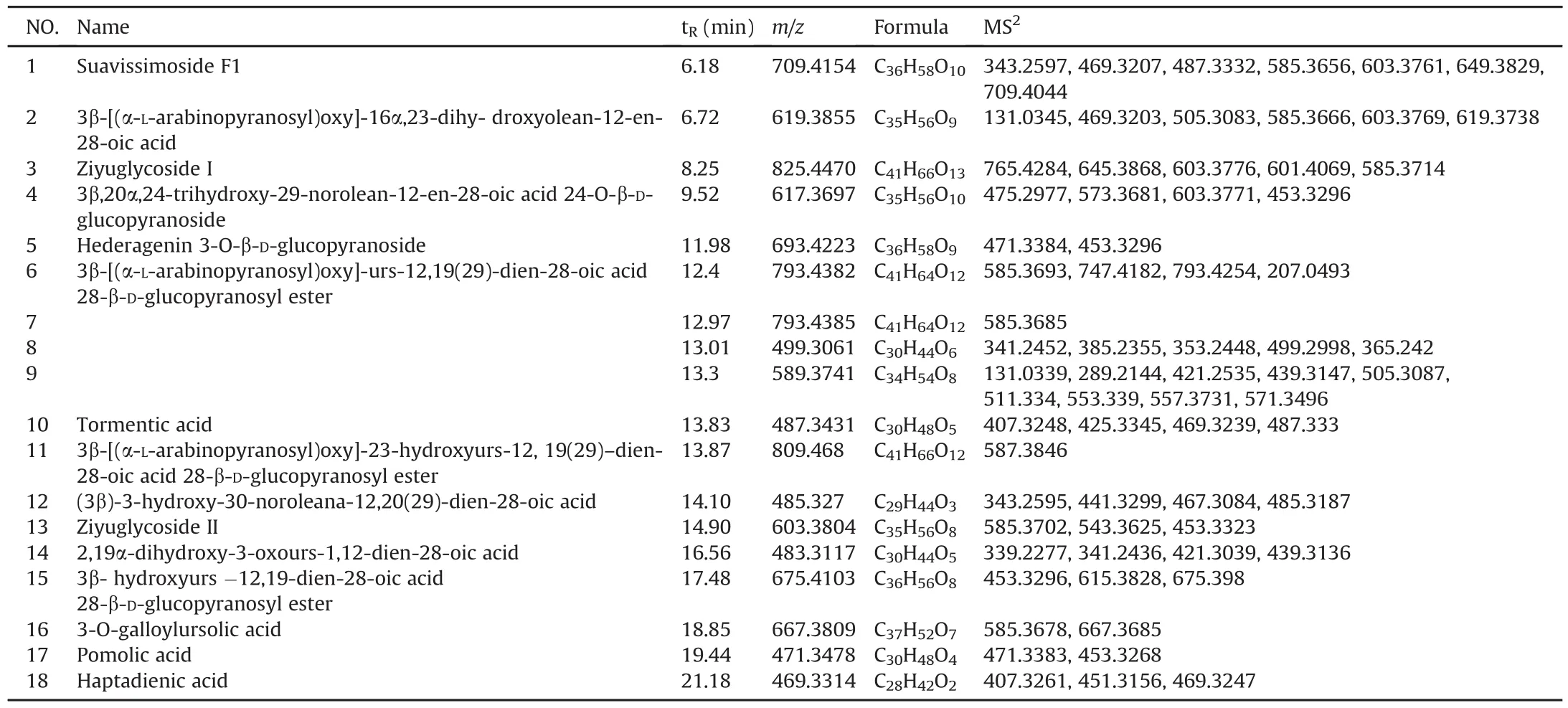
Table 1The information about the sanguisorbins identified in sanguisorbin extract using IDA data acquisition mode on LC-Q-TOF MS.
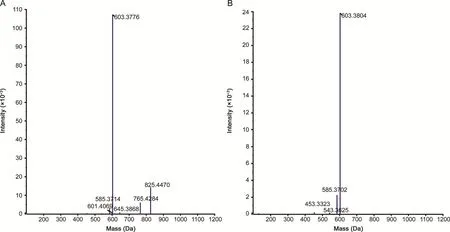
Fig.2.The MS2spectrum of ziyuglycoside I and II acquired in IDA-MS mode.(A)ziyuglycoside I,(B)ziyuglycoside II.
3.2.The influence of CE and Q1 isolation windows on the qualitative efficiency of sanguisorbins
According to the operating principle of IDA-MS,relevant ions,especially for constitutes in the low concentration range,are always lost even though in optimal settings for all situations when IDA is used.To date,SWATH-MS has already been widely used in proteomic and metabolomic research[19,23,24].SWATH data acquisition consists of a recurring cycle of a survey scan and a Q1 isolation strategy.CE and Q1 isolation window are the key factors affecting SWATH efficiency.Herein,different CE(fixed and rolling)and Q1 isolation windows(fixed and variable)were used to search for sanguisorbins and then to obtain the optimum SWATH conditions by comparing the number of saponins detected and the type of their product ions.
In proteomics research,Q1 isolation windows of SWATH were always set at sequential fragmentation in a serial of 25 Da quadrupole isolation windows[19,23,24].It must be noted,however,the complexity of TCM is always much lower than that of proteomics samples.To reduce the workload of data processing,we expanded the acquisition window to 65 Da in the detection of sanguisorbins in sanguisorbin extract.Besides,35 V of CE was found to produce more abundant fragment ions for most of the sanguisorbins in the IDA-MS experiment.Thus,we set CE at fixed 35 V,and then set Q1 isolation window at fixed 65 Da spanning the mass range 300-1250 Da to detect the sanguisorbins in sanguisorbin extract.A total of 29 sanguisorbins with fragment ions were detected,and most of the sanguisorbins could produce characteristic fragment ions with m/z 603,585,469 and 453(Table S1).Compared with IDA-MS identification results,11 kinds of other sanguisorbins were detected using SWATH-MS with fixed CE and Q1 isolation window.For instance,only 2 sanguisorbins(m/z 825.4653 and 603.3904)were identified within the retention time(tR)range of 8.1-8.2 min when IDA was used.As SWATH-MS with fixed CE and Q1 isolation window was used,5 sanguisorbins(m/z 723.3746,779.4024,1067.5973,825.4653,and 603.3904)were found within the tRrange of 8.1-8.2 min.Within retention time of 11-12 min,only the sanguisorbin at m/z 499.3061 was detected as IDA was used,and 5 sanguisorbins at m/z 723.4154,793.4382,861.3969,793.4385 and 499.3061 were detected as SWATH mode with fixed CE and Q1 isolation window was used.
Variable Q1 isolation windows in SWATH-MS are performed by assigning each SWATH window with different isolation width based on the equalized distribution of either the total ion current or the precursor ion population[31].Herein,variable Q1 isolation windows were used to investigate the influence of Q1 isolation window on the SWATH efficiency.By comparing the MS2total ion chromatographs(TICs)acquired using fixed Q1 isolation windows(Fig.3A)and variable Q1 isolation windows(Fig.3B),it could be found that there were some differences between the MS2TICs.When the CE and Q1 isolation window were set at fixed CE value(35 V)and variable Q1 isolation windows spanning the mass range 300-1250 Da,34 sanguisorbins with fragment ions were detected in sanguisorbin extract(Table S2).Clearly,5 more sanguisorbins(m/z 839.43075,617.3697,587.3944,455.35197 and 417.32405)were found in variable Q1 isolation window mode.The above results indicated that variable Q1 isolation windows could improve the efficiency of SWATH-MS for sanguisorbin detection.
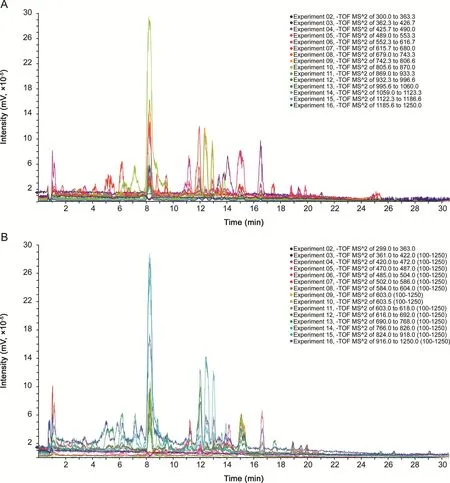
Fig.3.The MS2total ion chromatographs of sanguisorbins acquired in SWATH-MS mode at 35 V CE using fixed and variable Q1 isolation windows.(A)MS2TICs acquired in fixed Q1 isolation window,(B)MS2TICs acquired in variable Q1 isolation windows.
Next,rolling CE mode with collision energy spread of 4 eV was used to further investigate the influence of CE mode on the SWATHMS efficiency.The MS2TICs acquired in SWATH-MS mode at rolling CE using fixed and variable Q1 isolation windows are shown in Fig.S1.When the CE and Q1 isolation window were set at rolling mode and fixed 65 Da windows spanning the mass range 300-1250 Da,a total of 41 sanguisorbins with fragment ions were detected in sanguisorbin extract(Table S3).Compared with those detected in fixed CE 35 V and fixed Q1 isolation window(Table S1),12 other sanguisorbins were soughtout in rolling CE mode and fixed Q1 isolation window.This result fully showed that fragmentation efficiency of sanguisorbins in rolling CE mode was higher than that in fixed CE mode.Taking ziyuglycoside I as an example:4 fragment ions at m/z of 603,765 and 801 were dominated in its MS/MS spectrum when fixed CE and fixed Q1 isolation window were used(Fig.4A).The fragment ions at m/z of 603,765,801 and 811 could also be observed in ziyuglycoside I's MS/MS spectrum when fixed CE and variable Q1 isolation windows were used(Fig.4B).Once CE was changed to rolling mode,SWATH could produce at least eight fragment ions(m/z 131,223,541,585,603,765,801 and 811)for ziyuglycoside I(Figs.4C and D).The MS2spectrum of sanguisorbin II acquired under CE and Q1 isolation window conditions were compared to further confirm the optimum SWATH condition for qualitative analysis of sanguisorbins.As shown in Fig.S2,fragment ions of sanguisorbin II acquired in rolling CE mode were more abundant than those acquired in fixed CE mode.Besides,most of the other sanguisorbins acquired in rolling CE mode had far more fragment ions than those in fixed CE mode(Table S1 to Table S3).
The above results revealed that rolling CE and variable Q1 isolation windows could be more efficient for sanguisorbin detection than the fixed CE and fixed Q1 isolation window.To confirm the optimum SWATH parameters for qualitative analysis of sanguisorbins,rolling CE and variable Q1 isolation windows spanning the mass range 300-1250 Da were used to search for sanguisorbins in sanguisorbin extract.As shown in Table 2,47 sanguisorbins with fragment ions were tentatively identified.Compared to sanguisorbins collected at a fixed CE and variable Q1 isolation windows,12 other sanguisorbins were tentatively identified in rolling CE and variable Q1 isolation windows.Compared with those collected at a rolling CE and fixed Q1 isolation windows,5 other sanguisorbins(m/z 1073.5299,613.3118,867.40579,575.3024 and 743.35839)were found.Thus,rolling CE and variable Q1 isolation windows were the optimum SWATH conditions for sanguisorbin identification in sanguisorbin extract.
3.3.Searching for sanguisorbins and metabolites in rat plasma using SWATH data acquisition based on LC-Q-TOF MS
According to previous reports,the metabolic types of saponins in vivo mainly include deglycosylation,methylation,oxidation,reduction,etc[28,32].For instance,we found that ziyuglycoside I(M0)can be metabolized into 6 metabolites(M0-Glu-Ara+O,M0-Ara,M0-Glu-COOH,M0-Glu,M0-Glu-Ara+O and M0-Ara+H2O)in rat intestinal flora.In liver microsome,4 kinds of metabolites(M0-Glu,M0-CH2OH,M0-Glu+CH3,M0-Glu-Ara+CH3)of ziyuglycoside I were tentatively identified in our previous studies[32].In addition,17 metabolites of ziyuglycoside II,including 5 phase II metabolites,6 phase I redox metabolites and 5 deglycosylated metabolites,were tentatively identified in rat liver by our research group[28].
After intragastric administration of 200 mg/kg of sanguisorbin extract to the rats,hepatic portal vein blood was collected at 0.5,1,2,and 4 h to identify metabolites for sanguisorbins based on LC-QTOF MS.In this process,SWATH-MS technique under rolling CE and variable Q1 isolation window modes was used to acquire fragment ions of the metabolites.As shown in Table 3,a total of 26 metabolites of sanguisorbins were sought out in rat plasma.Clearly,the molecular weight of most metabolites was significantly lower than that of components in sanguisorbin extract,which indicated that the main metabolic pathway of sanguisorbins in rats was deglycosylation.In addition,the retention time of most metabolites was longer than that of prototype components,which further proves the above inference.Taking metabolites M1 and M2 for example:their m/z were both 407.28,which was lower than all prototype components in sanguisorbin extract.The metabolites M1 and M2 were deduced to be an aglycone produced by deglycosylation of sanguisorbins due to the molecular weight of these two metabolites was similar to that of aglycones.Similarly,M3,M5,M6,M9,M10,M19,M20 and M23 could also be tentatively identified as the deglycosylated metabolites of sanguisorbins according to their molecular weight and retention time.In addition,the m/z of M11 was 1017.6010(C52H90O19),which wasmuch higherthan allprototype components in sanguisorbin extract.According to its molecular weight and element composition,the metabolite M11 could be deduced to be a glucuronic acid conjugate of sanguisorbins.Similarly,M12,M13,M14 and M15 could also be phase II metabolites of sanguisorbins.For other metabolites,their molecular weight ranges from 600 to 900 Da,which was similar to that of the prototypes.According to the metabolic regularity of ziyuglycoside I/II developed in our previous studies,these metabolites could be induced to phase I metabolites of sanguisorbins,including oxidized,reductive and methylated products.Only a few major components,such as ziyuglycoside I and ziyuglycoside II,could be found in rat plasma which could be caused by two factors:(i)sanguisorbins have low bioavailability and low plasma exposure;(ii)sanguisorbins are widely metabolized in rats.The results above were consistent with the pharmacokinetic characteristics of saponins reported previously[31,33,34].
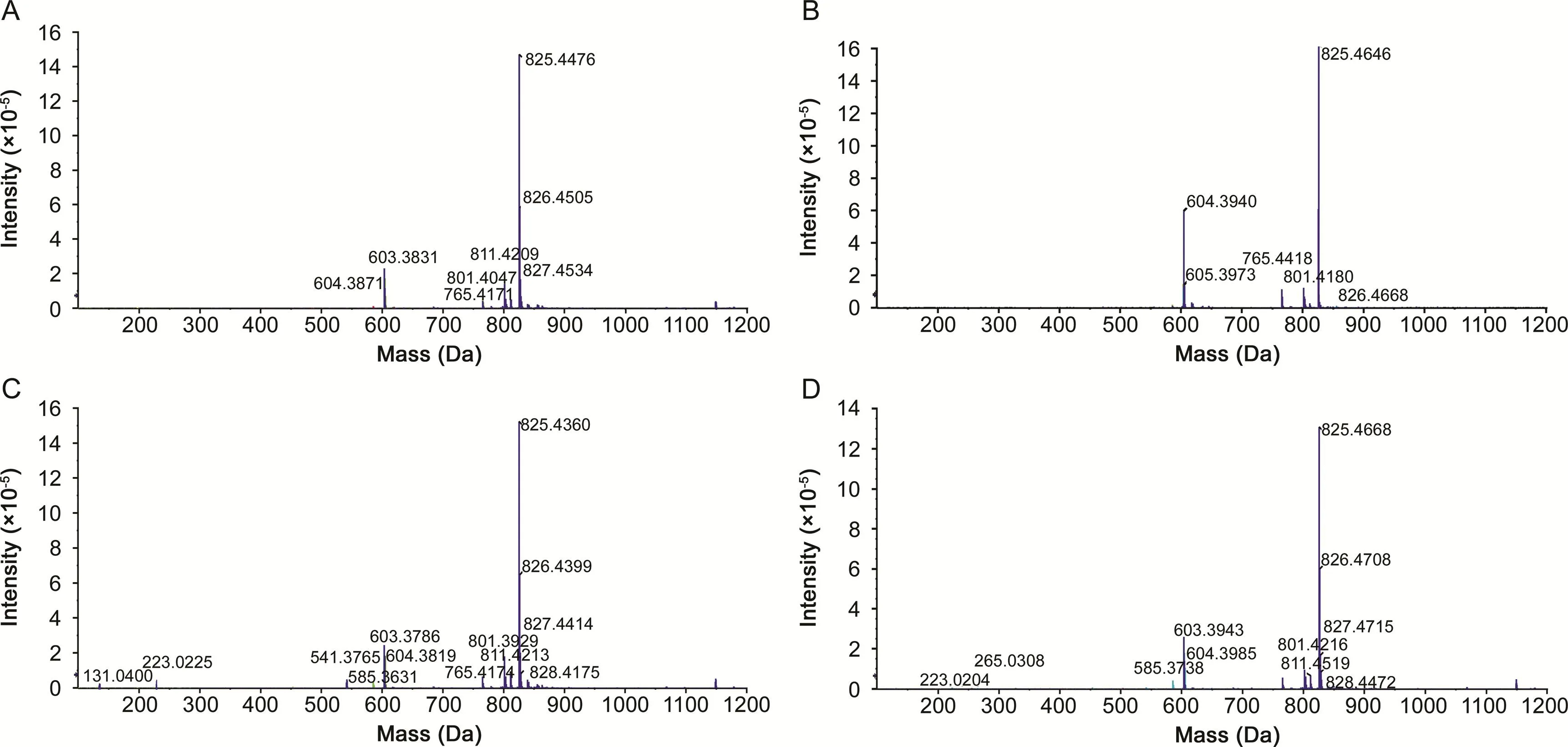
Fig.4.The MS2spectrum of ziyuglycoside I acquired in SWATH-MS mode at different CE values and Q1 isolation windows.(A)fixed CE and fixed Q1 isolation window,(B)fixed CE and variable Q1 isolation windows,(C)rolling CE and fixed Q1 isolation window,(D)rolling CE and variable Q1 isolation windows.

Table 2The information about the sanguisorbins identified in sanguisorbin extract using SWATH data acquisition at rolling CE and flexible acquisition window on LC-Q-TOF MS.

Table 3The information about the metabolites of sanguisorbins identified in rat plasma using SWATH data acquisition mode on LC-Q-TOF.
4.Conclusion
SWATH is a DIA method where the instrument deterministically fragments all precursor ions within the predefined m/z range in a systematic and unbiased fashion.So far,SWATH has been widely used in proteomics research since it was registered by SCIEX.To our knowledge,no studies have been published on the identification of components and metabolites for TCMs in vitro and in vivo based on SWATH technique.Herein,the feasibility and superiority of SWATH were systematically investigated by comparing the identification efficiency of SWATH-MS with that of IDA-MS for sanguisorbins in sanguisorbin extract.Furthermore,different CE(fixed and rolling)and Q1 isolation window(fixed and variable)modes were used to improve SWATH-MS data acquisition.Our results showed that rolling CE and variable Q1 isolation windows could be more efficient for sanguisorbin detection than the fixed CE and Q1 isolation window.More importantly,the qualitative efficiency of SWATH-MS was found significantly higher than that of IDA-MS.A total of 18 sanguisorbins with characteristic fragment ions at m/z 603,585,469 and 453 could be found using IDA-MS mode,and 46 sanguisorbins with more abundant fragment ions were detected using SWATH-MS data acquisition under rolling CE and variable Q1 isolation windows modes.In addition,26 metabolites of sanguisorbins were sought out in rat plasma,and their metabolic pathways could be inferred as decarbonylation,oxidization,reduction,methylation,glucuronidation,etc.
Compliance with ethical standards
All the animal experiments in the present study were approved by the Ethical Committee of Animal Experiments of China Pharmaceutical University.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation of China(81573559,81530098)and the Ministry of National Science and Technique(Grant No.2017ZX09309027).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2019.11.005.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Challenges for cysteamine stabilization,quantification,and biological effects improvement
- Offline two-dimensional liquid chromatography coupled with ion mobility-quadrupole time-of-flight mass spectrometry enabling fourdimensional separation and characterization of the multicomponents from white ginseng and red ginseng
- Single-run reversed-phase HPLC method for determining sertraline content,enantiomeric purity,and related substances in drug substance and finished product
- Development of a UHPLC-MS/MS method for the quantification of ilaprazole enantiomers in rat plasma and its pharmacokinetic application
- Use of subcutaneous tocilizumab to prepare intravenous solutions for COVID-19 emergency shortage:Comparative analytical study of physicochemical quality attributes
- Discovery of human coronaviruses pan-papain-like protease inhibitors using computational approaches
