Metabonomic analysis of plasma biochemical changes in pyrexia rats after treatment with Gegenqinlian decoction,aspirin and itraconazole by UHPLC-FT-ICR-MS
2021-01-21TingLiuRuiyunLiYueCuiZhiguoYuYunliZho
Ting Liu,Ruiyun Li,Yue Cui,Zhiguo Yu,Yunli Zho,*
aSchool of Pharmacy,Shenyang Pharmaceutical University,Shenyang,110016,China
bSchool of Pharmacy,Shenyang Medical College,Shenyang,110034,China
ABSTRACT
Keywords:
Gegenqinlian decoction
Aspirin
Itraconazole
Pyrexia
UHPLC-FT-ICR-MS
Metabonomic
1.Introduction
Pyrexia under pathological conditions is due to infection by various pathogens,such as those that cause influenza,pneumonia and typhoid.Pyrexia can also be caused by non-infectious diseases,such as heat stroke and malignant tumours.The central body that regulates heat production and dissipation is called the body temperature regulation center and is located in the hypothalamus.This center is similar to a thermostat.Under normal conditions,the body temperature is stable at the set point.If the actual body temperature is higher or lower than the normal value,the center will strengthen heat dissipation or production to maintain the normal body temperature.As a type of fungus,yeast consists of a thallus,capsular polysaccharide and protein.It either acts on the hypothalamic thermoregulatory center,releases a central mediator(endogenous pyrogen)of pyrexia and changes the set point[1]or induces acute ulcer and inflammation at the injection site[2].
The Gegenqinlian decoction(GQLD)is one of the most wellknown traditional Chinese medicine formulations.It was originally mentioned in the Shang Han Lun,which was written by Zhongjing Zhang in the Han Dynasty(202 BC-220 AD).The GQLD recipe consisted of Puerariae lobatae Radix(Gengen),Scutellariae Radix(Huangqin),Coptidis Rhizoma(Huanglian)and Glycyrrhizae Radix et Rhizoma Praeparata Cum Melle(Zhigancao)at the ratio of 8:3:3:2 by weight.GQLD exhibits antipyretic effect and now is commonly used clinically to treat fever[3,4].This medicine has other effects and is used to cure diabetes mellitus[5],diarrhoea[6]and hyperlipemia[7].Aspirin,also known as acetylsalicylic acid,is a derivative of salicylic acid.Aspirin is a long-established antipyretic and analgesic[8].This drug is widely distributed in the whole body and has a strong effect because it is easily absorbed after oral administration.Aspirin is extensively used to treat fever,headache and neuralgia.Itraconazole,a triazole antifungal agent,is a broadspectrum triazole.Ergosterol is a major component of cell membrane of yeast and fungal cells,itraconazole primarily prevents the synthesis of ergosterol by inhibiting the cytochrome P450 enzyme 14-alpha-demethylase,thereby preventing the growth of the microorganisms[9,10].
Metabonomics is an in-depth analysis of small molecules with m/z below 1000.This relatively modern technology has recently become increasingly popular in disease diagnosis and treatment.Non-targeted metabonomic studies aim to analyse many small molecules.The advantages of this process are highlighted when differences between various group samples are very subtle.Fourier transform ion cyclotron resonance mass spectrometry(FT-ICR-MS)measures m/z based on the ion cyclotron frequency in a given magnetic field.FT-ICR-MS possesses very high resolution and can measure compounds accurately.Biomarkers without reference substances are difficult to identify.FT-ICR-MS with high and stable mass accuracy is commonly used to analyse complex mixtures to detect molecular composition[11].This technique is suitable for the reliable analysis of biomarkers in vivo[2].
The aim of this study was to investigate the treatment of pyrexia rats by using GQLD,aspirin and itraconazole and to explore the underlying mechanism with metabonomic strategy.A UHPLC-FTICR-MS metabonomic approach was used for the metabolic profiling of rat plasma to provide a scientific basis for an in-depth understanding of the therapeutic effects of the three medicines on rats with yeast-induced pyrexia.
2.Methods
2.1.Chemicals and materials
The four herbs,i.e.,Gegen,Huangqin,Huanglian and Zhigancao,were purchased from Guoda Drug Store(Shenyang,China)and authenticated by Zhiguo Yu(School of Pharmacy,Shenyang Pharmaceutical University).
The reference compounds of Lysophosphatidylcholine(LPC)(16:0),LPC(18:0),LPC(18:1)and LPC(18:2)and tryptophane were obtained from Sigma Corporation(St.Louis,MO,USA).Acetonitrile of HPLC grade were obtained from Fisher Inc.(Town,USA).Formic acid of HPLC grade was obtained from Concord Technology(Tianjin,China).The purified water was purchased from Wahaha(Hangzhou,China).
2.2.Animals and sample collection
Healthy male Wistar rats weighing 240±20 g were purchased from Experimental Animal Center of Shenyang Pharmaceutical University(Shenyang,China).All rats were housed in the breeding room(temperature:23±2°C;humidity:50±10%;12 h light/dark cycle),acclimated to the rooms for 7 days,then transferred and allowed to acclimatize for an additional 3 days in individual metabolism cages.Standard food and water were supplied ad libitum.Animal experiment was carried out in accordance with the Guideline for Animal Experimentation of Shenyang Pharmaceutical University and the protocol was approved by the Animal Ethics Committee of the institution.The rats' rectal temperature was measured three times daily for obtaining the regular rhythm of body temperatures using a digital thermometer.Rats with a temperature difference greater than 0.5°C were excluded.
The GQLD was prepared according to the recipe of Gegenqinlian tablets and Gegenqinlian granules listed in the Chinese Pharmacopoeia(2015 version)[12].And GQLD was prepared in the same way as our previous study[11].Rats in the control group(CG)and model group(MG)were given normal saline solution;rats in the itraconazole group(IG)were orally administered with itraconazole at a dose of 18 mg/kg;rats in the aspirin group(AG)were orally administered with aspirin at a dose of 140 mg/kg;rats in the GQLD group(GQLDG)were orally administered with GQLD at a dose of 1.728 g/kg(equivalent to GQLD raw herbs of 4.28 g/kg)daily for 7 days.On the seventh day,the rats in the MG,IG,AG and GQLDG were subcutaneously injected with a 20% aqueous suspension of yeast(10 mL/kg)at the back below the nape,and the rats in CG were subcutaneously injected with normal saline solution(10 mL/kg)at the back below the nape.After 3 h,the rats in the IG,AG and GQLDG were orally administered with itraconazole,aspirin and GQLD,respectively,and the rats in CG and MG were orally administered with an equal amount of normal saline solution.Rats not administered with medicine were given an equal amount of normal saline solution during the experimental period.
The rectal temperature was measured,and the blood was collected via the fosse orbital vein in heparinised tubes at 6 h after oral administration and immediately centrifuged at 4,000 rpm for 10 min to separate the plasma.The samples were then transferred to clean tubes.All the collected samples were stored at-80°C before analysis.
2.3.Sample preparation
Plasma samples were deproteinised by adding 200μL of acetonitrile to 100μL of plasma.The mixture was vortexed for 3 min and centrifuged at 12,000 rpm for 10 min at 4°C.The supernatant was evaporated to dryness under a gentle nitrogen stream at 30°C and redissolved in 100 μL of acetonitrile-water(10:90,V/V).The supernatant was then vortexed for 3 min and centrifugated at 12,000 rpm for 10 min at 4°C.An aliquot(5 μL)of the supernatant was injected for UHPLC-FT-ICR-MS analysis.Aliquots of all plasma samples collected during the study were mixed to generate pooled quality control(QC)samples which were treated as above and used for monitoring the applied method.
2.4.Measurement of plasma biochemical parameters
The T3,T4,TSH and IL-1β levels in the plasma were determined with ELISA kits,and the level of pyruvate was measured with commercial detection kits(Nanjing Jiancheng Bioengineering Institute,Nianjing,China).All models were validated according to the manufacturers' instructions.
2.5.UHPLC-FT-ICR-MS profiling analysis
UHPLC-FT-ICR-MS experiments were performed on a Bruker Solarix 7.0T FT-ICR-MS system(Bruker,Germany)attached to an Agilent 1260 UHPLC system(Agilent,USA).In brief,a 5μL of injected plasma sample was separated by an HSS T3 column(2.1 mm×100 mm,1.8μm;Waters Corporation,Milford,UK)with a 0.1% formic acid in water(A)and 0.1% formic acid in acetonitrile(B)gradient system at column temperature of 35°C.The gradient elution of the mobile phase was performed for 25 min at a flow rate of 0.30 mL/min according to the following gradient conditions:0-1.5 min,an isocratic elution of 8%B;1.5-3.5 min,8%-30%B;3.5-9.0 min,30%-60% B; 9.0-12.0 min,60%-70% B;12.0-16.0 min,70%-99%B;16.0-20.0 min,an isocratic elution of 99%B;20.0-21.0 min,99%-8%B,and maintained until 4.0 min for column balance.
The MS analysis was performed on an FT-ICR mass spectrometer configured with electrospray ionisation interface in positive ion mode,acquiring in full scan spectra from m/z 50 to m/z 1000 for 0 min to 25 min.The capillary voltage was maintained at 4500V.The endplate offset was maintained at-500 V.The dry gas flow and temperature were maintained at 8 L/min and 200°C.The nebuliser gas pressure was4bar.The average scan was1.The ion accumulationtime was 0.15 s.High-purity argon and high-purity nitrogen were used as the collision and nebulising gases,respectively.All data acquisition and analysis were processed by FT-MS control,Bruker Compass-Hystar and Data Analysis Software(Bruker,Germany).
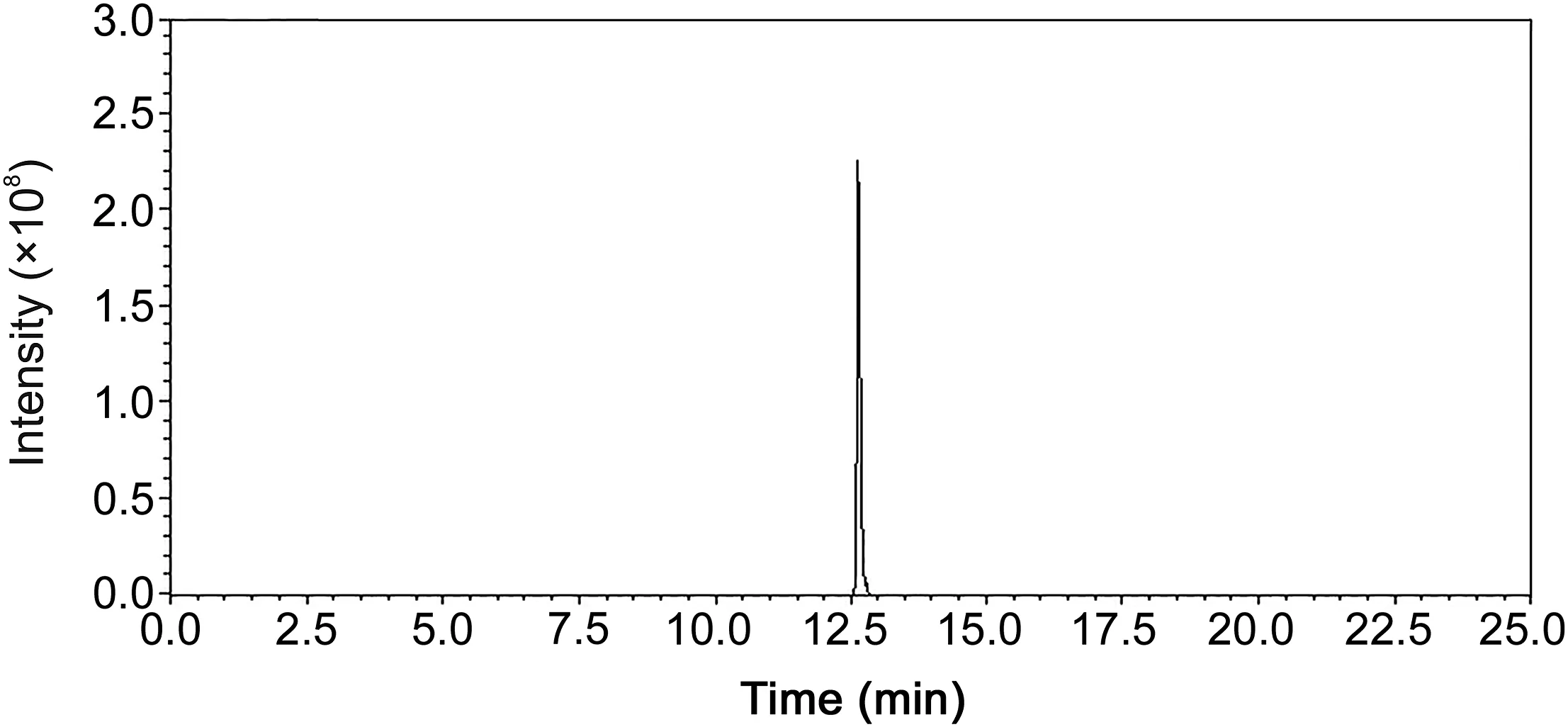
Fig.1.Extracted ion chromatogram of ion with the retention time and m/z pair of 12.66_373.27369 from quality control sample based on UHPLC-FT-ICR-MS.
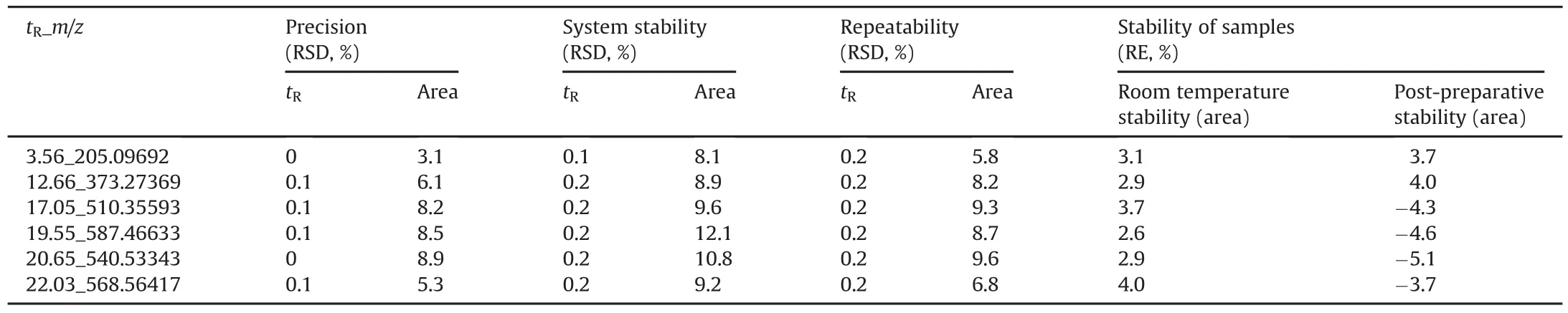
Table 1The analytical performance of plasma samples.
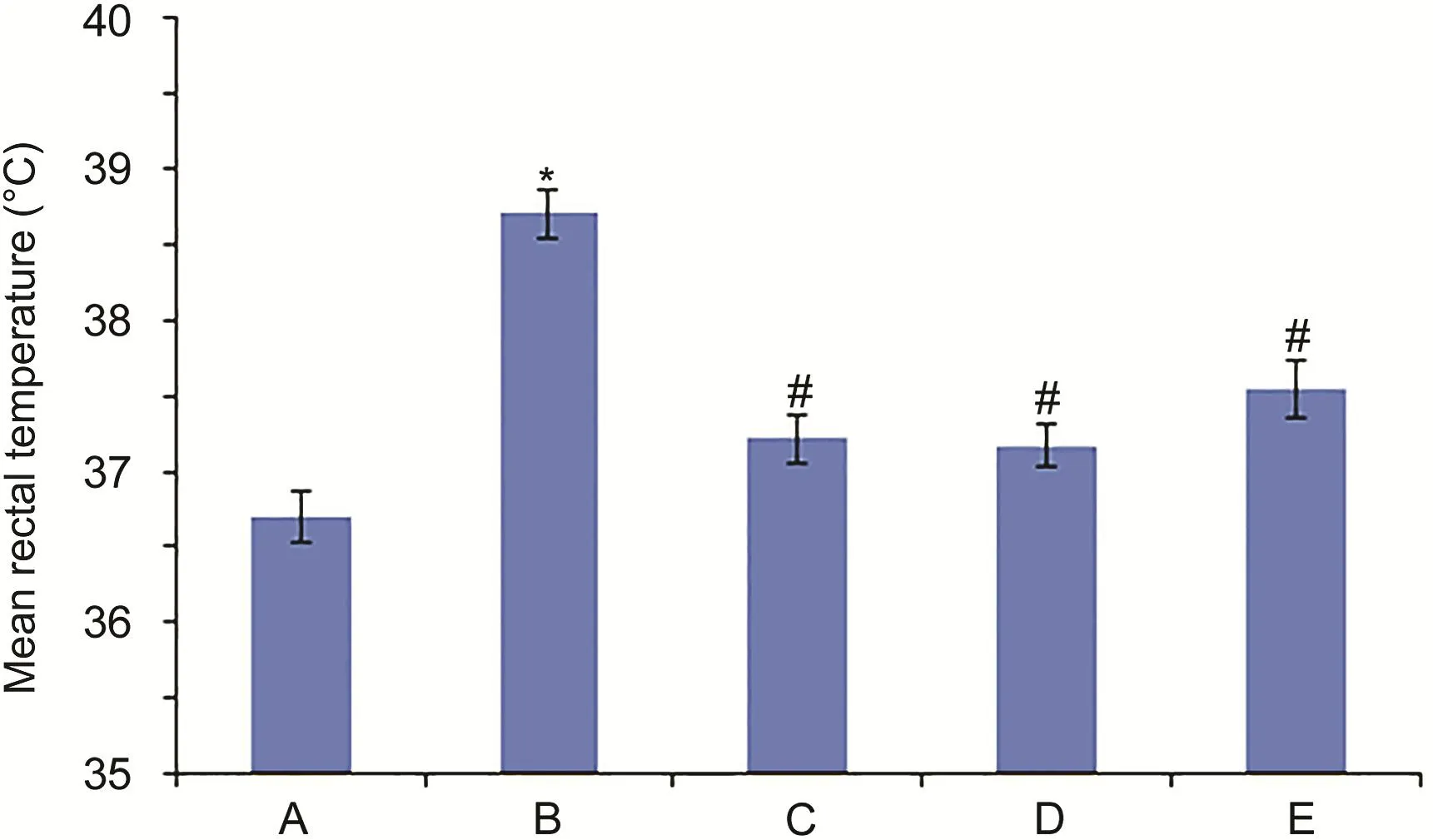
Fig.2.Rectal temperatures of rats in the different groups.(A)CG,(B)MG,(C)IG,(D)AG and(E)GQLDG.*Compared with A,P<0.01;#Compared with B,P<0.01.
The applied method was validated for the system stability,precision of instrument,method repeatability,post-preparative stability of the samples and the room temperature stability of the samples.System stability was examined by injecting a QC sample to every 10 specimens during the entire sample analysis.Precision of the instrument and method repeatability were examined by injecting six replicated injections from the same QC sample and six different QC samples obtained through the same preparation procedure.Six freshly prepared QC samples maintained at 4°C for 24 h were used to determine the post-preparative stability.Six QC samples stored for 4 h at room temperature were used to determine the room temperature stability.Six ions were selected to validate the method.The extracted ion chromatogram of ion with the retention time and m/z pair of 12.66_373.27369 from QC sample is shown Fig.1.The relative standard deviations(RSDs)of retention times and peak area intensities of selected ions were calculated and the RSD values were all less than 15%.As shown in Table 1,these results indicated that the method was reliable.

Table 2The results of biochemistry(mean±SD,n=6).
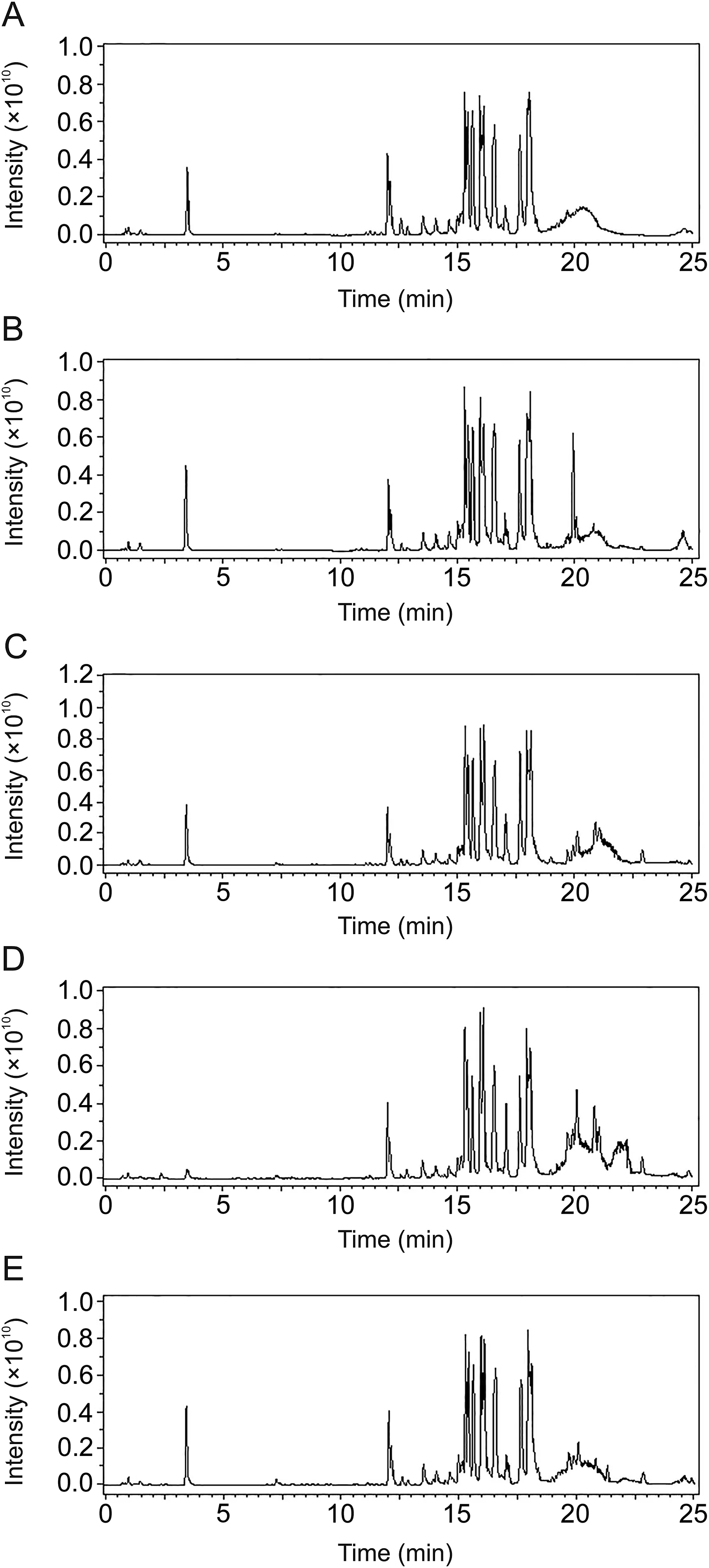
Fig.3.Positive ion full-scan chromatograms of representative plasma samples from(A)CG,(B)MG,(C)IG,(D)AG and(E)GQLDG based on UHPLC-FT-ICR-MS.
2.6.Data analysis
The Profile Analysis software(Bruker,Germany)was used to analyse the UHPLC-FT-ICR-MS raw data by employing for peak extraction,peak alignment,retention time,the mass and intensity of the peaks of each sample.The main filter parameters for data collection are as follows:the retention time range 0-25 min;mass range 50-1000 Da;rectangular bucketing min delta 0.2 min,and rectangular bucketing m/z delta 0.01 Da.The 80% rule was used for peak integration[13].The resultant data were imported to partial least squares discriminant analysis(PLS-DA)using the SIMCA-P program(version 11.0,Umetrics,Sweden).The statistical significance of the mean values was tested using the Student's t-test through SPSS17.0(IBM,New York,USA).Differences were considered statistically significant when the P-values were less than 0.05.Variables with project values(VIP)>3 and P-values<0.05 were regarded as the potential biomarkers.
The potential biomarkers were identified by comparing the molecular ions with compounds and pathways analysed in the available online databases,such as HMDB,KEGG and METLIN.Further identifications were then made by the MSntechnique.
3.Results
3.1.General observation
The rectal temperatures were determined by a digital thermometer and are shown in Fig.2.GQLD,aspirin and itraconazole exerted significant therapeutic effects on the pyrexia rats.
3.2.Biochemical assay
The levels of pyruvate,T3,T4,TSH and IL-1β in the rat plasma are listed in Table 2.Pyruvate is related to energy,while T3,T4 and TSH are usually used to evaluate the overall thyroid function.IL-1β is a proinflammatory cytokine that promotes inflammation.The plasma level of pyruvate proved that glycolysis was enhanced.Increased amounts of T3,T4 and TSH in the plasma of MG rats were also detected,thereby indicating that the thyroid functions of these rats were activated.An obvious increase in the IL-1βlevel in the plasma of the MG rats suggested that inflammation occurred in these rats.After treatment with GQLD,aspirin or itraconazole,the plasma levels of pyruvate,T3,T4,TSH and IL-1β were all downregulated in the GQLDG,AG and IG rats compared with the MG rats.This result suggested that GQLD,aspirin and itraconazole had a therapeutic effect on pyrexia rats.These three medicines could alleviate symptoms in pyrexia rats.
3.3.Metabonomic analysis based on UHPLC-FT-ICR-MS
The base peak intensity chromatograms of the plasma metabolic profiles from the CG,MG,GQLDG,AG and IG are shown in Fig.3.The score plot of PLS-DA illustrates the intrinsic clustering of group data based on the dissimilarity or similarity of their input coordinates.The PLS-DA score plots of the different groups are shown in Fig.4.The PLS-DA score plot in Fig.4 shows a good separation of CG and MG,thereby indicating the distinct perturbations between the two groups.GQLD,aspirin and itraconazole adjusted the abnormal pathways in different degrees and made the GQLDG,AG and IG approach the normal state.Compared with GQLDG,AG and IG were closer to CG.In addition,IG was the closest to CG,indicating the etiological treatment was better than symptomatic and GQLD treatments.
As shown in Fig.5,the variables far from the center of the plot contributed greatly to the group classification.The criteria of potential biomarkers were VIP>3 and the student's t-test(P<0.05).The structures of the metabolites were deduced via an integrated method.Firstly,retention time,the accurate quasi-molecular mass and the MS information about fragmentation were studied.The composition of the compound was calculated manually by using the smart formula of the Bruker Data Analysis Workstation,and the composition with very little error and a high score was considered.Then,the databases of HMDB and METLIN were searched for matching compounds.A total of 36 metabolites were identified or putatively identified in the plasma.
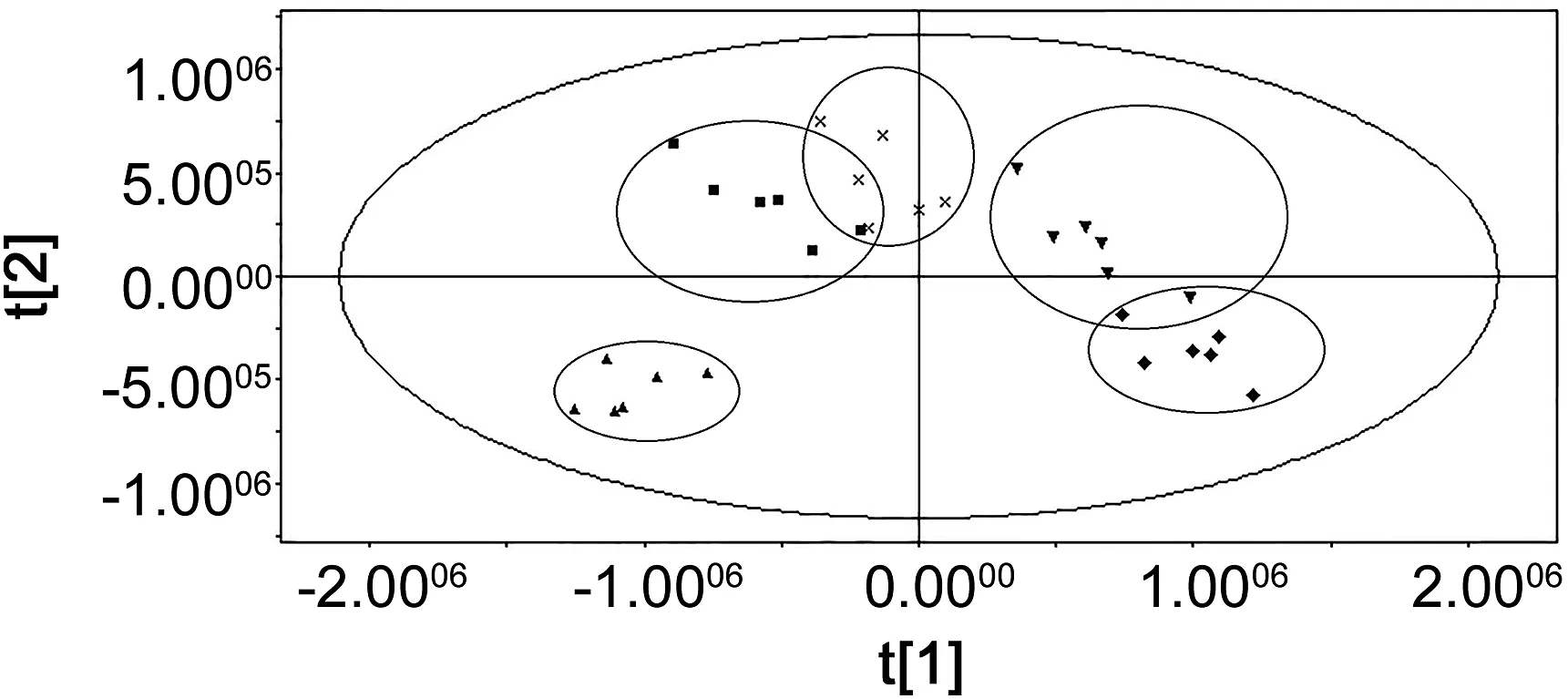
Fig.4.The score plot of PLS-DA results based on the plasma metabolic profiling.CG(◆),MG(▲),IG(▼),AG(×)and GQLDG(■).
As shown in Table 3,compared with those of the MG rats,the levels of LPC(14:0),LPC(16:0),LPC(18:0),Cer(d18:0/12:0),N-[(4E, 8E)-1,3-dihydroxyoctadeca-4,8-dien-2-yl]hexadecanamide,diglyceride(DG)(14:1/18:4),α-linolenoyl ethanolamide,tryptophan,acetylcarnitine,palmitoylcarnitine and stearoylcarnitine were down-regulated,while the levels of LPC(18:3),LPC(16:1),LPC(15:0),LPC(18:2),LPC(20:4),LPC(22:4),LPC(P-18:0),LPC(20:1),LPC(O-18:0),LPC(22:1),lysophosphatidylethanolamine(LPE)(0:0/22:1),LPE(0:0/22:0),phytosphingosine,palmitic amide,oleamide,octadecanamide,fatty acid amide C22:2 and fatty acid amide C22:1 were up-regulated in the GQLDG rats.Compared with those of the MG rats,the levels of LPC(14:0),LPC(18:0),LPE(18:2),Cer(d18:0/12:0),N-[(4E,8E)-1,3-dihydroxyoctadeca-4,8-dien-2-yl]hexade canamide,DG(14:1/18:4),tryptophan,acetylcarnitine,palmitoylcarnitine and stearoylcarnitine were down-regulated,whereas the levels of LPC(18:3),LPC(16:1),LPC(18:2),LPC(18:1),LPC(22:4),LPC(P-18:0),LPC(20:2),LPC(20:1),LPC(O-18:0),LPC(22:1),phytosphingosine,palmitic amide,oleamide and fatty acid amide C22:1 were up-regulated in the AG rats.Compared with those of the MG rats,the levels of LPC(14:0),LPC(18:0),LPE(16:0),Cer(d18:0/12:0),N-[(4E, 8E)-1,3-dihydroxyoctadeca-4,8-dien-2-yl]hexadecanamide,DG(14:1/18:4),tryptophan,acetylcarnitine,palmitoylcarnitine and stearoylcarnitine were down-regulated,whereas the levels of LPC(16:1),LPC(18:2),LPC(18:1),LPC(22:4),LPC(P-18:0),LPC(20:1),LPC(O-18:0),LPC(22:1),LPE(0:0/22:1),phytosphingosine,palmitic amide,oleamide,fatty acid amide C22:2 and fatty acid amide C22:1 were up-regulated in the IG rats.
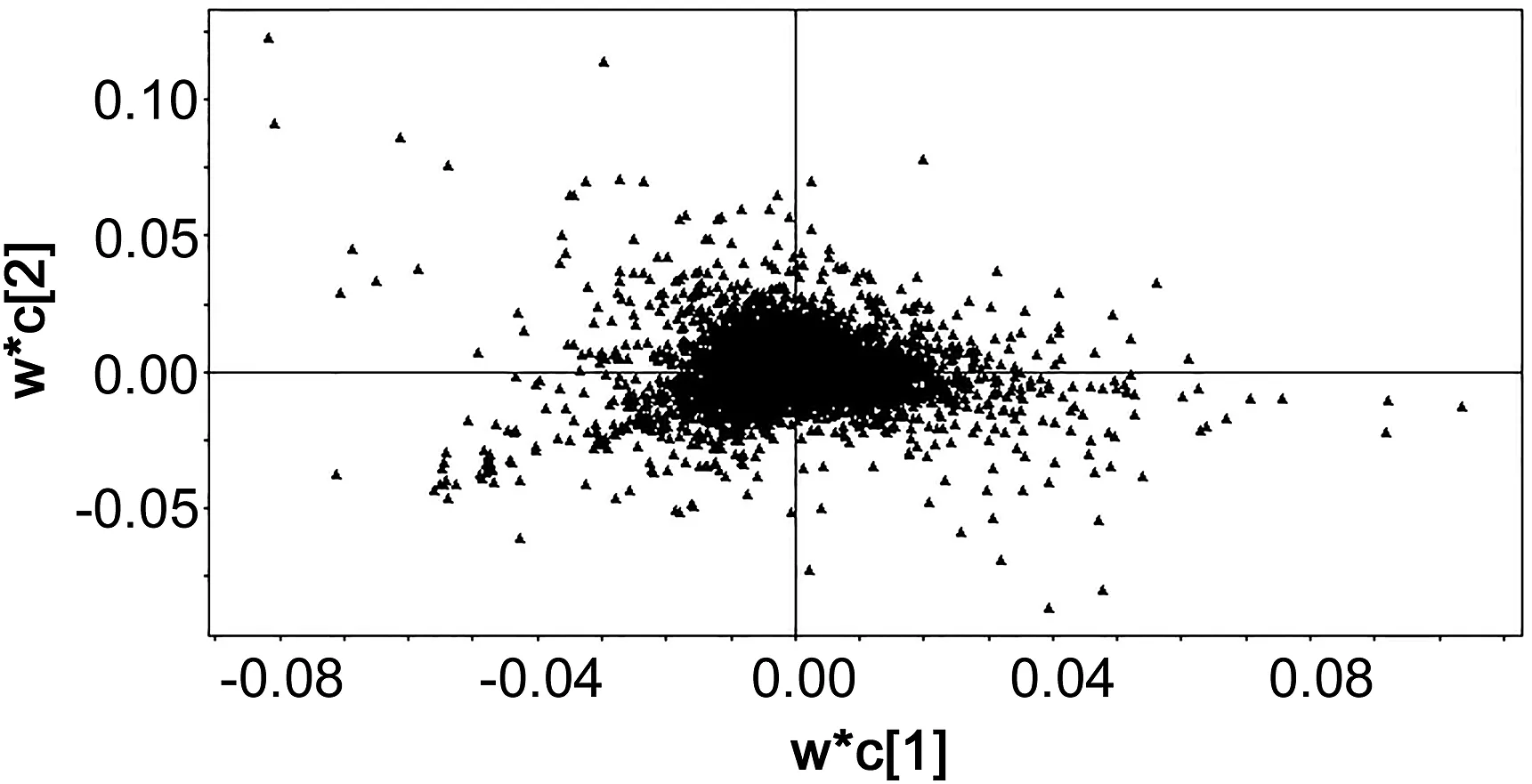
Fig.5.The loading plot of PLS-DA results based on the plasma metabolic profiling.
The results of plasma metabolomic study in the pyrexia rats showed that itraconazole,aspirin and GQLD exhibited obvious therapeutic effects on the pyrexia rats.However,the main biomarkers of the rats in GQLDG,AG and IG differed slightly.
4.Discussion
According to the usage and dose of Gegenqinlian Pills in the Pharmacopoeia[12],it is orally taken 3-4 tablets×3 times a day,and converted into a raw drug that is orally administered at a dose of 18-24 g daily(human daily dose).In the present study,the dose of GQLD administered to rats was chosen as 4.28 g/kg.And the doses of aspirin and itraconazole were determined to be 140 mg/kg and 18 mg/kg,respectively.
In this study,the plasma changes of pyrexia treated by the three medicines were explored by studying the metabolites in vivo.The results indicated that etiological treatment was better than symptomatic treatment and treatment with GQLD.In addition,we have identified or putatively identified 36 plasma metabolites and studied the effects of the three medicines on the 36 metabolites.Metabolic changes were observed as follows:metabolisms of the amino acid,phospholipid,sphingolipid,fatty acid oxidation,fatty acid amides,and glycerolipid.
LPC is the fundamental component of cellular membranes and mediates signal transduction.Phospholipid metabolism is related to autoimmune regulation and inflammatory response[14,15].Itraconazole,aspirin and GQLD could significantly prevent the change in the LPC contents in pyrexia rats,improve the metabolism of LPC and LPE in the body,and prevent inflammation in different degrees.
Ceramide and sphingosine are increasingly considered to be important cellular signals for inducing cell growth,differentiation,aging and apoptosis[16,17].Sphingolipid metabolism disorders may be associated with cell death and inflammation[18-20].Itraconazole,aspirin and GQLD can significantly prevent changes in the sphingomyelin content of pyrexia rats,improve the sphingomyelin metabolism disorder,and prevent cell death and inflammation.
DG is a second messenger associated with the uptake and utilisation of glucose,storage and movement of fat and muscle contraction[21].The level of DG in the pyrexia rats increased significantly,thereby indicating that the glycerolipid metabolism was accelerated.The body responded by automatically strengthening the tension in the muscle of the pyrexia rats[22].Itraconazole,aspirin and GQLD showed good effects on the regulation of the metabolism of DG.
Fatty acid amides are related to food intake,inflammation,energy balance,immune status,anxiety,depressive effects and so on[23].Oleamide is associated with thermoregulation,sleep,nociception and multiple neurotransmitter systems[24].The levels of fatty acid amides were decreased in the pyrexia rats,thereby indicating that the pyrexia rats experienced anxiety and restlessness.α-Linolenoyl ethanolamide belongs to N-acylethanolamines involved in acute inflammation.Increased α-linolenoyl ethanolamide was found in the pyrexia rats,indicating that these pyrexia rats suffer from severe inflammation.Itraconazole,aspirin and GQLD could effectively regulate the metabolism of fatty acid amide and prevent the decrease in the oleamide level in rats during fever.This phenomenon considerably prevents the increase in the αlinoleoyl ethanolamide level in the plasma of MG rats,thereby alleviating oxidative metabolism and preventing inflammation.
Tryptophan has been related to energy production or the formation of biologically active substances.Prior studies have reported the increased level of tryptophan in pyrexia rats[25].The GQLDG,AG and IG rats presented lower levels of tryptophan than the MG rats and tended to be a normal level.This phenomenonindicated that itraconazole,aspirin and GQLD could regulate the metabolism of tryptophan to ensure the stability of various neurotransmitters.
Table 3Biomarkers tentatively identified in rat plasma.
Acylcarnitines are essential intermediates in energy metabolism[26,27].For carnitine palmitoyltransferase II deficiency,increased levels of representative long-chain acylcarnitines of palmitoylcarnitine and stearoylcarnitine occur during apoptosis[26,27],which was also proved by the rectal temperature results in this study.Increased level of acylcarnitines in the MG rats compared with the CG rats was detected in the plasma,thereby indicating that pyrexia could accelerate fatty acid β-oxidation metabolism.Hence,high activity of fatty acid β-oxidation metabolism occurred in the pyrexia rats.The decreased levels of acylcarnitines,palmitoylcarnitine and stearoylcarnitine in the GQLDG,AG and IG rats indicated that the three drugs exhibited excellent regulation of the fatty acid β-oxidation.Itraconazole,aspirin and GQLD could prevent and improve the lack of oxygen supply in the body during pyrexia,ensure the oxidation function of free fatty acids,and avoid the accumulation of acylcarnitine,palmitoylcarnitine and stearoylcarnitine in the plasma.
In biological processes,the endogenous substances of pyrexia rats treated with GQLD,aspirin and itraconazole were slightly different.This study could provide a scientific basis for an in-depth understanding of the therapeutic effects of GQLD,aspirin and itraconazole on rats with yeast-induced pyrexia.
5.Conclusion
This study focused on studying the potential biomarkers for pyrexia treated by GQLD,aspirin and itraconazole by using UHPLCFT-ICR-MS metabonomic analysis and clarifying the therapeutic and metabolic effects on pyrexia rats treated with the three medicines.Thirty-six plasma metabolites were identified or putatively identified,and the effects of the three medicines on the thirty-six metabolites were studied.In the metabolic profile investigation,changes in the plasma metabolites were primarily involved in metabolisms of phospholipid,sphingolipid,amino acid,fatty acid amides,fatty acid oxidation,and glycerolipid.GQLD,aspirin and itraconazole showed different therapeutic effects on the pyrexia rats.Elucidating the mechanism of these three medicines in the treatment of pyrexia on the basis of plasma metabolites was very important for the development of pyrexia treatment strategies.Holistic metabonomic strategy based on UHPLC-FT-ICR-MS was beneficial for exploring potential biomarkers,uncovering differences in the small molecule profiles of different therapeutic medicines and evaluating the overall mechanism of the three medicines in vivo.The study provided a scientific basis for an in-depth understanding of the therapeutic effects of GQLD,aspirin and itraconazole on rats with yeast-induced pyrexia.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Funding:This work was supported by the Natural Science Foundation of China(NO.81573629).
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Challenges for cysteamine stabilization,quantification,and biological effects improvement
- Offline two-dimensional liquid chromatography coupled with ion mobility-quadrupole time-of-flight mass spectrometry enabling fourdimensional separation and characterization of the multicomponents from white ginseng and red ginseng
- Single-run reversed-phase HPLC method for determining sertraline content,enantiomeric purity,and related substances in drug substance and finished product
- Development of a UHPLC-MS/MS method for the quantification of ilaprazole enantiomers in rat plasma and its pharmacokinetic application
- Use of subcutaneous tocilizumab to prepare intravenous solutions for COVID-19 emergency shortage:Comparative analytical study of physicochemical quality attributes
- Discovery of human coronaviruses pan-papain-like protease inhibitors using computational approaches
