Hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis:A multicenter propensity scorematched cohort study
2021-01-18ZiyingLeiJiahongWangZhiLiBaozhongLiJialiLuoXuejunWangJinWangMingchenBaHongshengTangQingjunHeQuanxingLiaoXianshengYangTianpeiGuanHanLiangShuzhongCuionbehalfoftheChinesePeritonealOncologyStudyGroup
Ziying Lei,Jiahong Wang,Zhi Li,Baozhong Li,Jiali Luo,Xuejun Wang,Jin Wang,Mingchen Ba,Hongsheng Tang,Qingjun He,Quanxing Liao,Xiansheng Yang,Tianpei Guan,Han Liang,Shuzhong Cui,on behalf of the Chinese Peritoneal Oncology Study Group
1Department of Abdominal Surgery,Affiliated Cancer Hospital &Institute of Guangzhou Medical University,Guangzhou 510095,China;2Department of General Surgery,Affiliated Tumor Hospital of Zhengzhou University,Tumor Hospital of Henan Province,Zhengzhou 450008,China;3 Department of Surgery,Anyang Tumor Hospital,Anyang 455000,China;4 Department of Oncology,Guangzhou Medical University,Guangzhou 510095,China;5Department of Gastrointestinal Cancer,Tianjin Medical University Cancer Institute &Hospital,National Clinical Research Center of Cancer,Key Laboratory of Cancer Prevention and Therapy,Tianjin’s Clinical Research Center for Cancer,Tianjin 300060,China
Abstract Objective:Systemic chemotherapy has limited efficacy in the treatment of peritoneal metastasis (PM) in gastric cancer (GC).Hyperthermic intraperitoneal chemotherapy (HIPEC) combined with complete cytoreductive surgery(CRS) has shown promising outcomes but remains controversial.The present study aimed to evaluate the safety and efficacy of HIPEC without CRS in GC patients with PM.Methods:This retrospective propensity score-matched multicenter cohort study included GC patients with PM treated with either chemotherapy alone (Cx group) or with HIPEC combined with chemotherapy (HIPEC-Cx group) in four Chinese high-volume gastric medical centers between 2010 and 2017.The primary outcomes were median survival time (MST) and 3-year overall survival (OS).Propensity score matching was performed to compensate for controlling potential confounding effects and selection bias.Results:Of 663 eligible patients,498 were matched.The MST in the Cx and HIPEC-Cx groups was 10.8 and 15.9 months,respectively [hazard ratio (HR)=0.71,95% confidence interval (95% CI),0.58-0.88;P=0.002].The 3-year OS rate was 10.1% (95% CI,5.4%-14.8%) and 18.4% (95% CI,12.3%-24.5%) in the Cx and HIPEC-Cx groups,respectively (P=0.017).The complication rates were comparable.The time to first flatus and length of hospital stay for patients undergoing HIPEC combined with chemotherapy was longer than that of chemotherapy alone (4.6±2.4 d vs.2.7±1.8 d,P<0.001;14.2±5.8 d vs.11.4±7.7 d,P<0.001),respectively.The median follow-up period was 33.2 months.Conclusions:Compared with standard systemic chemotherapy,HIPEC combined with chemotherapy revealed a statistically significant survival benefit for GC patients with PM,without compromising patient safety.
Keywords:Gastric cancer;peritoneal metastasis;hyperthermic intraperitoneal chemotherapy;chemotherapy
Introduction
Peritoneal metastasis (PM) is detected in 10%-30% of patients with gastric cancer (GC) at the time of initial diagnosis,and over 50% of patients with stage II-III tumor develop PM within 5 years after curative surgery (1,2).Also,PM is the most common pattern of disease relapse and is the primary cause of GC-associated mortality (3).The median survival of GC patients with PM has been reported to be 3-9 months,even when treated with standard systemic chemotherapy (4,5).Therefore,PM is often considered incurable in patients with GC,which is attributed to the dismal prognosis of the disease.The current treatment options for these patients are palliative chemotherapy and supportive care;however,they have limited efficacy (6).Intraperitoneal chemotherapy seems to be an intuitive and attractive approach in the treatment of patients with PM.
Hyperthermic intraperitoneal chemotherapy (HIPEC)combined with cytoreductive surgery (CRS) has been proposed over the past 30 years as a treatment modality for PM (7).In some case series studies,it has been suggested that CRS combined with HIPEC could prolong overall survival (OS) of GC patients with PM (8-11).In the recent CYTO-CHIP study,which included 277 GC patients with PM,the propensity score analysis revealed that compared to CRS alone,patients who received HIPEC combined with CRS had significantly better OS (5-year OS,19.87%vs.6.43%,respectively) (12).However,CRS-related perioperative morbidity (55.3%vs.53.7%,for CRS and HIPEC+CRS,respectively) and 90-day mortality (10.1%vs.7.4%,respectively) seemed to indicate that only highly selected patients with limited PM from GC might benefit from this modality.Standardized CRS is extremely technical demanding and is rarely performed,even by specialized surgeons.In addition,most PM in GC patients is too extensive for CRS to achieve satisfactory cytoreduction (13,14).Therefore,it is important to evaluate the role of HIPEC alone in improving survival of GC with PM.However,the available evidence has heterogeneous results.Although many studies have suggested the benefits of treatment with HIPEC,others have demonstrated controversial results (8,12,15,16).
To fill this gap,we evaluated the efficacy and safety of HIPEC without CRS combined with systemic chemotherapy in the treatment of 498 GC patients with PM from four members of the Chinese Peritoneal Oncology Study (CPOS) group.
Materials and methods
Definition of P stage
The extent of PM was stratified according to the first English edition of the Japanese Classification of Gastric Carcinoma as follows:P0,no peritoneal seeding;P1,metastasis to the region directly adjacent to the peritoneum of stomach in supracolic department;P2,oligo metastases to distant peritoneum;and P3,numerous metastases throughout the peritoneal cavity (17).
Patient population
The present retrospective multicenter study was conducted by members of the Chinese Peritoneal Oncology Study group,who analyzed the records of 1,103 patients with stage IV GC treated between January 2010 and May 2017 at four centers (Figure 1).Five patients also had other concurrent malignancies,and 256 patients had metastatic disease beyond the peritoneal cavity (including hepatic,lung,brain,bone,and other distant metastases);none of these patients were included in the study.A total of 179 were excluded from the study because of incomplete data.Finally,663 (60.1%) GC patients with PM who had complete data were enrolled in the study.Peritoneal involvement of gastric origin was confirmed in all patients through laparoscopy, open exploration, imaging, or histological diagnosis.
Of the 663 eligible patients,405 (61.1%) and 258(38.9%) patients received HIPEC combined with chemotherapy (HIPEC-Cx) and chemotherapy alone (Cx),respectively.Systemic chemotherapy included a 5-fluorouracil-based (90.2%) or paclitaxel-based (9.8%)regimen.Compared with patients in the Cx group,patients in the HIPEC-Cx group were significant younger(51.7±11.8 years oldvs.56.5±13.4 years old,P<0.001),and had more ascites (little:22.2%vs.15.5%;moderate:10.4%vs.6.2%;large:16.5%vs.7.8%,respectively,P<0.001).Therefore,propensity score matching (PSM) was performed to compensate for controlling potential confounding effects and selection bias based on the two variables:age and ascites status.The study was approved by the Institutional Review Boards and the Ethics Committees of Affiliated Cancer Hospital &Institute of Guangzhou Medical University.Written informed consent was waived because of the retrospective design.
Definition of ascites quantity
Ascites was defined as “little” (fluid found only below the pelvic rim),“moderate” (fluid found above the pelvic rim)or “large” (fluid found throughout the abdominal cavity) by computed tomography or sonography (18).
HIPEC procedures
The closed HIPEC technique was standardized among the four participating centers.Two inflow tubes were placed in the upper abdomen,and two outflow tubes were placed in the lower abdomen.Subsequently,the heated perfusate was circulated at a flow rate of 400-600 mL/min and a perfusion volume of 2 L/m2for 60 min using the BRTRG-I hyperthermic perfusion intraperitoneal treatment system (Bright Medical Tech,Guangzhou,China).HIPEC was carried out at 43±0.1 °C with paclitaxel (75-100 mg/m2)or platinum (oxaliplatin:100-130 mg/m2or cisplatin:50-75 mg/m2) as chemotherapeutic agents.HIPEC was recommended to be conducted on d 1,3,and 5,respectively,with a mean frequency of 2.1±1.0 times.
Follow-up
The primary outcomes were median survival time (MST)and 3-year OS.The last patient was seen in June 2019.Data were collected from the outpatients’ visit or via telephone calls and letters for patients who could not attend regular hospital visits.Data of patients who were alive at the cutoff date or who were lost to follow-up was defined as censored data.Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (Version 4.0).
Statistical analysis
To balance the baseline characteristics and treatmentrelated factors between treatment groups,we performed a PSM analysis to minimize the potential selection bias.We calculated the propensity score for each patient as the predicted probability of having HIPEC from a multivariable logistic regression that included two confounding factors:age and ascites.One-to-one matching between the two groups was generated using nearestneighbor matching without replacement and with the use of a caliper width equal to 0.04.
Quantitative variables were described as mean with standard deviation (SD) or median with interquartile range(IQR),and were compared usingt-test or Mann-Whitney U test,as appropriate.Categorical variables were described as frequency and percentages,and were compared using Pearson test or Fisher’s exact test.Survival curves were estimated using the Kaplan-Meier method,and differences between curves were compared by using the log-rank test.Hazard ratio (HR) and corresponding 95% confidence interval (95% CI) were estimated with the Cox proportional-hazards model,and the multivariate backward likelihood ratio steps Cox model was chosen to assess the adjusted effect of HIPEC.Subgroup Cox regression analysis of OS was performed to assess the consistency of effect across subgroups,and interactions were evaluated.
Analyses were performed using SPSS statistics software(Version 24.0;IBM Crop.,New York,USA).Statistical significance was defined as P<0.05 (two-sided).
Results
PSM analysis
Of the 663 GC patients with PM who had complete data,those in the HIPEC-Cx group were statistically significantly younger and had more ascites,compared with those in the Cx group.Finally,of the 663 patients with complete data,498 patients were included in the final analysis after matching (n=249 patients/group;Figure 1).
The male-to-female ratio was 1.48 (297/201) and the mean age was 55.3 years old.The Eastern Cooperative Oncology Group performance status (ECOG PS) was 0 or 1 for 450 of 498 patients (90.4%),and 48 of 498 (9.6%)scored 2 and 3.Cancer was undifferentiated in 423 of 498(84.9%) patients.There were 335 patients (67.3%) with no ascites,83 patients (16.7%) with little ascites and 80 patients (16.0%) with moderate (36,7.2%) and large (44,8.8%) ascites.PM was classified as P1 in 156 patients(31.3%),P2 in 139 patients (27.9%),P3 in 197 patients(39.6%),and was unknown for 6 patients (1.2%).After PSM,there were no statistically significant differences in the baseline or clinical parameters,including sex,age,body mass index,ECOG PS,histology,ascites,or P stage between the Cx and HIPEC-Cx groups,except for the length of hospital stay (P<0.001) and time to first flatus(P<0.001;Table 1).
Survival
The median follow-up period was 33.2 (IQR,14.2-54.4)months.Survival analysis revealed that patients receiving HIPEC combined chemotherapy had a significantly higher median OS than those receiving chemotherapy alone,with 15.9 (95% CI,13.2-18.5) months and 10.8 (95% CI,9.5-12.1) months in the HIPEC-Cx and Cx groups,respectively (HR=0.71;95% CI,0.58-0.88;P=0.002;Figure 2).The corresponding 3-year OS rate was 18.4%(95% CI, 12.3%-24.5%) and 10.1% (95% CI,5.4%-14.8%) in the HIPEC-Cx and Cx groups,respectively (P=0.017). Additionally, we found that performing palliative gastrectomy plus HIPEC plus chemotherapy was associated with the highest survival[median OS,20.8 (95% CI,15.7-25.8) months,3-year OS rate,27.0% (95% CI,17.6%-36.4%)] (Supplementary Figure S1).
In the multivariable Cox proportional hazard model,patients who received HIPEC combined with chemotherapy were at lower risk of dying from GC than thosewho received chemotherapy alone (adjusted HR=0.69;95% CI,0.56-0.86;P=0.001;Figure 3).Subgroup analyses showed that the effect of HIPEC was consistent across the levels of prespecified stratification factors.Patients in the HIPEC-Cx group had statistically significant better survival rate than those in the Cx group for P1/P2 stage (HR=0.71;95% CI,0.54-0.93;P=0.015),and those with no or little ascites (HR=0.69,95% CI,0.53-0.90,P=0.006;HR=0.58,95% CI,0.33-1.00,P=0.050).There was no significant survival difference between the two groups for patients with P3 stage or moderate/large ascites.Notably,HIPEC had a significant impact on the remission of ascites (85.7%) in the moderate/large ascites subgroup (data not shown).
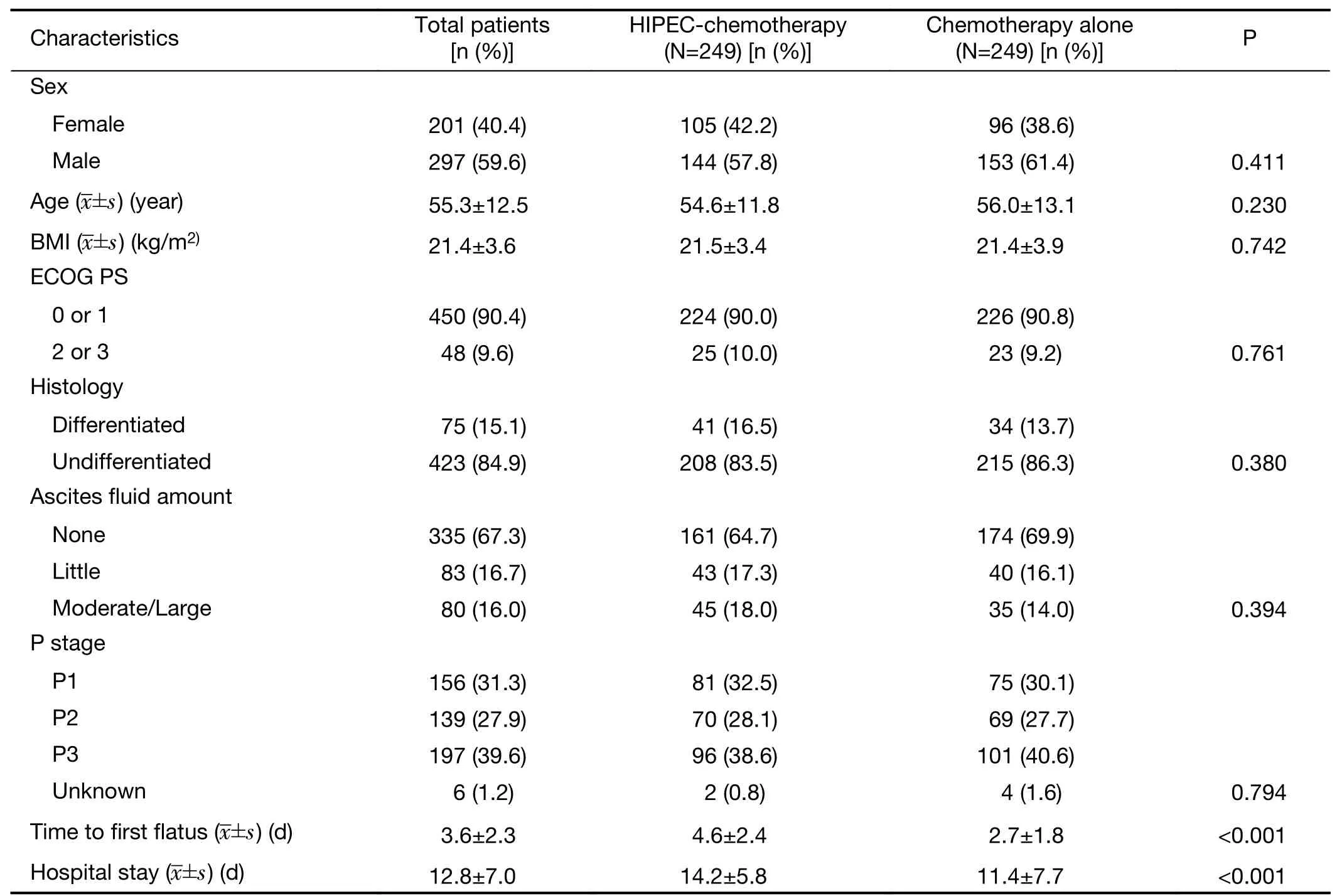
Table 1 Clinical and clinicopathological parameters of gastric cancer with peritoneal metastasis
Safety
Morbidity rates for both groups are shown inTable 2.The rates of hypoalbuminemia, anemia, lymphopenia,electrolyte disturbance,fever,and abdominal pain were statistically significantly higher.In contrast,the rates of nausea, vomiting, leukopenia, and neutropenia were statistically significantly lower in the HIPEC-Cx group,compared with the Cx group,respectively (P<0.05).The most frequent major complications (grade 3-4) were lymphopenia (21.0%vs.9.4%,P<0.001),neutropenia(1.6%vs.8.2%,P=0.001),leukopenia (1.2%vs.7.8%,P<0.001),and abdominal pain (5.6%vs.1.2%,P=0.007) in the HIPEC-Cx and Cx groups,respectively.The time to first flatus and length of hospital stay in patients treated with Cx was shorter than that for patients treated with HIPEC-Cx (2.7±1.8 dvs.4.6±2.4 d,P<0.001;11.4±7.7 dvs.14.2±5.8 d,respectively,P<0.001;Table 1).No other severe treatment-related complications were recorded.
Discussion
The National Comprehensive Cancer Network (NCCN)guidelines suggest that systemic chemotherapy is the firstline standard strategy for GC with PM,and chemotherapy combined with trastuzumab for patients with HER-2 positive GC (6,19).While the prognosis of metastatic GC patients has improved in the past decades with the development of new medications (20-24),survival remains poor (median survival:4.6-8.2 months) (5,25,26) in GC patients with PM compared with other metastatic sites.Poor blood circulation and low drug concentration are purported as the main reasons why tumor cells in the peritoneum have a poorer response to systemic chemotherapy than in other organs (27).
Several recent studies have shown that the multimodality treatment of CRS combined with HIPEC in treating GC with PM has promising results (8,11,12,28).The recent CYTO-CHIP study found that CRS-HIPEC resulted in longer OS than CRS alone (median OS:18.8vs.12.1 months,respectively) (12).The GYMSSA trial found that CRS combined with HIPEC and systemic chemotherapy could prolong survival in selected GC patients with PM(28).In their randomized phase III study,Yanget al.(11)found that the median survival of GC with PM was 11.0 and 6.5 months in patients with CRS-HIPEC and with CRS alone,respectively.This treatment modality is built on the synergistic effect of complete macroscopic removal of all visible tumors within the abdominal cavity,along with the elimination of residual microscopic tumor because of HIPEC.Undeniably,the completeness of CRS was strongly correlated with survival for GC with PM (8).Moreover,HIPEC is more effective when there is limited peritoneal spread or after radical CRS (CC index 0 or 1)(8,12,29).
Unfortunately,as reported in the CYTO-CHIP study,several studies have shown that satisfactory CRS is a highly challenging surgical endeavor with controversial perioperative mortality and morbidity,which limits its use in the treatment of GC with PM (12,30-33).In our study,most of the patients (336/498,67.5%) had a P2/P3 stage,and therefore might not be suitable for CRS.HIPEC without PM resection was performed as early as 2008,to reduce ascites associated with PM (ascites remission:100%)(34).Badgwellet al.(35) recently reported a phase II study of 19 patients (6 with positive peritoneal cytology and 13 with PM),who received laparoscopic HIPEC.Median OS was found to be 20.3 months,supporting the use of HIPEC for eliminating peritoneal diseases.In a retrospective study of 71 laparoscopic HIPEC GC patients with PM,the authors concluded that HIPEC was well tolerated,therefore gastrectomy could be performed for long-term survival benefits (36).
The results of our four-center study showed that GC patients with PM could benefit from HIPEC,even if CRS is not performed,with a statistically significantly improved mean survival time 15.9 (95% CI,13.2-18.5) months in the HIPEC-Cx groupvs.10.8 (95% CI,9.5-12.1) months in the Cx group,higher 3-year OS rate 18.4% (95% CI,12.3%-24.5%)vs.10.1% (95% CI,5.4%-14.8%)(P=0.017).As expected,the benefit of HIPEC focused on patients with P1 or P2 disease.This large-scale study,therefore,represents a promising modality for the treatment of GC with PM,with acceptable adverse events when CRS cannot be performed.To the best of our knowledge,HIPEC without CRS for GC with PM has not been previously evaluated in such a large study and may represent an effective and tolerated therapy.
Also,there is currently no standardized modality for HIPEC worldwide,which may explain the heterogeneity of results for GC patients with PM.These modalities involve,among others,the method of HIPEC (closed or open),the ideal temperature,the choice of chemotherapeutic agents,as well as the duration and frequency of treatment.Indeed,with regard to the latter,several studies included only one cycle of HIPEC after CRS,which might mitigate the efficacy of HIPEC (12,37,38).In our study,HIPEC was performed with pre-determined drugs and dosages at a constant temperature (43±0.1 °C) and duration (60 min),and last,repeatedly (mean number of times:2.1±1.0).Additionally,our study showed that HIPEC was effective in controlling ascites,therefore improving the quality of life of GC patients with PM.
The role of additional palliative gastrectomy in prolonging survival for incurable GC patients with PM remains controversial.Although several previous reports have found that palliative gastrectomy could prolongsurvival in patients with stage IV GC (39-41),the multicenter REGATTA randomized controlled trial (also in an Asian patient population) failed to find any survival benefit of gastrectomy plus chemotherapy compared with chemotherapy alone in the management of stage IV patients with GC (42).In addition,several small Asian randomized trials have demonstrated a survival benefit for adjuvant HIPEC in high-risk patients undergoing resection with curative intent;however,these results have not been validated in non-Asian patients (43,44).Theoretically,palliative gastrectomy might reduce local symptoms and sensitize HIPEC and systemic chemotherapy by reducing the tumor burden.Interestingly,in the present study,we found that the palliative gastrectomy+HIPEC+Cx had a statistically significant survival benefit (MST:20.8 months).To the best of our knowledge,this result is better than previous reports of patients managed either with CRS +HIPEC or standard systemic chemotherapy (8,18,42).This finding should be verified in further randomized controlled trials.
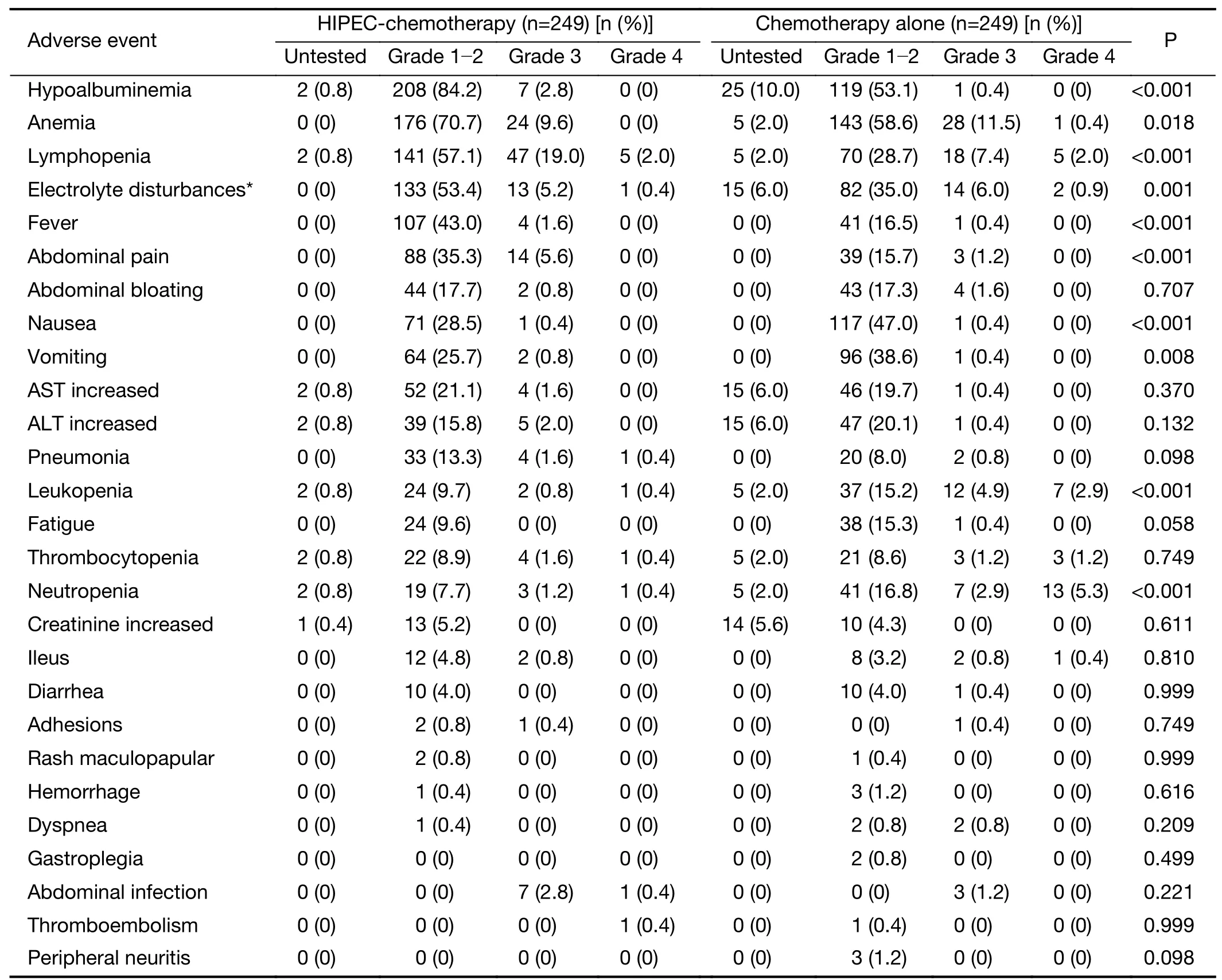
Table 2 Major toxicities and complications in gastric cancer patients with peritoneal metastasis
The incidence and severity of hematologic and nonhematologic toxicities were well within the range of those of other common chemotherapy and surgery regimens(18,42).Of note,postoperative complications occurred less frequently than in the CYTO-CHIP study (12),possibly because all of the patients in our study did not receive complete CRS.HIPEC did not increase the risk of serious complications in the present study.
The present study has several limitations.First,although larger than previous studies on GC with PM,our study is retrospective in nature.Second,the peritoneal cancer index,a major prognostic factor for GC patients with PM,was not used in all the centers participating in our study.Therefore,we adopted the Japanese guidelines,which are not universally used.Finally,of the 663 eligible patients,498 were matched.We did not analyze the 165 patients that were not included in the PSM.The exclusion of large number of patients is known to reduce the statistical power and may lead to a type II error if the number of unmatched cases or controls is large or the treatment effects are small(45).Moreover,current treatment regimens for HIPEC are not uniform concerning the choice of intraperitoneal chemotherapeutic agents,temperature,and duration and frequency of treatment,so our modality may not be ideal for all patients.Therefore,it is critical to launch welldesigned,prospective,multi-center,large-scale randomized controlled clinical trials to resolve the above-mentioned problems.
Conclusions
Compared with standard systemic chemotherapy,HIPEC combined with chemotherapy revealed a statistically significant survival benefit for GC patients with PM,without additional serious morbidity or mortality.This treatment modality may represent an effective and more acceptable therapy option for GC patients with PM.
Acknowledgements
This study was supported by the Guangzhou Key Medical Discipline Construction Project Fund,the Guangzhou High-Level Clinical Key Specialty Construction,the Clinical Research Promotion Project of Guangzhou Medical University for Building High Level University,the National Natural Science Foundation of China (No.81972918),and the Guangzhou Major Clinical Technology Program (No.2019ZD16).
Footnote
Conflicts of Interest:The authors have no conflicts of interest to declare.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Separate lateral parametrial lymph node dissection improves detection rate of parametrial lymph node metastasis in early-stage cervical cancer:10-year clinical evaluation in a single center in China
- Development and validation of prognostic nomogram based on log odds of positive lymph nodes for patients with gastric signet ring cell carcinoma
- Prognostic factors affecting long-term outcomes in patients with brain metastasis from esophageal carcinoma
- Signatures within esophageal microbiota with progression of esophageal squamous cell carcinoma
- Oral microbiome and risk of malignant esophageal lesions in a high-risk area of China:A nested case-control study
- Current epidemiology of pancreatic cancer:Challenges and opportunities
