Kratom induced severe cholestatic liver injury histologically mimicking primary biliary cholangitis: A case report
2021-01-14DarshanGandhiKritiAhujaAlexisQuadeKennethBattsLovePatel
Darshan Gandhi, Kriti Ahuja, Alexis Quade, Kenneth P Batts, Love Patel
Darshan Gandhi, Department of Radiology, Northwestern Memorial Hospital, Northwestern University Feinberg School of Medicine, Chicago, IL 60611, United States
Kriti Ahuja, Department of Internal Medicine, Maulana Azad Medical College, New Delhi 110002, Delhi, India
Alexis Quade, Internal Medicine-Pediatrics, University of California-San Diego and Rady Children's Hospital San Diego, San Diego, CA 92103, United States
Kenneth P Batts, Department of Pathology, Abbott Northwestern Hospital, Allina Health, Minneapolis, MN 55407, United States
Love Patel, Division of Hospital Medicine, Abbott Northwestern Hospital, Allina Health, Minneapolis, MN 55407, United States
Abstract
Key Words: Case report; Kratom; Cholestasis; Liver injury; Mitragyna speciosa; Cholangitis; Substance induced injury
INTRODUCTION
Acute liver failure is a severe condition which may rapidly become fatal[1].In the United States, a significant number of these cases occur due to drug-induced liver injury[2].Apart from prescribed medications, consumption of herbal and dietary supplements also plays an important role in causing liver injury[2].We present here a case of a 37-year-old female, with drug induced liver injury mimicking as antimitochondrial antibody (AMA) negative primary biliary cholangitis (PBC), secondary to consumption of an herbal supplement, Kratom.Derived from the leaves ofMitragyna speciosa, a plant found in Southeast Asia and Africa, this supplement is used for its stimulant properties, as a substitute for opioids, and to help opioid withdrawal symptoms[3].People in the United States report using this supplement predominantly for pain relief, and also report increased levels of energy and focus with consumption[4].Our patient learned of this herbal supplement from a friend and reported consuming it in order to boost her energy levels.Although uncommon, this herbal supplement is associated with the risk of hepatotoxicity, making it imperative for physicians to be aware of its harmful effects and caution their patients against its use[4].
CASE PRESENTATION
Chief complaints
A 37-year-old female with a history of depression and obesity (body mass index: 32) presented to emergency room with a week-long history of nausea, decreased appetite, fatigue, and two days of jaundice.
History of present illness
Four days prior to admission, she noticed that her stools were becoming tanner and eventually turned white.She also reported that her urine was dark.Two days prior to admission the patient noticed jaundice and scleral icterus which prompted her to seek treatment.Her only home medication was venlafaxine,which she had been taking for several years.She has no history of alcohol abuse.
On further questioning about new medications or supplement use, the patient reported using an herbal supplement containing Kratom two weeks prior to the onset of her symptoms.She used the supplement for the first time in her life.Encouraged by a friend to use the supplement to “boost energy levels,” she believed it was safe because it was “all natural”.She consumed approximately three grams in total over the course of three days in the form of powder (which she dissolved in water) and tablets.During her hospitalization, the patient’s liver enzymes continued to rise.On day 3 of her hospitalization, a liver biopsy was performed.
History of past illness
History of depression and obesity.
Physical examination
Unremarkable except jaundice.
Laboratory examinations
On admission, the patient had markedly elevated liver enzymes (Table 1).Other basic laboratory findings including blood count, basic metabolic panel, coagulation panel, serum thyroid stimulating hormone and antinuclear antibody were within normal range.Viral and autoimmune hepatitis studies were normal.Ceruloplasmin level was also normal.AMA was negative.
Imaging examinations
An abdominal ultrasound showed diffuse increased echogenicity of the liver with normal liver size and contour suggests diffuse hepatic steatosis (Figure 1).No intrahepatic or common biliary duct dilation or gall stones seen.An abdominal computed tomography scan showed similar findings.Magnetic resonance cholangiopancreatography did not demonstrate any further abnormalities.
FINAL DIAGNOSIS
Kratom induced severe liver injury histologically mimicking PBC.
TREATMENT
The patient was started on prednisone 40 mg daily.Advised to avoid any new medications or over the counter products with the potential risk of liver injury till her liver enzymes normalized.
OUTCOME AND FOLLOW-UP
The patient was discharged on day 5 of hospitalization and follow up was arranged with gastroenterology clinic.The patient had liver enzymes checked six days after discharge; her symptoms and liver enzymes showed marked improvement.Steroids were stopped.Patient was lost to further lab follow up with gastroenterology clinic but reported feeling back to her normal on follow up phone call 2-wk post hospitalization.
DISCUSSION
The herbal supplement Kratom is a psychoactive substance derived from the leaves ofMitragyna speciosa, a plant native to Southeast Asia[3].Its leaves are used for a variety of purposes, such as pain relief, enhancing energy levels, substituting opioids, managing opioid withdrawal[3,4].The psychoactive compounds of Kratom, mitragynine, and 7-hydroxymitragynine may also result in altered consciousness, particularly at high doses of consumption[3].As a result, Kratom is a controlled drug in several countries and illegal in several others[5].The Drug Enforcement Administration of the United States considers it a Drug and Chemical of Concern[6].While Kratom products are legal in most parts of the United States, a few states and cities have banned them[4].With concerns regarding its safety, the Food and Drug Administration warns consumers against the use of these products[4,7].
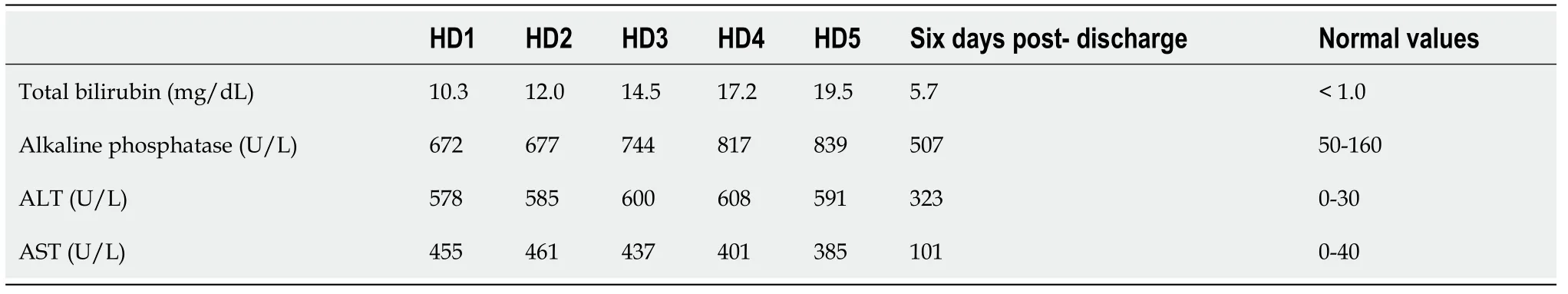
Table 1 Patient’s liver function labs from the day after admission until the day of hospital discharge
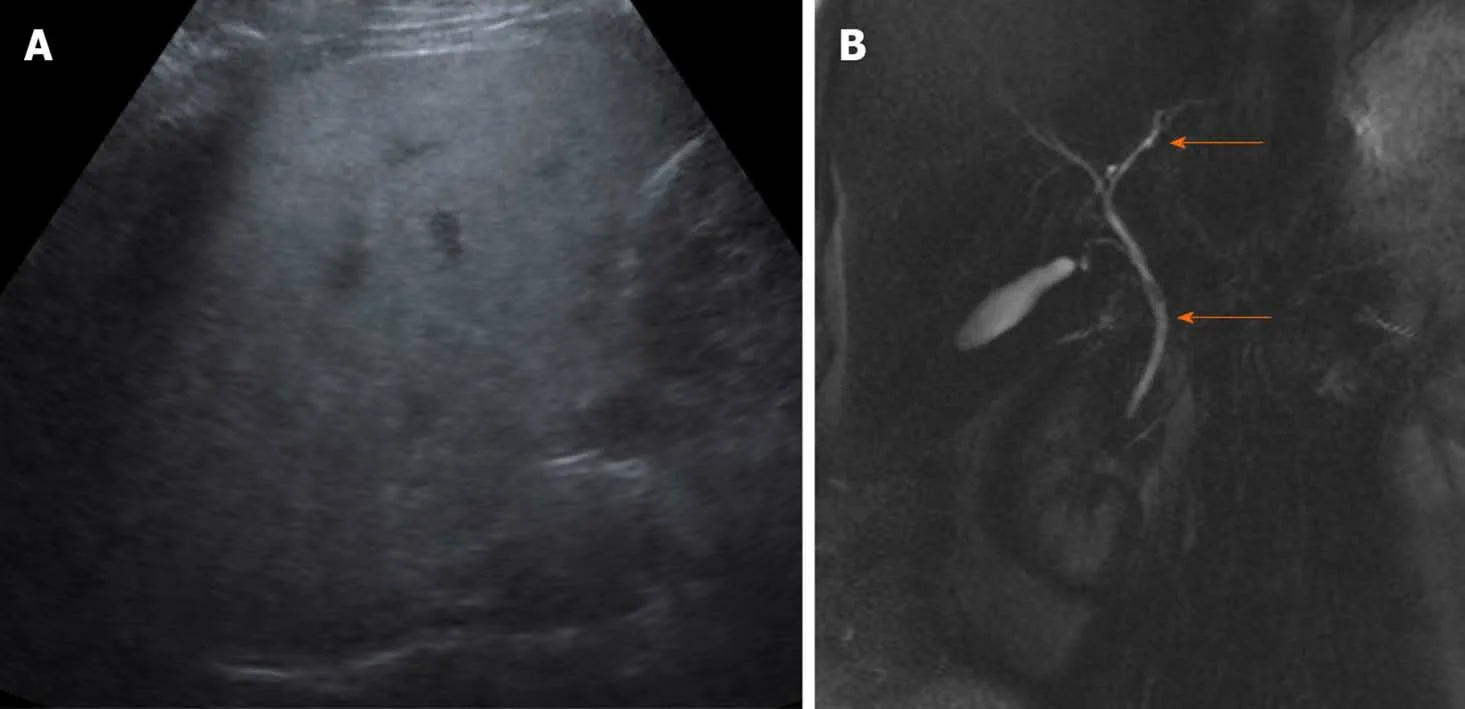
Figure 1 The abdominal ultrasound.
Further, studies have shown that patients reporting Kratom use may present with confusion, lethargy, irritability, agitation, nausea and vomiting, tachycardia, hypertension or in severe cases, bradycardia, seizures, increased bilirubin, renal failure and even coma[4].Further, Kratom is also reported to exert effects similar to opioids, such as sedation, hypnosis, nausea, stupor and respiratory depression[3,4,8].In addition to this, the Food and Drug Administration has recalled Kratom supplements due to contamination with Salmonella[9].
Although Kratom induced liver injury is described, reports with detailed description of histopathological changes are rare which are mentioned below in Table 2.Rapid clinical and liver enzyme improvement supports the diagnosis of Kratom induced liver injury.
An interesting aspect of our case is the pathological features of liver injury (Figure 2).The zone 3 cholestasis was felt to reflect drug effect; zone 3 cholestasis is a common finding in cholestatic drug reactions but not a feature of early stage PBC.The lymphocytic cholangitis, a typical feature of PBC and unusual medication-injury finding, raised initial concern for underlying early stage PBC.This is of lesser concern given her negative antinuclear antibody and AMA status, trend toward rapid resolution of liver enzymes, and a case report by Aldyabet al[10]in 2019 that reported a case of kratom toxicity with granulomatous cholangitis (another form of florid duct lesion) that mimicked PBC.This was from a 40-year-old female presenting with liver injury after Kratom use who initially perceived to have AMA-negative PBC but diagnosed with Kratom induced liver injury after rapid normalization of liver enzymes.
In the first published case report of intrahepatic cholestasis due to consumption of Kratom, the patient’s laboratory results showed a peak bilirubin of 29.3 mg/dL with prolonged elimination based on known half-life of the drug and analysis of urine samples[11].It was speculated that this could be due to the patient’s underlying steatohepatitis[11].Similarly, our patient also had steatohepatitis observed on imaging and pathology, which could explain why even the relatively low doses of Kratom used by our patient compared to other cases discussed in the literature led to such profound liver injury.

Table 2 Kratom-induced hepatotoxicity with review of literature in patients with liver biopsy
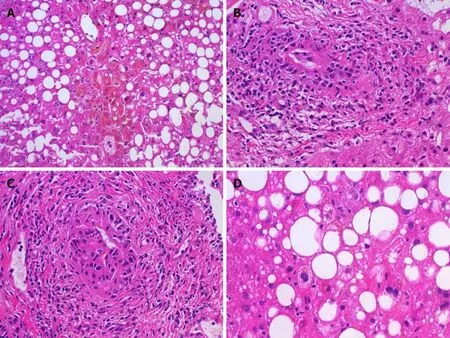
Figure 2 Histopathological findings.
As Kratom use appears to be on the rise in the United States, physicians need to be aware of its potential for adverse effects[4,9].This case highlights how even small amounts of the supplement can be hepatotoxic.Physicians need to be able to discuss safety concerns of over-the-counter supplements with their patients.Moreover, the rising use of supplements and recreational substances can pose diagnostic challenges for clinicians when their use is not reported.Medical providers always need to consider the use of supplements when patients present with possible drug-induced liver injury.
CONCLUSION
Kratom induced liver injury is an important differential diagnosis for physicians to consider in any patient presenting with acute liver injury.As observed in our patient, this manifestation of Kratom consumption may occur even at low doses.Further, this case report demonstrates that a thorough history is essential for an accurate and timely diagnosis.Patients may consider dietary and herb supplements to be natural and riskfree products, not realizing the potential for harm.In addition to asking their patients about the consumption of any supplements, it is imperative that physicians update themselves so as to be able to discuss the benefits and risks and counsel their patients effectively.Identifying use of supplements helps in early diagnosis and treatment, while also preventing future harm.From the pathology perspective, biliary changes associated with Kratom injury can mimic PBC.
杂志排行
World Journal of Hepatology的其它文章
- Neoadjuvant treatment strategies for intrahepatic cholangiocarcinoma
- Metabolic syndrome and liver disease in the era of bariatric surgery: What you need to know!
- Combined liver-kidney transplantation for rare diseases
- Hepatocellular carcinoma Liver Imaging Reporting and Data Systems treatment response assessment: Lessons learned and future directions
- Tumor necrosis family receptor superfamily member 9/tumor necrosis factor receptor-associated factor 1 pathway on hepatitis C viral persistence and natural history
- Apatinib as an alternative therapy for advanced hepatocellular carcinoma
