Hepatocellular carcinoma Liver Imaging Reporting and Data Systems treatment response assessment: Lessons learned and future directions
2021-01-14AnumAslamRichardKinhGianDoAvinashKambadakoneBradleySpielerFrankMillerAhmedGabrResmiCharalelCharlesKimDavidMadoffMishalMendirattaLala
Anum Aslam, Richard Kinh Gian Do, Avinash Kambadakone, Bradley Spieler, Frank H Miller, Ahmed M Gabr, Resmi A Charalel, Charles Y Kim, David C Madoff, Mishal Mendiratta-Lala
Anum Aslam, Department of Radiology, University of Michigan, Ann Arbor, MI 48019, United States
Richard Kinh Gian Do, Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY 10065, United States
Avinash Kambadakone, Abdominal Imaging and Interventional Radiology, Harvard Medical School, Massachusetts General Hospital, Boston, MA 02114, United States
Bradley Spieler, Department of Radiology, Louisiana State University Health Sciences Center, New Orleans, LA 70112, United States
Frank H Miller, Department of Radiology, Northwestern University Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, United States
Ahmed M Gabr, Department of Interventional Radiology, OHSU and Tanta University, Egypt, Portland, OR 97239, United States
Resmi A Charalel, Department of Radiology, Weill Cornell Medicine, New York, NY 10065, United States
Charles Y Kim, Department of Radiology, Duke University Medical Center, Duke University, Durham, NC 27710, United States
David C Madoff, Department of Radiology and Biomedical Imaging, Yale School of Medicine, New Haven, CT 06520, United States
Mishal Mendiratta-Lala, School of Medicine, 1500 East Medical Center Drive, University of Michigan, Ann Arbor, MI 48109, United States
Abstract Hepatocellular carcinoma (HCC) is a leading cause of morbidity and mortality worldwide, with rising clinical and economic burden as incidence increases.There are a multitude of evolving treatment options, including locoregional therapies which can be used alone, in combination with each other, or in combination with systemic therapy.These treatment options have shown to be effective in achieving remission, controlling tumor progression, improving disease free and overall survival in patients who cannot undergo resection and providing a bridge to transplant by debulking tumor burden to downstage patients.Following locoregional therapy (LRT), it is crucial to provide treatment response assessment to guide management and liver transplant candidacy.Therefore, Liver Imaging Reporting and Data Systems (LI-RADS) Treatment Response Algorithm (TRA) was created to provide a standardized assessment of HCC following LRT.LIRADS TRA provides a step by step approach to evaluate each lesion independently for accurate tumor assessment.In this review, we provide an overview of different locoregional therapies for HCC, describe the expected post treatment imaging appearance following treatment, and review the LI-RADS TRA with guidance for its application in clinical practice.Unique to other publications, we will also review emerging literature supporting the use of LI-RADS for assessment of HCC treatment response after LRT.
Key Words: Hepatocellular carcinoma; Liver Imaging Reporting and Data Systems Treatment Response Algorithm; Locoregional therapy; Liver Imaging Reporting and Data Systems Treatment Response equivocal; Arterial phase hyper enhancement; Stereotactic body radiotherapy
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and third leading cause of cancer related mortality worldwide[1].The incidence of HCC continues to rise in the United States[2], largely due to the increasing rate of cirrhosis from obesity, alcohol use and chronic viral hepatitis[3].Historically, curative treatment options for HCC include liver transplantation, surgical resection or thermal ablation for tumors less than 3 cm in size[4].However, approximately 80% of patients are not surgical candidates; for them, locoregional treatment (LRT) options include: Thermal ablation [e.g., microwave ablation (MWA), radiofrequency (RFA), cryoablation], percutaneous ethanol injection (PEI), transarterial chemoembolization (TACE), transarterial bland embolization (TAE), transarterial radioembolization (TARE), and stereotactic body radiotherapy (SBRT)[5-7].LRT can be used alone, in combination with each other, or in combination with systemic therapy, and has been shown to improve disease-free and overall survival (OS) in patients who cannot undergo surgery[8-10].Furthermore, LRT can help prolong time to progression, extend survival, palliate symptoms, keep lesions from progressing outside of Milan criteria to maintain liver transplant candidacy(bridge to transplant),and convert non-transplant candidates to transplant candidates based on Milan criteria (downstage to transplant)[11-13].Treatment decisions are usually made by a multidisciplinary liver tumor board, and depend on various patient factors, including tumor location, size and multiplicity, disease stage, liver function, performance status, technical feasibility and potential for future transplant candidacy[14,15].
Following LRT, it is imperative to provide accurate treatment response assessment to help guide clinical management.While numerous validated imaging based treatment response classification systems exist, [i.e., European Association for the Study of Liver Disease (EASL)[16], modified Response Evaluation Criteria in Solid Tumors (mRECIST)][17], they are based on tumor response assessment at the patientlevel.However, HCC is unique in that tumors are often isolated to the liver, and LRT can be used to target the tumor(s) directly.Different LRTs may also be performed to different lesions within the same liver.Furthermore, liver transplant candidacy is based on assessment of each lesion.Consequently, the Liver Reporting and Data System (LI-RADS) Treatment Response Algorithm (TRA)[2], was created to provide a standardized assessment of each HCC treated by LRT, a feature which makes this treatment response classification unique to the existing ones.Furthermore, this allows LI-RADS TRA to be more applicable from a clinical perspective in patient management.
In this manuscript, we provide a brief overview of the various LRTs for HCC, describe the expected post treatment imaging appearances after LRT, review the definitions within the LI-RADS TRA and provide guidance for their use in clinical practice.We will also review the emerging literature supporting LI-RADS for assessment of HCC treatment response after LRT.This review is unique to other publications because it provides a comprehensive overview of the LI-RADS TRA and guidance for its application in clinical practice based on expected post treatment imaging findings, as well as critically reviews current literature supporting this algorithm.
LOCOREGIONAL THERAPIES FOR HCC
There are many treatment options for HCC, depending on stage of disease, as well as other factors mentioned above.LRTs for liver limited disease have proliferated in recent years, and are generally categorized as follows[18-20]: (1) Loco-ablative therapy: Chemical ablation (PEI), physical ablation utilizing energy sources [Heat: RFA, MWA; Cold: Cryoablation; Electrical: Irreversible electroporation (IRE)]; (2) Arterial based therapy (non-radiation): TAE, conventional trans-arterial chemoembolization (cTACE), drug eluting beads TACE (DEB-TACE); and (3) Radiation-based therapy: TARE and SBRT.
Loco-ablative therapy
Historically, the first ablative therapy used for HCC was PEI, which consists of injecting ethanol directly into the tumor under image guidance to achieve tumor deathviacoagulative necrosis and ischemia[21].Studies show that PEI has a high safety profile, good overall efficacy, and low complication rates with complete necrosis of small HCC tumors; however, limitations of PEI include the need for multiple treatments[22].In 1999, the first thermal ablation was performed with RFA, showing a high safety profile, good overall efficacy, and 5 year survival rates similar to surgical resection for tumors less than 3 cm in size[23].Thermal ablation modalities, including RFA and MWA, use energy at different frequencies to create high temperatures with rapidly oscillating field strengthviapercutaneous insertion of electrodes (RFA)/antennas (MWA) into the tumor through image guidance.This results in cell deathviacoagulation necrosis[24].Multiple randomized controlled trials have been performed showing superiority of RFA to PEI in terms of OS, complete response (CR) and local recurrence (LR)[25].A recent meta-analysis demonstrated that RFA significantly increases survival in patients with HCC by 3-years as compared with PEI[26].Although there is still a role for PEI in some cases where ablation is technically challenging or cannot be performed safely, thermal ablation is much more commonly performed worldwide.
Arterial based therapy (non-radiation)
The goal of non-radiation arterial-based therapy is to prevent local progression in intermediate and advanced stage HCC, as a form of palliative therapy or as a method for downstaging/bridging to resection or transplant.By providing arterial delivery of embolic material, either bland (TAE) or chemotherapy coated (cTACE/DEB-TACE), to HCCviathe hepatic artery, arterial inflow to the tumor is eliminated, resulting in cell death[18,27,28].When TACE is performed, tumor cell death (necrosis) occurs by two mechanisms: Ischemic injury from arterial embolization and chemotoxic injury from the administered chemotherapeutic agent[29].Historically, TACE is indicated in nonsurgical patients with large multifocal HCC and Child-Pugh Score A without extrahepatic spread[30]; although recently, the scope for using TACE has expanded, and now includes treating small and/or solitary tumors.The presence of portal vein obstruction by bland thrombus or intravascular tumor is a relative contraindication to non-radiation arterial based therapy[31,32]; in this instance, both the arterial and portal venous flow to the liver would be compromised, resulting in ischemia of the hepatic parenchyma within the embolization zone[33].Non-radiation based trans-catheter intraarterial therapies include: (1) Conventional TACE: Utilizes an emulsion of iodized oil mixed with a chemotherapeutic agent followed by administration of gelatin sponge or embolic microparticles to near complete stasis[34]; (2) DEB-TACE: Utilizes microspheres coated with chemotherapeutic agent, which will elute into local tissues over time[35]; and (3) Bland TAE: Utilizes polyvinyl alcohol particles or microspheres to occlude the arterial supply without a chemotherapeutic agent[36].Multiple randomized control studies have shown that neither TACE nor DEB-TACE improved tumor objective response or provided survival benefit when compared to TAE[9,37,38].However,conventional TACE remains the current standard of care for unresectable intermediate or advanced stage HCC in patients with preserved liver function based on BCLC guidelines[39].
Radiation based therapy
TARE and SBRT are the most common radiation-based treatment modalities used today[40].As in any form of LRT, patient selection, including assessment of patient’s disease burden, biochemical parameters and performance status, are critical to determining which form of therapy is preferred.TARE is ideal if the disease is limited to less than half of the liver[40].Other lab parameters are also important for patient selection, including bilirubin < 2 mg/dL and albumin > 3 g/dL[40,41].TARE involves injection of Y90 microspheres (20-60 microns in diameter) into the hepatic arteries, which delivers targeted radiation[42].Portal vein thrombus (bland or tumor) is not a relative contraindication for TARE[41], since this form of intra-arterial therapy does not result in arterial embolization[42].
SBRT consists of applying multiple tightly focused high energy beams of radiation to treat HCC, allowing for the delivery of higher doses of radiation with relative sparing of adjacent parenchyma when compared to other options, albeit with the limitation of multiple treatment sessions over days.
HCC TREATMENT ASSESSMENT BASED ON LI-RADS TRA
Following LRT for HCC, cross-sectional imaging with multiphasic MRI and/or CT (including pre contrast and dynamic arterial, portal venous and delayed phase imaging) is routinely performed to assess treatment response and to identify new or developing sites of disease in the untreated liver.Routine time intervals for follow-up varies depending on institutional protocol, type of LRT performed, and transplant status for patients being down staged or bridged.Most major transplant centers and large institutions perform imaging 1-mo post-treatment, followed by imaging at 2-3 mo intervals.Imaging after radiation-based therapy (TARE/SBRT) begins 3 mo after treatment and about every 3 mo thereafter.While the choice of imaging modality (CT or MRI) can vary depending on patient factors and institutional preference, it is important to try and maintain consistency in the modality and technique for imaging performed before and after LRT.Accurate interpretation of post-treatment imaging is essential for guiding further management decisions and requires comparison of posttreatment with pre-treatment imaging to appreciate the original tumor dimensions and enhancement characteristics.
EXPECTED POST TREATMENT IMAGING APPEARANCE
Imaging appearances of HCC after LRT will vary depending on the treatment modality, with different expected findings for the various forms of therapy.Thus, it is imperative to become aware of the expected treatment specific enhancement patterns in order to prevent inaccurate interpretation of residual or recurrent neoplasm.While the expected imaging appearances of the treated tumor are similar for ablation and non-radiation arterial-based therapy, as tumoral necrosis is expected immediately after LRT, imaging findings are distinct after radiation-based therapy (TARE and SBRT), as tumoral necrosis develops over time.
The creation of an ablation margin of greater than 5-10 mm around the tumor is considered essential for adequate ablation; thus, an ablation zone larger than the original tumor dimension is an expected finding.Furthermore, the ablation zone should not demonstrate residual enhancement because of the anticipated coagulation necrosis and cell death within the treatment cavity; this frequently results in the development of a central zone of hyper-intense signal on pre-contrast T1 weighted MRI and a hyperdense appearance on unenhanced CT, both are expected posttreatment findings[43].Subtraction (MRI) and non-contrast (CT) imaging are essential to avoid interpreting these imaging characteristics as areas of arterial phase hyperenhancement (APHE, Figure 1).Since the ablation zone represents devitalized liver parenchyma and tumor, reporting measurements of the ablated zone is not mandatory, rather, the residual nodular areas of enhancement suspicious for viable tumor should be described.
A uniform thin peripheral rim of enhancement is an expected post-ablation finding.Additionally, there can be geographic APHE within the parenchyma surrounding the treatment zone, which usually resolves, but can persist on portal venous and delayed phase imaging (Figure 1).APHE which resolves on portal venous and delayed phase of imaging is referred to as transient hepatic intensity/attenuation difference (THID/THAD), postulated to be secondary to arterioportal shunts created during needle puncture or coagulated portal vein branches resulting in compensatory increased arterial flow[44].Over time, the ablation cavity is expected to involute and stabilize in size.Imaging features suggestive of residual viable tumor post-treatment include: Thick peripheral irregular nodular APHE with or without washout appearance, “washout” alone, enhancement characteristics similar to pre-treatment tumor, or discontinuity in the smooth thin peripheral rim of enhancement[45](Figure 2).
As with ablation, non-radiation arterial-based therapies have a similar evolution of post-treatment appearances.TAE and TACE create ischemic and/or cytotoxic effects that result in cell necrosis; the tumor usually does not change in size early posttreatment, although rarely can slightly increase in size as a result of edema and hemorrhage.As with ablation, the treated tumor should become immediately nonenhancing after transarterial therapy.Often, there is a pronounced surrounding geographic enhancement pattern that persists on portal venous and delayed imaging, which represents perfusional changes secondary to inflammation and arterial embolization[17].
One unique transarterial post-treatment feature is seen when iodized oil is used for embolization.In these instances, the treatment zone appears extremely hyperdense on unenhanced CT, secondary to iodized oil deposition within and around the tumor, limiting assessment for tumor viability on post contrast CT images[46].Evidence does suggest that the degree of iodized oil deposition within the tumor is an indicator of tumor necrosis, thus could possibly be used as an indirect feature for tumor response assessment; nonetheless, evaluation for residual tumoral enhancement is limited by the hyperdense appearance of the iodized oil.The iodized oil is not apparent on MRI, and thus MRI is preferred to evaluate for APHE in and around the treatment zone to assess for recurrent/viable disease.Just as with ablation, locally recurrent or residual viable HCC presents as irregular, nodular areas of APHE, APHE plus “washout”, “washout” alone, or enhancement similar to the pretreatment tumor, within or along the margin of the treated tumor (Figure 3).Some studies have reported that recurrence after TACE and RFA could result in dedifferentiation into more aggressive infiltrative tumor[47-49], which tends to have an atypical appearance on post-therapy imaging (Figure 4); thus one must pay close attention for any changes in the treated tumor, particularly in size.

Figure 1 Spectrum of expected post treatment imaging appearances after successful LR TR Nonviable microwave ablation of LR 5 hepatocellular carcinoma in different patients.
Radiation-based therapies result in post-treatment imaging appearances distinct from other therapies, particularly on the arterial phase of imaging.For example, early after SBRT and TARE transient increases in tumor size can be seen.Furthermore, tumor shrinkage is often delayed and slow, secondary to the cytostatic effects of TARE[50].Therefore, size measurements within 3-mo of treatment are not reliable for prediction of tumor response[18].Enhancement patterns after TARE are also highly variable, as successfully treated tumors can demonstrate a range of imaging findings, including: (1) Persistent intra-tumoral APHE; (2) Geographic or nodular peri-tumoral APHE; (3) Thin rim of peri-tumoral APHE, and (4) Complete lack of enhancement (Figure 5).Of note, persistent central arterial hyperenhancement can be seen at 3 mo in tumors that have been treated by TARE, specifically in cases that show progressive decrease and eventual lack of enhancement over time from the delayed effects of radiation therapy.Thus, evaluation of these tumors poses a diagnostic and interpretation challenge.Key features suggestive of residual tumor after TARE includes new or enlarging nodular or mass-like APHE within or around the treated tumor and growth over time, particularly when identified more than 6 mo after treatment.Care should be taken not to mistake TARE-related peri-tumoral perfusional change with viable tumor.Close evaluation and comparison of the pre-treatment imaging to identify the tumor margins is essential; additionally, peritumoral parenchymal perfusion tends to be geographic in shape, and either persists on portal venous and delayed phases of imaging or becomes isoenhancing to the remainder of the hepatic parenchyma.
After SBRT, APHE with or without “washout” can persist for up to a year, and even longer, but these imaging features gradually decrease over time[51](Figure 6).Early post treatment, geographic APHE surrounding the treated tumor is a common finding and likely represents hyperemia; this eventually converts to progressive delayed phase geographic enhancement, likely secondary to radiation fibrosis, usually with associated findings of capsular retraction and peripheral intrahepatic biliary dilatation[52].The treated tumor should gradually decrease or stay stable in size during the time period where treatment response is evolving (Figure 6).Imaging features suggesting recurrent disease after SBRT include: Increase in size of the treated tumor or new or increasing intensity of APHE after treatment[53].Although the treated HCC often demonstrates persistent APHE after SBRT, the degree, or intensity, of APHE often decreases as it resolves.Thus, in treated HCC which originally demonstrates persistent but decreasing degree of enhancement or resolution of enhancement, the development of increasing intensity of enhancement or new APHE, is a feature suggesting LR.
LI-RADS TRA
LI-RADS TRA was created to improve the consistency and standardization in reporting treatment response after liver-directed therapy and, unlike other response assessment systems, it has a distinct advantage of providing assessment on a lesionby-lesion basis, an approach which can potentially improve communications for individualized management considerations.LI-RADS TRA is modelled after mRECIST, as it primarily relies on post treatment APHE to identify viable tumor.However, LI-RADS TRA is unique, because in addition to APHE, the definition of viable tumor includes washout appearance or enhancement similar to that seen before treatment.This may render this advantageous when interpreting treatment response after radiation-based therapies in particular.
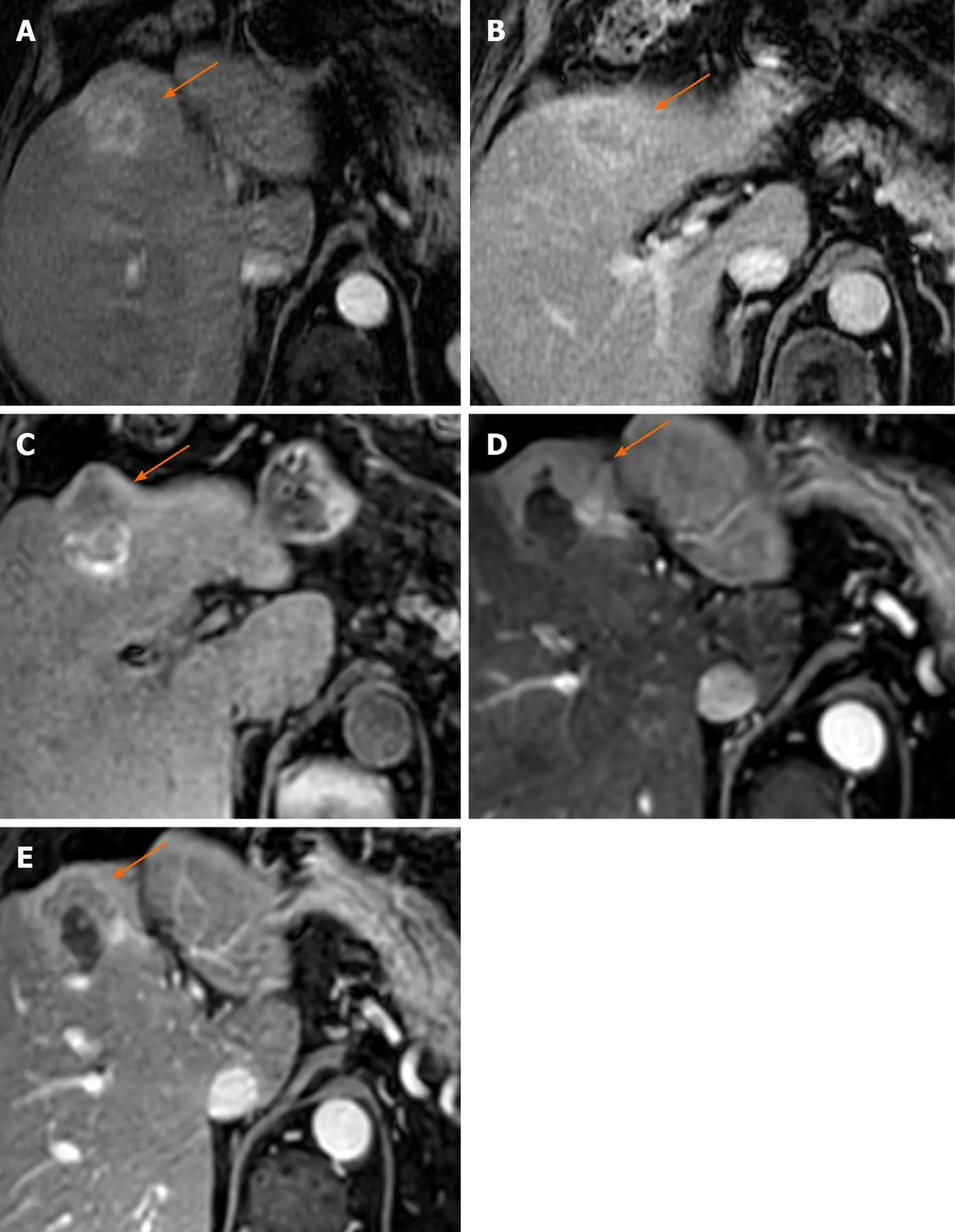
Figure 2 Eighty-three-year old male with nonalcoholic steatohepatitis related cirrhosis presented with a 3.5 cm mass demonstrating arterial phase hyperenhancement (APHE) (A) and “washout” (B), compatible with an LR-5 hepatocellular carcinoma; C: Pre-contrast T1 images 6 mo post microwave ablation demonstrate a hypointense nodular area along the anterior margin of the ablation cavity,with thick irregular nodular APHE on arterial phase (D) and “washout” and “capsule” on portal venous phase (E), LR-TR Viable.
During image interpretation of treated HCC, each treated liver observation should be reported separately according to the LR-TR categories[54].Treatment response categories include: “LR-TR Nonviable”, “LR-TR Equivocal” and “LR-TR Viable”.In instances where technical limitation precludes characterization of the tumor, an “LRTR Nonevaluable” category can be assigned[2,55].
LR TR Nonviable
If a treated lesion exhibits no tumoral enhancement or only shows a treatment specific enhancement pattern (which is unique for each LRT, such as thin rim enhancement after ablation), an LR-TR Nonviable category can be assigned[54].After ablation (MWA/RFA/cryoablation/PEI/IRE) and non-radiation arterial-based therapies (TAE/cTACE/DEB-TACE), a nonviable tumor category is assigned when there is complete tumor devascularization,i.e., complete loss of APHE.One must carefully evaluate the margins of the treated lesion for the presence of nodular or irregular APHE and/or a discontinuous appearance of the thin rim of expected post-treatment enhancement, which suggests viable disease.
In contradistinction,after SBRT and TARE,there is often,but not always,persistent APHE and “washout” within the treated tumor, which can be seen for up to a year, and sometimes longer.This creates a diagnostic dilemma during image interpretation since persistent APHE, albeit a treatment-specific expected appearance, denotes viable disease for other LRTs.Lack of radiology-pathology correlation studies in patients treated with SBRT for HCC limits interpretation of imaging features that translate to true viability or nonviability.Despite early post-treatment APHE after radiation therapy, this frequently resolves over time without additional therapy, and with subsequent decrease in size of the treated lesion (Figure 6).Thus, early post-treatment, a category of LR TR Viable may be misleading and result in unnecessary retreatment if the referring clinician does not understand the time course for tumoral necrosis resulting from radiation.Alternatively, even though APHE is an “expected” imaging feature, categorization as LR TR Nonviable may also be inaccurate if there is ongoing necrosis but residual viable tissue on pathology.Therefore, there is a current gap in
knowledge in the application of LI-RADS TRA after radiation therapy, given the absence of radiology-pathology correlation.Notably, this feature of APHE renders TRA after radiation therapy a challenge with all existing treatment response classification systems.
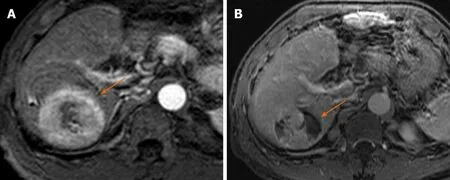
Figure 3 Fifty-four-year old male with hepatitis C virus presents with 5.4 cm hepatocellular carcinoma in the right lobe of the liver demonstrating arterial phase hyperenhancement on arterial phase of imaging (A), LR 5; 1 mo post-transarterial chemoembolization there is significant residual viable enhancing tumor with areas of necrosis on arterial phase imaging (B), LR-TR Viable.

Figure 4 Seventy-three-year old woman with history of nonalcoholic steatohepatitis related cirrhosis presented with 1.

Figure 5 Sixty-two-year old female with LR 5 hepatocellular carcinoma within segment 4a of the liver, demonstrating arterial phase hyperenhancement (APHE) (A) and “washout” (B); 3 mo post transarterial radioembolization (TARE) there is persistent arterial phase nodular enhancement(arrow)(C)with associated“washout”on portal venous phase(D)within the largely necrotic treatment cavity,LR TR Equivocal; 6 mo post-TARE, the treatment cavity decreases in size and the nodular area of APHE is no longer identified on arterial phase (E) and portal venous phase (F), LR TR Nonviable; geographic APHE in the surrounding parenchyma is compatible with postradiation changes (E and F).
LR TR Viable
The LR-TR Viable category is considered if there is presence of nodular, mass-like or thick irregular tissue within or along the treated tumor with any of the following features: APHE, washout appearance or enhancement similar to pre-treatment imaging[54].After ablation or non-radiation arterial-based LRT, this is fairly straightforward; however, for the reasons mentioned above, assigning LR-TR Viable to lesions early after SBRT and TARE therapy remains a diagnostic challenge as APHE is an expected post-treatment imaging finding that can evolve into nonviable disease on subsequent exam[56].The expected temporal evolution of HCC treated with radiationbased LRT results in decreasing degree/intensity of enhancement with a gradual decrease in size.If there is new or increasing enhancement of the treated tumor or an increase in size of the enhancing tumor post SBRT or TARE, then the LR TR Viable category should be assigned.

Figure 6 Fifty-eight-year old male presenting 3 mo post transarterial chemoembolization for follow up of a 7.2 cm LR 5 hepatocellular carcinoma.
LR-TR Equivocal
A unique aspect of the LI-RADS TRA is the addition of a novel category, LR TR Equivocal.This category allows reporting of lesions when there is uncertainty in viability or nonviability, allowing short-term follow-up for re-evaluation.This is particularly useful given the increasing complexity in post-treatment imaging appearances, particularly after radiation-based therapies.
The LR-TR Equivocal category is defined as enhancement atypical for treatment specific enhancement pattern and not meeting criteria for probably or definitely viable tumor[54].There are instances when imaging findings are equivocal following LRT, particularly after arterial-based and radiation-based therapies, which also affect the hepatic parenchyma adjacent to the targeted tumor.The result is the presence of abnormal areas of APHE as a result of altered perfusion around the treated tumor, which can mimic viable disease.In these cases, it may be prudent to assign an LR-TR Equivocal category unless the perfusional alteration is clearly geographic in appearance.While this category may result in increased frequency of follow-up imaging, as well as the risk that viable tumor is left untreated, HCC is generally a slow growing tumor with a double timing of 85.7-117 d[57,58].Thus, a “wait and watch” approach with 2-3 mo interval imaging can help distinguish true residual disease from benign parenchymal perfusional alterations.
EMERGING EVIDENCE
While the LI-RADS TRA was designed to complement other existing treatment response systems, its utility is limited, as it needs to be validated in clinical practice.There have been a number of recent publications evaluating the performance of LIRADS TRA.These studies compare imaging to pathologic data, as well as measure the reliability of the TRA categories with inter-reader studies.
Evidence from recent literature suggests moderate inter-reader agreement in assigning LR-TR categories following LRT (ablation and non-radiation arterial-based therapies) for HCC.Two recent studies, Coolset al[59]and Chaudhryet al[60],have shown high inter-reader agreement in determining LR TR category after thermal ablation (RFA/MWA), with an inter-reader reliability of 90% and 95% and kappa of 0.75 (Standard Error ± 0.09) and 0.71 (95%CI: 0.59-0.84), respectively.Inter-reader agreements are slightly lower when comparing LR TR categorization after nonradiation arterial-based therapy for HCC.Seoet al[61],in which 78.6% of tumors were treated with TACE and imaged with either CT or MRI and Shropshireet al[62]in which all tumors were treated with TAE and imaging with MRI, reported kappas of 0.69 (CT) and 0.56 (MRI), and 0.55, respectively.These differences in inter-reader agreement between thermal ablation and arterial therapy is not surprising, since the expected imaging appearance post-ablation is simpler compared to the often complex imaging features seen after transcatheter arterial based therapies.
In addition to inter-reader reliability studies, validation studies evaluating the sensitivity and specificity of LR TR algorithm to predict tumor necrosis with radiology-pathology correlation are necessary.Chaudhryet al[60]reported that 81% of HCC post-TACE which were categorized as LR-TR Nonviable demonstrate 100% necrosis on pathology.Similarly, Shropshireet al[62], reported that 67%-71% of tumors categorized as LR-TR Nonviable after TAE were 100% necrotic at pathology.The reported incidence is not surprising, since the gold standard histopathology would call anything less than 100% necrotic as viable disease.Thus, while microscopic viable tumor is present in a moderate percentage of treated HCC that are deemed nonviable based on imaging features, the clinical significance based on local tumor progression, disease free survival and impact on OS are yet to be determined.Microscopic viable tumor may be of little clinical significance, particularly since national and international guidelines accept the presence of viable tumor, at a specific size threshold, in patients undergoing transplantation.Although, post-liver transplant, achieving a complete pathologic response has been shown to strongly predict tumor-free survival[63].
These studies also reported high sensitivity and specificities when evaluating the radiology-pathology concordance with LR TR Viable categorization.Chaudhryet al[60]report 73% of treated lesions characterized as viable disease had < 99% necrosis and Shropshireet al[62]report 60%-65% of disease reported as LR TR viable had < 99% necrosis.
The LR-TR Equivocal category has a relatively low sensitivity for predicting tumor necrosis.Radiology-pathology correlation after thermal ablation report that 83% of treated tumors categorized as LR TR Equivocal demonstrate viable neoplasm at pathology; similarly, after TAE, 71% of treated lesions categorized as LR TR Equivocal demonstrate viable neoplasm at pathology[60,62].Seoet al[61]report that 93%-100% of the HCC’s treated with TACE, RFA or in combination, which were categorized as LR-TR Equivocal, demonstrated viable disease.All three studies thus report similar findings with a high percentage showing viable tumor at histopathology when treated tumor is assigned LR-TR Equivocal category.When LR-TR Equivocal categorization was treated as equivalent to viable disease in one study, sensitivity and specificity of detecting viable disease increased from 40%-77% to 81%-85%, across readers[60].As previously mentioned, these findings are likely related to the ability of pathology to determine microscopic viable tumor which is not evident on imaging.Of note, the ACR LI-RADS manual states that the LR-TR categories were designed to help provide a probability of the presence of viable tumor and do not correspond to histologic viability; hence the presence of microscopic tumor cannot be excluded based on imaging alone[54].As mentioned above, the impact of microscopic viable HCC in the setting of cirrhosis is yet to be determined in relation to a patient’s OS, disease free survival and time to local progression.Although the results show that there is a high rate of viable disease in the LR-TR Equivocal category, further data is needed before its elimination as a concept.
The above studies include HCC treated only with thermal ablation or non-radiation arterial-based therapies.As mentioned earlier, HCC treated with radiation-based therapy has unique post-treatment imaging features of persistent APHE which confounds image interpretation and can result in a high false positive rate of LR TR Viable categorization.The current challenge is the limited number of studies evaluating radiology-pathology correlation after radiation-based LRT.A study by Mendiratta-Lalaet al[64], in which 10 SBRT treated HCC’s had corresponding explant or AFP values as a surrogate biomarker, reported imaging findings of persistent APHE (4/10) and washout (9/10) at 12 mo, in which all of the treated HCC showed complete tumor necrosis on explant pathology or normalization of AFP values.Mooreet al[65]evaluated the role of SBRT as bridge to liver transplantation for early stage inoperable disease in a small cohort, and reported no SBRT-related mortality or recurrence.Amongst the explants, there were 3 (27%) that showed CR, 6 (54.5%) pathological partial response and 2 (18%) pathological stable disease.Currently, no inter-reader agreement studies have been performed assessing LI-RADS treatment response categories in patient treated with radiation-based therapies and explant pathology to determine actual tumor necrosis.Similar gaps in knowledge are present for the use of mRECIST in patients treated with SBRT.
Riazet al[66]evaluated the degree of tumor necrosis on explant pathology in 37 lesions post TARE and found complete pathologic necrosis in 61% of the lesions, even in the setting of residual nodular enhancement on imaging pre-transplant.Radiologic findings of these treated lesions were compared to the pathologic findings to determine the predictability of actual tumor necrosis by imaging.WHO and EASL treatment response categories were assigned as CR in 78% and 100% of lesions at a median time of 34 d (95%CI: 29-43) and 126 d (95%CI: 80.2-313.2), respectively.It was also noted that the longer the time to liver transplant, the greater the degree of tumor necrosis identified within the lesion, with the least percentage of tumor necrosis seen in explants at 3 mo post TARE[66].Thus, it is possible that residual APHE in the early post TARE treatment period does correspond to some viable disease, but it is viable disease that will decrease over time as the radiation effects progress.In this context, LR-TR Equivocal may be the best option for TARE treated lesions in the first three months of treatment.As mentioned previously, currently all of the treatment response classification systems are limited in their ability to accurately assess treatment response after radiation-based therapy given the persistence of APHE early posttreatment.
MANAGEMENT BASED ON LR-TR CATEGORIES
With the advent of different types of LRT for treatment of HCC, it is extremely challenging to develop a dedicated management pathway that can be applicable to all patients.Thus, while no specific management recommendations exist, LI-RADS TRA provides lesion by lesion assessment which, when discussed in a multidisciplinary setting, may allow improved communication and management in this cohort of patients.However, unlike mRECIST, EASL, WHO and RECIST which have a multitude of validating literature, the LR-TR algorithm is relatively new.Future anticipated studies validating the LR-TR algorithm will therefore improve our ability to create standardized guidelines for post treatment management and better predict outcomes.
CURRENT LIMITATIONS OF LI-RADS TRA
The main limitation of LI-RADS TRA is the small number validation studies, given its recent introduction in 2017, although the published sensitivity/specificity for a subset of LRTs is promising.Further studies investigating its use in tumors treated with radiation-based therapy are sorely needed.Second, the LR TR algorithm is not yet applicable to tumor treated with systemic and/or biologic therapy.Given that LRT is increasingly used in combination with systemic therapy, this will remain a challenge to address.Third, there is no dedicated post-treatment specific imaging follow-up interval recommendation, partly due to the variable evolution of post treatment necrosis after different forms of LRT, and institution-specific imaging protocol.Fourth, the long-term utility of the LR-TR Equivocal category remains to be seen, given the evidence that most LR-TR Equivocal lesions are viable in the studies published to date.
CONCLUSION
With the increasing incidence of HCC and the increasing number of LRTs available, the complexity in assessing treatment response will also rise.Nevertheless, post treatment imaging will always play a critical role in providing the clinician a road map to direct further management.It is thus essential for diagnostic radiologists to understand interpretation of post-treatment imaging findings specific to each form of LRT.Current existing treatment response classification systems such as RECIST, mRECIST, EASL and WHO are fraught with their own unique limitations when assessing LRT for HCC, including lack of change in size post-treatment (thus rendering RECIST and WHO limited), and persistent post-treatment enhancement after radiation-based therapies (thus rendering mRECIST and EASL limited).LI-RADS TRA provides a new framework to describe treatment response for each individual lesion and the emerging evidence is promising for ablation and non-radiation based arterial therapies.LI-RADS TRA should be used cautiously for radiation-based therapies (TARE, SBRT) in which early post-treatment persistent APHE is common and expected.Its current limitations will be addressed as future studies investigate its performance and inform refinements of future versions.
杂志排行
World Journal of Hepatology的其它文章
- Neoadjuvant treatment strategies for intrahepatic cholangiocarcinoma
- Metabolic syndrome and liver disease in the era of bariatric surgery: What you need to know!
- Combined liver-kidney transplantation for rare diseases
- Tumor necrosis family receptor superfamily member 9/tumor necrosis factor receptor-associated factor 1 pathway on hepatitis C viral persistence and natural history
- Apatinib as an alternative therapy for advanced hepatocellular carcinoma
- Hepatitis B virus detected in paper currencies in a densely populated city of India: A plausible source of horizontal transmission?
