Effect of treating chronic hepatitis C with direct-acting antivirals on extrahepatic cutaneous manifestations
2021-01-14MohamedElKassasOsamaMOHegazyEmanSalah
Mohamed El Kassas, Osama MO Hegazy, Eman M Salah
Mohamed El Kassas, Department of Endemic Medicine, Faculty of Medicine, Helwan University, Cairo 11795, Egypt
Osama MO Hegazy, Eman M Salah, Department of Dermatology, Venereology and Andrology, Faculty of Medicine, Helwan University, Cairo 11795, Egypt
Abstract
Key Words: Directly acting antivirals; Extrahepatic manifestations; Hepatitis C virus; Lichen planus; Pruritus; Cutaneous manifestations
INTRODUCTION
Hepatitis C virus (HCV) is one of the human hepatotropic viruses that infects around 71 million individuals globally[1].Spontaneous clearance of the virus fails in nearly half of cases and acute infection progresses to chronic hepatitis C, and further progression of the disease could lead to cirrhosis, and hepatocellular carcinoma (HCC)[2].Egypt was considered a country with a high prevalence of HCV.After recognizing the magnitude of this problem, the Egyptian National Committee for Control of Viral Hepatitis (NCCVH), established specialized treatment centers for the evaluation and management of viral hepatitis[3-7].Treatment of chronic HCV showed a significant shift after the introduction of direct-acting antivirals (DAAs), which proved a great success with an acceptable safety profile compared to the previous standard of care treatment (pegylated interferon and ribavirin)[8-10].Many treatment regimens have been considered for the management of HCV, which is usually a combination of two or more DAAs classes[11-13].
Being a hepatotropic and lymphotropic virus, HCV does not only cause hepatic manifestations, it also leads to a significant number of extra-hepatic manifestations.Around 74% of patients with HCV will show HCV-related extrahepatic manifestations in their lifetime[14].The development of HCV-related extrahepatic manifestations most likely involves autoimmune mechanisms.This theory is supported by the appearance of autoimmune features, such as palpable purpura, complex lymphoproliferative disorders (e.g., lymphomas), and immune complex deposit diseases that cause local and/or systemic complications[14,15].Among the extrahepatic manifestations, dermatologic manifestations significantly add to the morbidity of the disease[16].The association of chronic HCV infection with a broad spectrum of cutaneous manifestations has been widely reported in the literature, with varying strengths of epidemiological association.In registry-based studies, approximately 17% of HCV patients have at least one skin manifestation, which can be induced directly or indirectly by chronic HCV infection[17].Dermatosis disorders are known to be linked to HCV infection including mixed cryoglobulinemia, lichen planus, porphyria cutanea tarda, and necrolytic acral erythema[18].Besides these dermatoses, HCV can also be associated with autoimmune cutaneous diseases.Vitiligo is one of the autoimmune cutaneous diseases reported in studies to have a similar prevalence in patients with HCV infection and in the healthy population with vitiligo[19].Psoriasis can also be found in association with HCV infection.This was confirmed by studies that found anti-HCV antibodies in psoriatic patients as well as HCV-RNA in the lesions of patients with psoriasis and HCV infection[20].Pruritus is also a common dermatologic manifestation that is recognized as an early sign of chronic HCV infection[21,22].This study aimed to assess the effect of treating HCV with DAAs on the extrahepatic cutaneous manifestations of HCV.
MATERIALS AND METHODS
This prospective observational study included 1039 HCV patients, who were recruited from New Cairo Viral Hepatitis Treatment Center, one of the specialized treatment centers for HCV management, affiliated to the Egyptian NCCVH[7], from October 2018 to July 2019.These patients received HCV antiviral therapy with sofosbuvir/ daclatasvir (SOF/DAC) combination regimen for 12 wk and were followed-up until assessment of virological response 12 wk after treatment cessation.Patients were treated with DAAs according to the standardized protocol for HCV treatment issued by the NCCVH.The main exclusion criteria were: Child-Turcotte-Pugh (CTP) class C, hemoglobin level below 10 g/dL, platelet count less than 50000/mm3, diagnosed with HCC 6 mo following a successful intervention, extrahepatic malignancy after 2 years of cure, co-infection with hepatitis B or HIV, pregnancy or inability to use effective contraception, and hypersensitivity to any of the treatment medications[5].In addition to the previous contraindication, we excluded patients who received dermatological treatment for their cutaneous manifestations, patients with renal failure, patients with autoimmune diseases, and those who received simeprevir as it can induce skin lesions as a complication[9].
A total of 30 patients were diagnosed with extrahepatic cutaneous manifestations and fulfilled the study inclusion criteria: Six patients were diagnosed with classic lichen planus, eight patients with psoriasis vulgaris, and sixteen patients had hepatic pruritus.A full medical history was obtained for these patients, followed by a clinical examination, and a complete liver biochemical profile.All subjects were tested for HCV viremia by polymerase chain reaction, and hepatitis B surface antigen was determined to exclude chronic HBV infection.Kidney function tests were also performed to exclude patients with renal failure.In addition an antinuclear antibody test was carried out to exclude patients with autoimmune hepatitis.
A dermatological assessment was performed by photographing skin lesions (in cases with lichen planus lesions and psoriatic plaques), before and after antiviral treatment.Dermoscopic photography of the lichen planus lesions and psoriatic plaques was performed before receiving and after completing treatment using a Dermlite DL4 3Gen.dermoscope.Patients with hepatic pruritus were evaluated before and after treatment using the 12-Item Pruritus, Severity Scale developed and validated by Reich and colleagues[23].
Descriptive statistics
Data were collected, revised, coded, and entered into the SPSS version 26 software.Qualitative data are presented as numbers and percentages, while quantitative data are presented as mean, standard deviations and ranges.Comparisons between two groups with qualitative data were carried out using the Chi-square test was used instead of the Chi-square test when the expected count in any cell was found less than 5.Comparisons between two independent groups with quantitative data and parametric distribution was performed using the independentt-test.Comparisons between more than two groups with parametric distribution was performed using One Way Analysis of Variance.Pearson correlation coefficients were used to assess the relationship between two studied parameters in the same group.The receiver operating characteristic curve was used to assess the cut-off point with the best sensitivity, specificity, positive predictive value, and negative predictive value.The confidence interval was set to 95% and the margin of error accepted was set to 5%.Thus, the significance of the followingPvalues was considered as follows:P> 0.05: Non significant,P< 0.05: Significant, andP< 0.01: Highly significant.
RESULTS
Thirty patients were included in the study.The mean age was 45.67 years and ranged from 24 to 60 years.Sixteen of the 30 recruited subjects were female.All patients received the SOF/DAC regimen, and all reached SVR12.
Cutaneous manifestations associated with chronic HCV infection were observed in all 30 recruited subjects and consisted of hepatic pruritus in 16 patients,lichen planus in 6 patients and psoriasis vulgaris in 8 patients.Age and sex distribution of the studied patients are shown in Table 1.
Lichen planus
Four of six patients with lichen planus showed complete improvement of lesions following treatment, while no improvement was seen in the remaining two patients.The impact of HCV treatment on lichen planus is shown in Table 2.Figure 1 shows lichen planus papules on the hand of a patient before and after receiving DAAs, with dermoscopic images of lichen planus papules before and after treatment for HCV.
Psoriasis
Eight patients in this study had psoriasis, and all patients showed complete improvement of psoriatic plaques 12 wk after finishing treatment.The impact of HCV treatment on psoriasis is shown in Table 3.Figure 2 shows psoriatic plaques on the back and thigh of a patient before and after receiving DAAs for HCV, in addition to dermoscopic images of psoriatic plaques before and after treatment.
Hepatic pruritus
Sixteen patients in this study complained of pruritus.All patients with pruritus showed complete improvement and reported total relief of pruritus according to the 12-Item Pruritus Severity Scale.According to this scale, the mean score before treatment was 9.94 ± 1.61, with a range of 7-12.Complete disappearance of pruritus was reported with a score of zero in all patients at the follow-up visit.
DISCUSSION
The association of chronic HCV infection with a wide spectrum of cutaneous manifestations has been widely reported in the literature, with varying strengths of epidemiological association.In registry-based studies, approximately 17% of HCV patients showed at least one skin manifestation, which was induced directly or indirectly by chronic HCV infection[17].This study was an observational prospective hospital-based study, which included 30 HCV positive patients with associated extrahepatic cutaneous manifestations.All cutaneous lesions were assessed clinically and dermoscopically before receiving treatment and during the follow-up visit after six months of treatment.To our knowledge, this is the first study to assesses and follow-up HCV patients with extrahepatic cutaneous manifestations using a dermatoscope.Out of 1039 HCV patients who were referred to receive antiviral therapy, only those with dermatological manifestations were included in the study (30 patients).Of these 30 patients, six had cutaneous lichen planus, eight had psoriasis vulgaris, and sixteen had hepatic pruritus.Some dermatological disorders which are more frequent and are closely related to HCV such as porphyria cutanea tarda and necrolytic acral erythema were not observed.This could be explained by the pathognomonic nature of disorders such as HCV, and hence most of the patients with these disorders were diagnosed with HCV infection and treated early in the HCV treatment project which started in Egypt in 2015.
All patients with hepatic pruritus in this study reported complete resolution after finishing antiviral therapy.Although pruritus is often associated with HCV infection, the exclusion of all other causes of pruritus either dermatological or systemic, and its disappearance after viral clearance could confirm this association, and raise the suspicion of pruritus as an extrahepatic manifestation of HCV infection.
All patients with psoriasis showed total resolution of all psoriatic plaques.The results obtained by Enomotoet al[24], were concordant with our results, as they reported a male patient with a nine-year history of refractory psoriasis, with a Psoriasis Area and Severity Index (PASI) score of 3.8.The patient's symptoms and signs gradually resolved after a 12-wk course of oral, fixed-dose ledipasvir–sofosbuvir.The same results were also confirmed in a report from Egypt, where the authors described an 18-year-old male with psoriasis who received sofosbuvir and ribavirin and showed a sustained virologic response and the disappearance of skin lesions without the use of topical or systemic treatments for psoriasis six months after the end of treatment[25].This was also in agreement with a published report of a patient with refractory psoriasis.The patient with HCV infection was treated with daclatasvir and asunaprevir combination, and his PASI score decreased from 3.4 to 0[26].

Table 1 Age and gender distribution in each group, n (%)

Table 2 Results in patients with lichen planus

Table 3 Results in patients with psoriasis
Four of the six patients diagnosed with lichen planus in the present study had a complete cure, while the remaining two patients showed no improvement in their lesions.Ansariet al[27]in 2017, supported these study findings in a 55-year-old HCV positive male patient with associated lichen planus.The patient received HCV treatment with ledipasvir-sofosbuvir, which led to a marked improvement in cutaneous lesions.
The main limitation in our study was the small number of recruited patients (30 subjects), who were enrolled after screening 1039 HCV patients over a 7-mo period.This relatively small number could be explained by the fact that most cases with HCVrelated dermatological manifestations were discovered and treated at earlier stages of the HCV treatment project in Egypt.As this was a pilot study, it was the first time that a dermoscope was used to evaluate the response of extrahepatic cutaneous manifestations to HCV treatment with DAAs.Further studies with a larger number of patients and more diverse dermatological lesions are warranted to confirm our findings.
CONCLUSION
In conclusion, treatment of HCV infection with DAAs was effective in all patients with hepatic pruritus and psoriasis, and in most patients with lichen planus.These findings confirm the association between HCV infection and the extrahepatic dermatological manifestations of this virus.Based on our results, treatment of HCV infection in patients with extrahepatic dermatological manifestations is highly recommended.

Figure 1 Shows lichen planus papules on the hand of a patient (A) before and (B) after receiving direct-acting antivirals as treatment for hepatitis C virus infection.
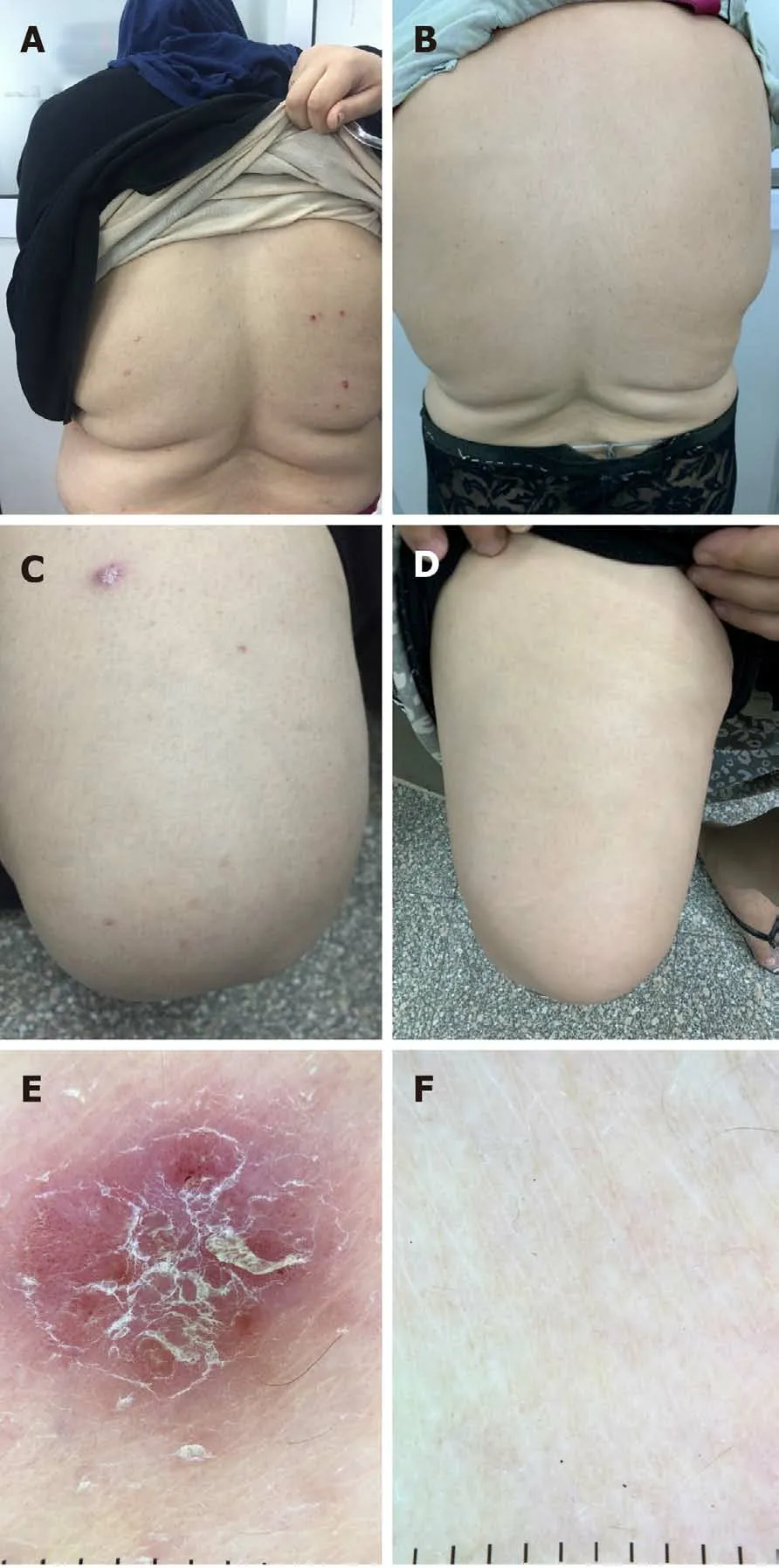
Figure 2 Shows psoriatic plaques on the back of a patient (A) before and (B) after receiving direct-acting antivirals as treatment for hepatitis C virus infection and on thighs (C) before and (D) after receiving treatment.
ARTICLE HIGHLIGHTS
Research background
Hepatitis C virus (HCV) is a disease with a significant global impact, affecting approximately 2%-2.5% of the world’s population.New direct-acting antivirals (DAAs) have been introduced over the past few years leading to successful viral eradication.
Research motivation
The association of chronic HCV infection with a wide spectrum of cutaneous manifestations has been widely reported in the literature.
Research objectives
This study aimed to assess the effect of treating HCV with DAAs on the extrahepatic cutaneous manifestations of HCV.
Research methods
A prospective observational study included HCV positive Egyptian patients who were eligible to receive DAAs.Patients with lichen planus or psoriasis were dermoscopically evaluated before treatment and 6 mo after treatment, while patients with hepatic pruritus were assessed using the 12-Item Pruritus Severity Scale over the same period.All patients received DAAs from October 2018 to July 2019 in the form of a three-month course of sofosbuvir/daclatasvir combination.
Research results
A total of 30 from 1039 patients eligible for antiviral treatment were diagnosed with extrahepatic cutaneous manifestations and fulfilled the inclusion criteria of this study.Of these 30 patients, 6 patients had classic lichen planus, 8 patients had psoriasis vulgaris and 16 had hepatic pruritus.All patients with psoriasis showed significant improvement of all psoriatic plaques, and all patients with hepatic pruritus scored 0 on the 12-Item Pruritus Severity Scale indicating total improvement of pruritus.In addition, four of six patients with lichen planus showed complete improvement.
Research conclusions
Treatment of HCV with DAAs was effective in improving HCV-related extrahepatic cutaneous manifestations.
Research perspectives
Further studies with a larger number of patients and more diverse dermatological lesions are warranted to confirm our findings.
杂志排行
World Journal of Hepatology的其它文章
- Neoadjuvant treatment strategies for intrahepatic cholangiocarcinoma
- Metabolic syndrome and liver disease in the era of bariatric surgery: What you need to know!
- Combined liver-kidney transplantation for rare diseases
- Hepatocellular carcinoma Liver Imaging Reporting and Data Systems treatment response assessment: Lessons learned and future directions
- Tumor necrosis family receptor superfamily member 9/tumor necrosis factor receptor-associated factor 1 pathway on hepatitis C viral persistence and natural history
- Apatinib as an alternative therapy for advanced hepatocellular carcinoma
