Stain capacity of three fungi on two fast-growing wood
2021-01-11TaizeSongFangchaoChengJianpingSun
Taize Song · Fangchao Cheng · Jianping Sun
Abstract We investigated the stain of fast-growing wood(Cunninghamia lanceolate, CL; Paulownia, PT) inoculated with three fungi (Arthrinium phaeospermum, AP; Vibrio anguillarum , VA; Aspergillacea , AS) to explore the new wood dyeing ways and the better combination of wood and fungi for dyeing. Only AP could dye on CL and PT. Especially for CL, its percentage of internal spalting, percentage of external spalting and dyeing depth were the highest(48%, 15% and 5.06 mm, respectively). Surprisingly, the bigger weight loss occurs on PT. The results showed that the dyeing effect of AP dyeing CL was the best, and the wood color change was obviously (Orange to dark red). AP could produce more pigments than the other two fungi (VA; AS),CL was more suitable for fungus staining than PT, indicating that AP could offered a new potential market and a chance for areas to earning higher income for CL. This research paves the way for improving color change was obviously(Orange to dark red). AP could produce more pigments than the other two fungi (VA; AS), CL was more suitable for fungus staining than PT, indicating that AP could offer a new potential market and a chance for areas to earn higher income for CL.
Keywords Spalted wood · Cunninghamia lanceolata ·Paulownia · Arthrinium phaeospermum · Vibrio anguillarum · Aspergillacea
Introduction
The demand of environmentally friendly wood dyestuffhas been higher year by year owing to the economic development and the improvement ofliving standards, especially the people’s awareness of environmental protection has been constantly enhanced. Therefore, many scientists have paid their attention to the researches on environmentally friendly wood dyestuffall over the world. Among these wood dyestuff, direct dyes, acid dyes and VAT dyes are usually employed to dye wood. However, these chemical dyes can cause serious air pollution, limiting the application of dyestuffin living space. Therefore, the pigment of environmentally friendly has great potential in the future. Among them, fungus pigment is regarded as a perfect wood dyestuffdue to its environmental friendliness, which has attracted extensive research interest.
There is a long history of fungus pigment as a wood dye(Blanchette and Biggs 1992). Until now, fungus pigments have been used as a special dye to increase the value-added of wood, which depends on the colonization and stain wood ability of fungi (Robinson et al. 2007a, b; Robinson and Laks 2010a, b, c). How to approach the idealized technology of spalted wood is the goal of researchers in recent years.Therefore, exploring effective pigmented fungi and wood is to find the optimal environment of pigmented fungi.
Spalting is mainly divided into three parts: the stain fungi producing the stain substance, the bleaching formed by the white rot fungus, and the zone lines formed by the fungal interaction between the funguses (Robinson 2012).The dyeing and the zone lines are widely used in wood dyeing. In terms of fungal staining, Scytalidium cuboideum and Scytalidium ganodermophthorum are relatively effective fungus, which can produce red pigment and yellow pigment,respectively (Vega Gutierrez 2016). When spalted fungi is inoculated on wood, it can obtain nutrition in wood rays or other internal tissues of wood, thus dyeing wood. However, spalted fungi have white rot ability for wood (Liese 1970; Worrall et al. 1997; Richter and Glaeser 2015). Existing studies have shown that the longer the incubation time of dyeing fungi is, the better the dyeing effect of wood is,but the higher the risk of white rot of wood is. Therefore,in practical application, fungal pigments can be extracted by organic solvents such as DCM and applied directly to wood dyeing, or dissolved in flaxseed oil after extraction by organic solvents and then dyeing wood, which can solve the problem of white rot of wood and shorten the whole dyeing time (Robinson et al. 2014a, b; Robinson et al. 2014a, b;Hinsch et al. 2015; Weber et al. 2015; Agurto et al. 2017;Robinson et al. 2017; Hinsch and Robinson 2018). In terms of zone lines, the researchers found that Bjerkandera adusta/Trametes versicolor and Polyporus brumalis/Trametes versicolor combinations, as well as X. polymorphism, Coriolus versicolor, etc. could produce stable zone lines (Robinson et al. 2010; Robinson et al. 2011). There are many reasons for the formation of zone lines, but generally they are all caused by changes in the growth environment, which are“Guard” and “Death” of hyphae (Mallett and Hiratsuka 1986; Cease et al. 1989).
In addition, fungi have different preferences for wood because of different functional groups and nutrients received from wood. Studies have shown that the spalted fungi are preferring alder, maple, poplar, phoebe, birch and beech(Chapela 1994; Robinson et al. 2011; Hai-Shan et al. 2013).
Due to the increase in value associated with spalted wood,efforts had recently been undertaken to utilize the process on low value woods. Of particular interest in southeastern China is the potential for PT (Paulownia) and CL (Cunninghamia lanceolate ). Both species are native to China,although they are also commonly cultivated in other ethnic areas. These two species of tree from China has wide range of uses and has fixed value due to the relatively fast growth cycle. But CL is a soft, fine, aromatic, and straight grain wood that is easy to process and suitable for furniture,architecture and other applications. PT has a straight texture,a uniform structure and easy processing. PT is suitable for use in construction, furniture, wood-based panels and other applications. These two species of wood are easy to buy, but are currently underutilized in China, making them ideal for value-added processes.
A substantial body ofliterature exists on the controlled spalting of hardwoods and their use in woodcraft and furniture. There is currently few known record of controlled spalting being attempted with fast-growing wood. For example, using some of the poorly-formed wood, the wood is subjected to fungal infestation without destroying the board to achieve wood dyeing, and ultimately increase the vassal value of these fast-growing woods.
AP (Arthrinium phaeospermum ), VA (Vibrio anguillarum), and AS (Aspergillacea) were used to inoculate two fast-growing wood (CL and PT). The purpose of this research was to investigate which unknown spalting fungi might be suitable for inoculation on the fast-growing wood, with the intended result of producing extra pigments that would increase the marketability of the lumber. And screened new spotted fungi and study the ability of fastgrowing wood in fungal staining. Whether fungi that produce large amounts of extracellular pigments can also treat cork under controlled conditions. Successful pigmentation of the fast-growing wood by any of the tested fungi would offer an opportunity for sawmills to generate additional income, and then market lumber to interested consumers.
Materials and methods
Materials
PT and CL were selected to improve the economic value of fast-growing wood and explore the possibility of producing spalted in fast-growing wood, and the average moisture contents of the tested wood species were 19.04% for CL and 18.00% for PT, respectively. The specifications of the experimental block were 20 mm × 20 mm × 20 mm.
We recorded whether the pigments of the three fungi changed significantly on both woods. These three wood fungus, which were isolated and screened from spalted PT.The strains fungi were shown in Table 1.
Fungi isolation and screening and culturing
The portion with the spalted was cut into small pieces oflength and width less than 10 mm. Alcohol lamps, ultrapurewater, 0.001 g/ml mercury chloride solution, scalpels etc.,that were placed in a clean bench and sterilized by UV lamp for 1 h. The small pieces were washed by three times with 0.1% mercury chloride solution, soaked for 1 min each time,then washed with sterile water for 3-5 min, and finally the wooden blocks were cut with a sterile scalpel on a sterile workbench. The block was placed on the surface of the medium, and the sealed medium was cultured in a dark environment of 27 °C ± 2 °C for about one week. After the obvious mycelium grows around the small wooden block in the medium, the purification was repeated by 2-3 times according to different morphological colors until obtaining a purer fungus.

Table 1 Wood and fungus
Inoculation
The moisture content test was performed using a modified decay jar test with vermiculite instead of soil, as outlined in Robinson et al. (2009), to avoid eventual influence of soil substrates on pigment formation. Jars with plastic lids(250 mL) were prepared with 15 g vermiculite and 30 mL water. Before incubating the fungi, blocks were oven dried at 60 °C for 48 h and weighed, and then a piece of wood was put into the jar.
Culture jars with vermiculite, water and wood samples were autoclaved for 40 min at 121 °C. The entire sterile bench was UV sterilized for 30 min before inoculated. After sterilizing, the inoculum strip (approximately 20 mm × 20 mm agar with actively growing mycelium) was placed on both sides of the block (longitudinal section), and the block was covered with perlite, and the jar was sealed.Inoculum consisted of mycelium and agar approximately 20 mm × 20 mm cut from an actively growing culture in a Petri dish. Each fungus was repeated in 20 jars for one wood,and a total of 120 jars were used. After inoculating, jars were placed in an incubation room (27 °C ± 2 °C, 80% ± 5%RH) and incubated in the dark. There were 20 replicate jars of each pairing made (Table 1). After meeting the incubation time, mycelium was scraped offand blocks were evaluated externally for bleaching, pigmentation, and zone lines.Blocks were then cut in half perpendicular to the grain on the transverse plane and one internal side was also evaluated. All internal spalting, pigmentation, and zone lines were noted for the internal face being evaluated. At 4, 6, 8, and 10 weeks, five jars of each combination (a fungus combines a wood) were removed, and those blocks were cleaned, oven dried (60 °C for 48 h), evaluated for color change, and then weighed again (to determine percent weight loss).
Pigment evaluation
Pigment evaluation involved RGB distribution, amount of spalting and dyeing depth. Firstly, the wood samples inoculated fungi were dried and scanned with Epson Expression 10000XL Scanner at 2400 dpi to obtain stain images,by which external spalting evaluation were carried out with Scion Image software, following the protocol described in Robinson et al. (2009). Secondly, RGB of these images were analyzed to explore color change three fungal-forming spalted wood by MATLAB. Finally, the sample after being scanned was cut in half to expose the inner plane and the inner surface was also scanned to analyze the internal staining. Meanwhile, the depth of internal spalted was measured by vernier caliper. And five points were selected on both sides and the average value was taken as the dyeing depth of the wood.
Weight loss analysis
Before inoculating the fungus in the wood block, the wood block was dried for 48 h, and the M was weighed; after completing the culture, the surface impurities of the wood block were taken out, dried, and weighed m 0 . Where, M is oven dry weight of wood sample prior to exposure and m 0 is the oven dry weight following exposure to fungus. The calculation for weight loss rate (R) is as Eq. 1:

Results and discussion
Multivariate analysis
Multivariate analysis was performed by using fungi, wood species, and culture time as independent variables to determine which fungus produced the greatest amount of spalting in the experiment, and which wood was more suitable for spalted fungal inoculation and optimal incubation time.
RGB distribution
The obtained RGB values (Tables 2 and 3) were compared with the RGB color query comparison table to obtain their expression color. Three fungi were inoculated on CL, and the color changes after different incubation time were shown in Fig. 1.
The three columns of Fig. 1 were the average RGB color block of the corresponding CL block at week 4, 6, 8, and 10, inoculated by AP, VA and AS. And the specific code of corresponding color blocks (RGB color comparison table) of each color were shown on the right side of the column. From Fig. 1, we could find that when AP was inoculated on CL,the color of the wood changed from orange to dark red as theincubation time prolonged, but from light yellow to taupe for VA and AS. Moreover, during the first three incubation times,the color changed from orange to red; and the longer the incubation time was, the deeper the wood color was. During the last period, there was almost not color change except AP.The main reason was the pigment secreted by the fungus. It also could be seen that the third inoculated period, that is week 8, was the best time for dyeing CL for the three fungi,which agreed with the result of Robinson et al. (2007a, b).
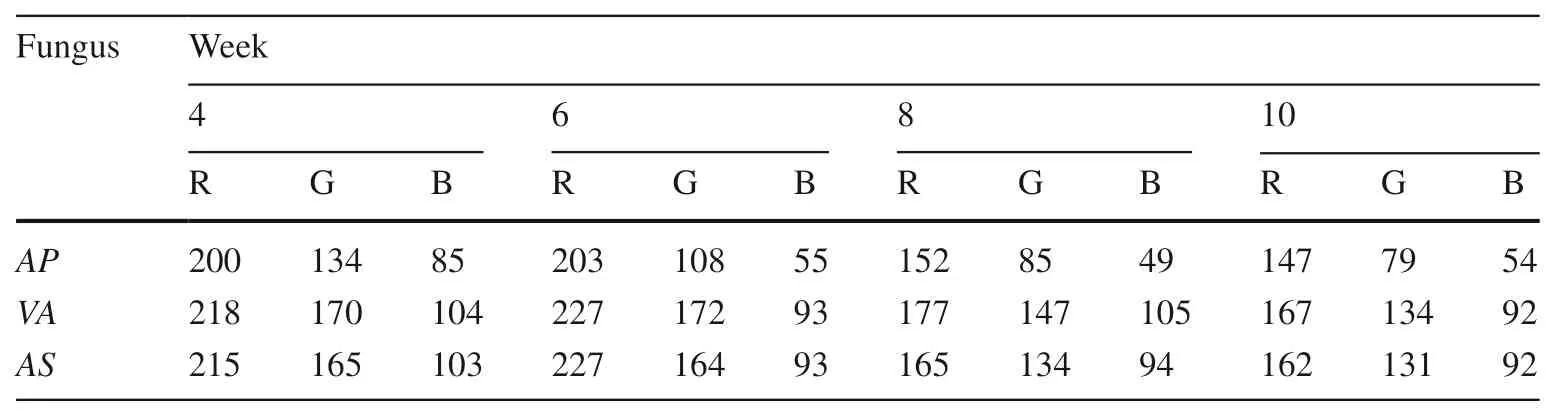
Table 2 RGB value of CL(Cunninghamia lanceolate)

Table 3 RGB value of PT(Paulownia)

Fig. 1 Color expressed by RGB value of CL (Cunninghamia lanceolate)
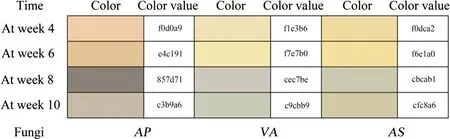
Fig. 2 Color expressed by RGB value of PT (Paulownia)
From Fig. 1, it could be found that the maximum change of RGB was R, followed by B and G. With the extension of incubation time, the R and G values of wood decreased significantly for the three fungi. But the B value was influenced significantly by AP, and there is no influence for the other two fungi. This indicated that the wood color change was more biased towards red and green gray scales.
Figure 2 showed the color change of PT. With the extension of the incubation period, the R and G values of PT have been significantly reduced. On PT , AP caused the most obvious change in wood color (The largest decrease at week 8), while VA and AS plants had not significant change.When VA and AS were inoculated into the PT, the B value was increased slightly at week 4, 6 and 8, but it almost did not change at week 10. On the contrary, AP was a special case, the B value was decreased significantly, and rebounded significantly at week 10, the color approached wood of the firstly incubation time. On account of, AP produced white rot at week 10, which causes wood color to gray.
From the Fig. 2 we could find that when AP was inoculated into PT, the color of the wood gradually changed from light yellow to gray as the incubation time prolonged, and eventually became grayish white; then for VA, the color of the wood gradually changed from light yellow to gray as the culture period prolonged, and eventually became pale grayish green at week 10; finally for AS, the color of the wood gradually changed from light yellow to gray as the incubation time was extended, and finally became pale grayish brown in the fourth incubation time. From this, we knew that VA and AS were not produce effective pigments to cause pronounced color difference in the wood. It could be seen that the color change of wood was not obvious at week 4 with 6, but significant changes occurred at week 8. At week 10,there were significantly external white rot on wood blocks of inoculated with AP, but other two fungi were not produced.This suggests that AP might be a white rot fungus.
In conclusion, on the one hand, AP could produce obvious red matter on CL and PT , among them the staining effect was not stable on PT, but CL was perfect. After the sample was placed for one year, the dyeing effect still existed on CL . It indicated the potential of AP for large-scale dyeing of wood. On the other hand, the incubation time has a close relationship with the color change of wood. The longer the incubation time, the more the amount of spalting produced by fungus, resulted in a more pronounced change in the color of the wood. The third incubation time might be the best period of pigmented material produced by the fungus during this period (Not sure the most pigmented period).
Amount of spalting
Figure 3 was the percentage average of external spalting of the wood formed by the three fungi on CL (Fig. 3 a) and PT(Fig. 3 b). From Fig. 3 a, there was significantly more spalting on CL at week 10 than those at week 4, 6 and 8 (P< 0.05). And the maximum spalting percentage was 48% for AP, 46% for VA, and 43% for AS at week 10, and the minimum spalting was 4%, 11% and 25%, respectively, for AP, VA and AS at week 4.With the extension of inoculated time, the percent of spalting gradually increased. For CL, there was more spalting for AP than those of the two other fungi. From Fig. 3 b, the maximum spalting on PT was at week 10 for VA and AS, but at week 8 for AP. The highest value was significant by SPSS one-way ANOVA (P< 0.05). The spalting caused by AP and AS was climbed steadily at week 4, 6, 8 and 10, and there was similar trend for VA expect at week 10, at which it was dropped down.It might be an unexpected result of the experiment.

Fig. 3 a The percentage of spalting on CL; b The percentage of spalting on PT

Fig. 4 a The part effect of three fungus dyeing on CL; b The part effect of three fungus dyeing on PT
Comparison of three fungus inoculated in CL and PT(Fig. 4). The external spalting on the CL were more than that in the PT at same incubation time and fungus (P< 0.05).Among the three fungi, AP external spalting was significantly higher than the other fungus at same incubation time and wood species (P< 0.05). Fungi had different preferences for wood, for example, AP preferring to CL. It was possible that there were some special matter in CL, and it could be benef icial to colonization of fungi and promote the growth of fungi or the production of pigment. In addition, PT might had some substances that inhibit the growth of fungi. Therefore, there had more pigment on CT that PT.
Dyeing depth analysis
The combination of AP and CL was the only one that could produce internal spalting. Therefore, the depths of these wooden blocks were measured, and the average of the depths on both sides of the spalted wood block was taken as the dyeing depth of the wood block. The average value of five test samples per batch was taken as the dyeing depth, and the curve of dyeing depth and percentage of internal spalting as the incubation time. Figure 5 shows the percentage average of internal spalting at different incubation time, and dyeing depth. The mean number of internally spalting and dyeing depth varied significantly depending on time. Internal spalting and dyeing depth were steadily increased for weeks 4, 6,8 and 10. Later weeks (8 and 10) showed significantly more internally spalting than earlier weeks. It could be seen from Fig. 5 that the minimum average depth was 1.8 mm at week 4, and the maximum average depth was 5.06 mm at week 10.In addition, the dyeing depth of a single wood block reached 7 mm at week 10. Summarizing the internal spalting of AP,it could be found that the extension of the incubation time has a significant influence and regularity on the generation of internal spalting.
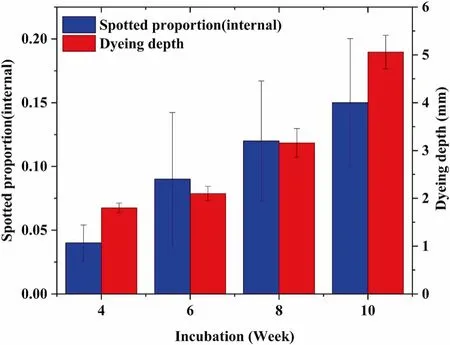
Fig. 5 The depth of internal spalting formed by fungi AP on CL blocks, and the percentage of internal spalting by fungi AP on CL blocks
In the early stages, AP could observe significant pigment diffusion on the solid medium, so the pigment might be an extracellular pigment with good diffusibility. So, as the incubation time, the more pigments were produced by the fungus, the deeper the pigment penetrates the wood and the larger the proportion of internal spalting. However, it was not prove that the depth of the pigment was caused by physical inf iltration or colonization of hyphae in the experiment. Again, results indicate that AP was a better fungus for spalting due to the higher levels of dyeing depth and internally spalting.
Weight loss
Figure 6 showed the weight loss of the spalted wood block,when the three fungi were inoculated on two fast-growing wood.
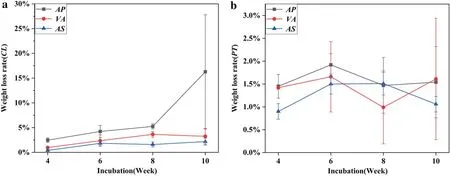
Fig. 6 a Weight loss rate of CL block; b) Weight loss rate of PT block
Significant difference was analyzed by SPSS one-way ANOVA, and it was found that the influence of incubation time on wood weight loss was not significant (P> 0.05).When the three fungus were inoculated into PT, the maximum weight loss rate was 16.27% (AP, at week 10), and the minimum value was 0.40% (AS, at week 4). On CL , the maximum weight loss rate was 1.92% (AP, at week 6), and the minimum value was 0.90% (AS, at week 4). When AP was inoculated, the weight loss of PT and CL was significantly different. Surprisingly, PT had lower external spalting (pigment), but significantly higher weight loss than CL. Especially, the weight loss of PT was significantly higher than that of CL at week 10, and the other two fungi were not exception. Among the three fungi, only PT inoculated with AP showed a regular increase in weight loss with the extension of incubation time, but other fungus and wood combination was not obvious change. Combined with the weight loss rates of the wood, the results showed that there was no significant relationship between the weight loss of CL blocks with fungi and incubation time.
Conclusion
In this work, AP (Arthrinium phaeospermum), VA (Vibrio anguillarum) and AS (Aspergillacea) were inoculated on CL(Cunninghamia lanceolate) and PT (Paulownia ), respectively, and then wood samples were evaluated. The results showed that AP could produce the most percentage average of external and internal spalting on CL, which were 48% and 15%, respectively (The average staining depth was 5.06 mm). And the weight loss of the combination was low(0.46 g), which had little influence on wood strength. The results showed that AP was an ideal wood staining fungus,which could produce pigment stably and had a broad application prospect. CL was an ideal application in wood fungus dyeing.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creat iveco mmons.org/licen ses/by/4.0/.
杂志排行
Journal of Forestry Research的其它文章
- A commentary review on the use of normalized difference vegetation index (NDVI) in the era of popular remote sensing
- Reconciliation of research on forest carbon sequestration and water conservation
- A theory to link relationships of stand volume, density, mean diameter and height in forestry data
- A new model for predicting the total tree height for stems cut-to-length by harvesters in Pinus radiata plantations
- Comparative performances of new and existing indices of crown asymmetry: an evaluation using tall trees of Eucalyptus pilularis(Smith)
- Tree mortality and biomass loss in drought-affected forests of East Texas, USA
