Tree mortality and biomass loss in drought-affected forests of East Texas, USA
2021-01-11MuktiRamSubediWeiminXiChristopherEdgarSandraRideoutHanzakMingYan
Mukti Ram Subedi · Weimin Xi ·Christopher B. Edgar · Sandra Rideout-Hanzak ·Ming Yan
Abstract Changes in tree mortality due to severe drought can alter forest structure, composition, dynamics, ecosystem services, carbon fluxes, and energy interactions between the atmosphere and land surfaces. We utilized long-term(2000-2017, 3 full inventory cycles) Forest Inventory and Analysis (FIA) data to examine tree mortality and biomass loss in drought-affected forests for East Texas, USA.Plots that experienced six or more years of droughts during those censuses were selected based on 12-month moderate drought severity [Standardized Precipitation Evaporation Index (SPEI) -1.0]. Plots that experienced other disturbances and inconsistent records were excluded from the analysis.In total, 222 plots were retained from nearly 4000 plots.Generalized nonlinear mixed models (GNMMs) were used to examine the changes in tree mortality and recruitment rates for selected plots. The results showed that tree mortality rates and biomass loss to mortality increased overall,and across tree sizes, dominant genera, height classes, and ecoregions. An average mortality rate of 5.89% year −1 during the study period could be incited by water stress created by the regional prolonged and episodic drought events.The overall plot and species-group level recruitment rates decreased during the study period. Forest mortality showed mixed results regarding basal area and forest density using all plots together and when analyzed the plots by stand origin and ecoregion. Higher mortality rates of smaller trees were detected and were likely compounded by densitydependent factors. Comparative analysis of drought-induced tree mortality using hydro-meteorological data along with drought severity and length gradient is suggested to better understand the effects of drought on tree mortality and biomass loss around and beyond East Texas in the southeastern United States.
Keywords Generalized nonlinear mixed model ·Endogenous factors · Drought index · Standardized precipitation evaporation index(SPEI) · Above-ground biomass · Competition index · Biomass lost to mortality ·East Texas
Introduction
Global change assessments on natural ecosystems have focused primarily on how vegetation will respond to the expected rate of climate extremes (Lewis et al. 2004; Adams et al. 2009; Halofsky et al. 2013). Experimental and observational studies of forest ecosystem response to drought have demonstrated increased tree mortality at multiple sites across the globe (Engelbrecht et al. 2007; van Mantgem et al. 2009; Adams et al. 2009; da Costa et al. 2010; Allen et al. 2010; Peng et al. 2011; Zhang et al. 2012; Zeppel et al.2013; Taeger et al. 2013; Brien et al. 2014). However, predicting drought-induced tree mortality is difficult because it involves multiple factors/agents in a nonlinear threshold process (Moorcroft et al. 2001).
Droughts have led to forest ecosystem changes in net primary productivity (Zhao and Running 2010), carbon balances (Frank et al. 2015), background tree mortality (van Mantgem et al. 2009; Peng et al. 2011), spatial patterns of tree mortality (Guarín and Taylor 2005; Baguskas et al.2014; Gea-Izquierdo et al. 2014), plant growth (Misson et al.2011; Bernal et al. 2011; Mou et al. 2018), plant phenology(Misson et al. 2011; Bernal et al. 2011), physiological and biochemical responses (Deligöz and Cankara 2019; Liang et al. 2019) species distribution and composition (Engelbrecht et al. 2007), and species diversity (Slik 2004; Engelbrecht et al. 2007; Clark et al. 2011). The Intergovernmental Panel on Climate Change (IPCC) (2013) predicted increasing frequency and intensity of drought in the 21st century.Foresters are making efforts to understand and predict the consequences of global climate change on forest ecosystems(Lindner et al. 2010; Vose et al. 2012; Luo and Chen 2013;Sohngen and Tian 2016).
Researchers across the globe have made efforts to quantify the impacts of drought and increased water stress on tree mortality. In temperate forests of the Netherlands, Weemstra et al.(2013) demonstrated that summer drought was responsible for reduced radial growth across multiple species. Regional warming and associated water stress are contributing factors to widespread tree mortality in the temperate forests of the U.S. (van Mantgem et al. 2009; Williams et al. 2010, 2013).Peng et al. (2011) found regional water stress as a likely dominant contributor to tree mortality rates across a range of species, size classes, elevations, longitudes and latitudes in western and eastern boreal forests of Canada. Similar results were reported from temperate forests for Beijing, China (Zhang et al. 2014). In Southern and Eastern Europe, drought was the dominant factor in mortality, outweighing the positive effects of a warming climate on forest growth and wood production (Lindner et al. 2010; Carnicer et al. 2011). In Australia,Mitchell et al. (2014) predicted that the frequency of droughts capable of inducing tree die-offs in dry and moderate warming scenarios could increase from one in 24 years to one in 15 years by 2050. However, observed background tree mortality rates are highly variable with numerous compounding factors and thus are hard to quantify in a uniform fashion. In light of this, Wang et al. (2012) proposed three key processes (stand density change, basal area reduction, and biomass reduction)be quantified for a uniform understanding of background tree mortality by calibrating and validating data from long-term observational data.
Texas, USA, has data on significant drought periods since 1930 (Nielsen-Gammon 2011). In 1999, the Texas Forest Service analyzed weather data from the previous 100 years and identified three separate 25- to 30-year interval drought periods with the last drought beginning in the 1950s and ending in the late 1970s (Barber et al. 2009). During a drought cycle, rainfall and wet periods continue to occur. However,drought effects are frequently compounding, mainly where dry conditions occur with higher frequency and intensity.Drought has become the “normal” pattern rather than the exception (Barber et al. 2009). Analyzing drought-induced tree mortality and biomass dynamics is increasingly crucial as forests play a vital role in mitigating effects of climate change, making the accurate assessment of tree mortality and biomass stored in the forest of utmost importance.
Several attempts have been made to quantify effects of drought on tree mortality in the southeastern U.S. (Klos et al.2009; Crosby et al. 2012, 2015) and in Texas (Cooper and Bentley 2012; Huang et al. 2014; Waring and Schwilk 2014;Morin et al. 2015) using Forest Inventory and Analysis (FIA)data. However, often these studies investigated a single drought event (usually 2011) and did not utilize plots with multiple measurements. In East Texas, severe droughts occurred in the years 1998-2001, 2008-2009 and 2011 (Nielsen-Gammon 2011) and caused widespread tree mortality. Recently,Klockow et al. (2018) estimated temporal trends (from 2012 to 2015) in post-drought tree mortality, and Edgar et al. (2019)estimated widespread tree-damage in East Texas due to Hurricane Rita (2005), Hurricane Ike (2008) and drought (2011)using full, multiple sets, and single set of panels. To our knowledge, this research uses a longer period to investigate droughtinduced tree mortality in East Texas than past studies had
This study provides a detailed analysis of tree mortality and recruitment rates (% year −1) across East Texas forests and the associated biomass change utilizing long-term FIA data with key climatic variables, tree, and stand attributes(Table A1). In East Texas, FIA is on a five-year inventory cycle with 20% of the plots measured each year. Our selected FIA dataset included the first three complete inventories(2000-2013) and partial data from the fourth inventory(2014-2017). In order to assess drought-induced tree mortality, this research separates plots experiencing mortality by different disturbances and selects plots that consistently experienced multiple episodes of droughts (i.e., > 6 years)over the study period. By doing so, the research seeks to separate drought from other density-independent factors. The possible confounding factor would be a density-dependent factor, for which competition index (basal area and density of plots) were considered in the analysis. We hypothesized that in the absence of other disturbances, the observed mortality trends could be attributed to the severity and length of the drought. Surviving trees may sequester less biomass than they would under conditions due to the higher cost of respiration during prolonged drought. Uncaptured mortality,if any, should be reflected in the biomass loss. Therefore,with the subset of drought-affected plots, this study aimed at answering the following questions: (1) Are there systematic changes in the mortality and recruitment rates during the study period (2000-2017) across ecoregions, diameter classes, height classes, stand origins, latitude classes and major species groups? If so (2) what factors (competition or drought) are responsible for these changes? and (3) what are the trends in the annual proportion of biomass lost in East Texas forests during the study period?
Materials and methods
Study area
The present study occurred in East Texas (8.96 million ha)(O’Connell et al. 2015), which includes two FIA survey Units: Northeast and Southeast. The Northeast Unit includes 22 counties, while the Southeast includes 21 counties. A location map of the study area is presented in Fig. 1.
East Texas has a mild, mid-latitude, humid subtropical climate with hot summers and mild winters. The mean annual temperature varies approximately from 17 to 21 °C. Average annual precipitation varies from 680 to 1700 mm (Wiken et al.2011). The frost-free period ranges from 220 to 365 days. Compared to other climatic divisions in the state, East Texas has the least decadal variation in precipitation both in absolute and relative terms and has received a higher proportion of precipitation increase (15% per century) from December to March (Nielsen-Gammon 2011). Many areas in East-Central Texas Plains have a thick, underlying clay pan with Alf isols and Vertisols, and have a thermic temperature regime with udic and ustic soil moisture regimes. Similarly, dominant soil types in Western Gulf Coastal Plains include Alf isols, Vertisols, Entisols, and Mollisols with hyperthermic soil temperatures and ustic, udic,and aquic soil moisture regimes (Wiken et al. 2011).
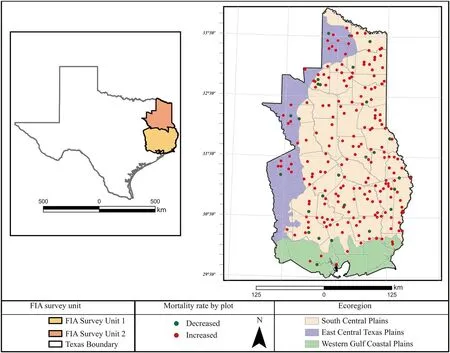
Fig. 1 Approximate locations of selected FIA plots. Circles show increased (red) or decreased (green) plot level mortality between first and last census during the study period (2000-2017). Our analysis showed that mortality rates increased in 88% of drought experienced FIA plots (196/222). On the inset map (left) cantaloupe color represents FIA Survey Unit 2 or Northeast Unit, and mango color represents FIA Survey Unit 1 or Southeast Unit. On the main map(right) the sand, purple, and green color, respectively, represent South Central Plains, East Central Texas Plains, and Western Gulf Coastal Plains ecoregions
About 4.9 million ha in East Texas are forestland, and almost 4.8 million ha of the forestland is timberland. Timberland is a non-reserved forest land with a potential of producing a timber volume of at least 1.398 m 3 ha −1 [20 ft 3 acre −1 year −1 ] (O’Connell et al. 2015). About slightly more than 50%of forest area in East Texas is hardwood, and slightly less than 50% is softwood. Oak-hickory is the dominant hardwood forest type, which follows by oak-pine, and oak-gum-cypress. In the softwood forest type, loblolly pine-shortleaf pine is the dominant forest type. The most abundant species by order of total aboveground dry biomass are (1) loblolly pine (Pinus taeda), (2) sweetgum (Liquidambar styraciflua), (3) water oak(Quercus nigra), (4) post oak (Quercus stellata), (5) shortleaf pine (Pinus echinata), (6) southern red oak (Quercus falcata),(7) willow oak (Quercus phellos), (8) white oak (Quercus alba) (Dooley and Kerry 2018) (Table A2).
Plot selection and analysis
This research compiled the data from FIA databases maintained by the U. S Forest Service of the United States Department of Agriculture (USDA) between 2000 and 2017. The FIA dataset contains tree-level data for 95 species growing in East Texas(Table A2). During the study period, remeasurement time ranged from 1 to 6 years, with an average of 4.3 years. The criteria used to select the plots affected by drought stress were:(1) plots must have been measured at least three times during the study period; (2) trees had to measure at least 2.54 cm dbh at the initial inventory; (3) plots had to have at least 10-year long survey data; (4) plots must have no signs of fire, flood, hurricane, insect, or cutting; (5) plots with initial density of at least 15 trees; and (6) plots must have experienced multiple episodes of drought (i.e., > 6 years 12-month average SPEI < − 1.0) during 2000 and 2017 (Subedi et al. 2018). Plots with inconsistent measurement records were not included in the analysis.
Of the nearly 4000 FIA plots in East Texas, the abovementioned criteria resulted in 222 plots being included in the analyses, of which 38 were plantations and 185 regenerated naturally. Tree individuals in selected plots were classified into three diameter classes (< 15 cm, 15-30 cm, ≥ 30 cm), two height classes (< 20 m, ≥ 20 m), four species groups (Pines,Sweetgum, Oaks and Others), and two latitude categories(< 31.5°N, ≥ 31.5°N). Diameter and height classes and latitude categories were created to have roughly equal numbers of trees in each class. The analysis was also carried out at the FIA survey Unit level (Northeast and Southeast Units) and ecoregion level. Ecoregion Level III data were downloaded from the Environmental Protection Agency’s website (EPA 2012).East Texas includes parts of four ecoregions: (1) Texas Blackland Prairies, (2) East Central Texas Plains, (3) Western Gulf Central Plains, and (4) South Central Plains. Texas Blackland Prairies which covered a significantly small portion compared to other ecoregion, was merged into East Central Texas.
Selected plots were between the latitudinal minimum (29.71°N), and maximum (33.65° N), to longitudinal minimum (93.55°E) and maximum (96.11° E). The length of the census interval ranged from 1 to 7 years (mean ± S.D. = 4.53 ± 1.36). The initial census year ranged from 2001 to 2003 and the final year census ranged from 2013 to 2017. Artificially regenerated forest plots (n = 38) had an average diameter of 19.52 ± 11.72 cm with skewness (1.06) and kurtosis (4.98). Similarly, the plots in natural forests (n = 184) had an average diameter size of 19.38 ± 11.80 cm with skewness (1.14) and kurtosis (5.61)(Table 1). Key characteristics of the plots are presented in the supplementary material Table A1 of the supplementary material. Table A2 ranks top 20 species contribution by a number of trees and standing volume in 222 plots.
Statistical models
To understand drought-induced tree mortality and droughttrigged biomass loss in the east Texas forest this research utilized statistical models similar to those used by van Mantgem et al. (2009) and Peng et al. (2011). Generalized nonlinear mixed models (GNMMs) were used to regress changes in mortality and recruitment rates as functions of time for specific plots, and plot identity was used as a random effect to analyze several plots together.
Changes in annual mortality rates were estimated using the following logistic function:

where, p is the probability of mortality, subscript i indicates plot number, tjrepresents the year of jth census, β0and β1,are slope estimates, and γiis the random effect parameter among the multiple plots.
Annual changes in mortality or recruitment were modeled using a negative binomial regression model where nijindicates the number oflive trees at the previous census for the ith plot and the jth census, and mijis the count of dead trees in theith plot and j th census.


Table 1 Fixed effects in the generalized nonlinear mixed models describing mortality rate trends across the diameter,species, height, ecoregion, stand origin, and latitudinal category

where pijrepresents the rate of recruitment over the census interval.
Parameters of the mortality and recruitment models were estimated using maximum likelihood. Percent changes in mortality (m %) and recruitment (r %) were estimated as (m% or r %) = (exp(β 1) − 1)*100) following the methods of van Mantgem and Stephenson (2007).
Linear mixed modeling (LMM) approach was used to understand whether the competition indices (endogenous factors) were affecting tree mortality. For this, trends in forest density, basal area, and census interval length were estimated. Parameters of LMM were estimated using the restricted maximum likelihood method:

where, i is the plot number, j is the jth census ,yijis a fractional change in annual mortality, x is the dependent variable(basal area, forest density, or census interval length), γiis the plot random intercept and εijis the random term that follows a normal distribution.
Biomass loss to mortality
The annual proportion of biomass loss to mortality (apbm)at the plot level was calculated following Sheil et al. (1995)(Eq. 7), which through compounding, adjusts the time bias in the calculation of mortality rates (Gustafson and Sturtevant 2013).
where, B(n−1)is the biomass oflive trees (of a given category,e.g., species) in the previous census, Bnis the biomass oflive trees at the next census, and t is the number of calendar years between two censuses.
Changes in tree mortality and recruitments rates were calculated using Eqs. 3 and 5, respectively. Changes in the annual fractional change in mortality because of change in density, basal area, and census interval were performed using Eq. 6. The annual proportion of biomass lost to mortality was calculated using Eq. 7. Moreover, binomial tests were carried out to understand the number of plots that experienced increasing rates of biomass loss to mortality.Two sample student t test with unequal variances was used to examine the difference in biomass loss to mortality by FIA Units. One-way analysis of variance (ANOVA) was performed to test for differences among ecoregions.
Results
Changes in mortality rates
The mortality rate increased significantly for all plots combined (p< 0.001) and by species group, tree height class, and latitude class (Table 1). At the plot level, when all plots were considered together, the average tree mortality rate was about 5.89% year −1 during the 18-year study period (Table 1). Mortality rates among species, diameter,height, stand origin, latitude, and ecoregion varied from 3.86 to 7.22%. Mortality rates also increased for small(< 15 cm), medium (15-30 cm), and large-diameter class(dbh ≥ 30 cm) trees. Both small and medium diameter class trees showed similar higher mortality trends, while larger diameter class individual trees showed relatively lower mortality. The highest mortality was observed in pine trees. Plantation forest plots suffered from higher mortality rates during the study period than natural-origin forest stands.
Both short (< 20 m) and tall (≥ 20 m) trees showed increased mortality (Fig. 2 e). In the first few years, the difference in mortality rates was somewhat similar, but in later years the difference in mortality rates widened although trends in mortality increased. Among ecoregions increase in mortality rates was statistically significant only in the South Central Plains ecoregion (Fig. 2 d, Table 1). Mortality trends were significant across the latitude categories in East Texas (Table 1). In general, all studied categories showed increasing mortality trends which were more rapid after 2010 (Figs. 2, 3).
Changes in recruitment rates
Unlike mortality rates (Table 1), recruitment rates decreased significantly for all plots (p = 0.005,rate = − 0.89% yr −1) and for all species groups, ecoregions, latitudinal and stand origin classes (Fig. 4,Table 2). Recruitment rates decreased in the order of species groups: Pine (− 0.95%), other (− 0.91%), Sweetgum (− 0.86%), Oak (− 0.84%). Ecoregions South Central Plains and Western Gulf Coastal Plains showed a decreased recruitment rate (p< 0.05); however,recruitment rates in the East Central Texas Plains showed no statistically significant changes (p = 0.102; Table 2).
Changes in density and basal area
An LMM (Eq. 6) was used to examine trends in forest density and basal area to understand whether the mortality was triggered by endogenous/competition factors. The result indicates that in addition to drought competition factors,density, basal area, and census intervals were associated with mortality. Plot-level tree density declined significantly considering all plots together (p< 0.001); however, no significant change or slight decline in density occurred in Western Gulf Coastal Plains or East-central Texas Plains,unlike South Central which showed a significant reduction in density (β = − 0.021,p< 0.001). Both natural and plantation forest plots showed a decline in forest density. Further,LMM revealed a slight decrease in basal area in the South Central Plains ecoregion and plantation forests (Table 3).Decrease in basal areas in Western Gulf Coastal Plain, and East-Central Texas Plains and natural forest plots did not show a statistically significant relationship (Table 3).
Results from LMMs suggest a significant trend in census interval (Fig. 5 a), forest density, (Fig. 5 b), and basal area(Fig. 5 c) for annual fractional changes in mortality.
Changes in annual proportion of biomass lost to mortality (apbm)
Trends in the rate of the annual proportion of aboveground biomass lost to mortality were modeled (Eq. 6)based on the calendar year (a census year) to estimate trends in the rate of mortality change (t ha−1year −1 ). Separate models for forest origin types and ecoregions suggested the annual proportion of biomass lost to mortality(t ha −1 year −1) increased for plantation forest and South Central Plains ecoregion (Table 4). Although combined plots showed a slight decrease in biomass loss to mortality, natural forest plots and plots from Western Gulf Coastal Plains and East Central Texas plains showed an increase in biomass loss to mortality. Nonetheless, these statistics show increasing trends in apbm were not statistically significant (Table 4).
One-way analysis of variance (ANOVA) for plot level biomass lost to mortality across ecoregions showed no statistically significant results (F = 0.88,p= 0.415). However,sample t-tests across stand origin showed significantly different mortality rates at the plot level (t = − 3.1891,p= 0.0016).Similarly, two sample t-test with unequal variances suggested there was not a statistically significant difference in biomass lost to mortality for either northeast (= − 0.7813,s.e. = 0.276) or southeast Units (= − 1.33, s.e. = 0.494(t = 0.975,p= 0.3307).

Fig. 2 Modeled trends in mortality for (a) diameter, (b) Species, (c) Stand origin, (d) ecoregion (level III), height (e), and latitude (f)
Discussion
East Texas has experienced significantly increased tree mortality and biomass loss based on the analysis of 222 droughtaffected FIA plots that were measured for at least three times since 2000. Factors contributing to tree mortality were segregated to endogenous (stand characteristics) and exogenous (climate-temperature, precipitation, and a measure of drought). Endogenous factors contributing to tree mortality include forest stand structure and composition, of which structural components of forest density (ind. ha −1 ), stand age, and basal area (m 2 ha −1) are best-known indicators of tree mortality and survival (Franklin et al. 1987, 2002;Saud et al. 2016). Although allometric equations have been developed to predict the age of the species, there is no clear consensus about their meaning and usefulness (Shaw 2015).Thus, only density (ind. ha −1) and basal area (m 2 ha −1) were considered in this research.

Fig. 3 Plot level mortality rates by FIA Survey Unit 1 (a), and Survey Unit 2(b) across the studied period. Each horizontal line segment represents the mortality rate during a single census interval for a specific plot. The thick red curved line represents the modeled trends in tree mortality for each survey Unit
Moisture stress in higher density plots during drought could lead to increased mortality (Stone et al. 2002). Silvicultural activity e.g., thinning in denser stands can reduce drought-triggered mortality by reducing competition for nutrients and moisture (Giuggiola et al. 2013; Elkin et al.2015). In lower elevation forests during drier climate periods of the Lake Tahoe basin in Nevada, Van Gunst et al. (2016)using a remote sensing approach and found positive densitydependent mortality; an increase in stand density elevated the probability of mortality. However, during a 10-year long drought study (1997-2007) in mixed-conifer and pine forests of Arizona stand density was not strongly related to mortality rates (Ganey and Vojta 2011). Although annual proportional change in mortality is small (< 2% year−1) due to census interval, basal area, and density, statistically significant trends across these variables indicate that density related factors could play a role. The higher mortality rate of small sizes and shorter trees along with density suggests that density-dependent mortality during the drought could be an artifact of data filtering process where trees ≥ 2.54 cm dbh were included from four subplots from clusters rather than larger-size plots used in other studies.

Table 2 Fixed effects in the generalized nonlinear mixed models describing annual recruitment rates across species,ecoregion, stand origin, and latitudinal category (Eq. 5)
Generally, stem volume increases over to leaf area,which suggest that larger trees should be less susceptible to drought-induced mortality compared to shorter trees because larger trees can store more water in the stem (Phillips et al. 2003; Scholz et al. 2011). In Californian Bishop pine (Pinus muricata.) forests, Baguskas et al. (2014) found a higher probability of mortality for shorter trees (< 8 m)even when the height difference was only one to two meters.In the Italian Oak (Quercus frainetto), shorter trees were more susceptible to drought-induced mortality than taller trees (Colangelo et al. 2017). Hanna and Kulakowski (2012)reported a larger size of surviving quaking aspen (Populus tremuloides) trees in Colorado and Wyoming trees than that of trees that did not survive. However, other researchers have shown that this tends not to be the case where taller trees are generally more susceptible to drought because oflonger hydraulic paths and increased atmospheric demand(Mencuccini et al. 2005; Bennett et al. 2015; Rowland et al.2015; Moore et al. 2016). We utilized high variation in trees heights (Range = 36.27 m and s.d = 6.25 m) with highermortality rates of smaller trees. A possible interpretation for this result could be smaller trees have limited access to stable subsurface moisture reserved in the soil and therefore could not compete during periods of prolonged drought with larger and taller trees.

Fig. 4 Recruitment rates through time for plots combined for Species category (a), Stand origin (b), Ecoregions (c), and latitudinal class (d). Negative sings on the y-axis indicate decreasing recruitment rates. The higher the negative number, the greater the distance is from zero; therefore, y-axis labels are reversed to show the decreasing trend obtained from the models

Table 3 Trends in forest density (ind. ha −1) and basal area (m 2 ha −1) from the linear mixed models. The negative sign of coefficient indicates negative trends in data across categories

Fig. 5 Modeled annual fractional change in mortality rate (%) of individual plots relative to forest density, basal area, and census interval
The two-tailed binomial test showed that mortality rates increased in 88% of drought experienced FIA plots(196/222, Fig. 1) (p< 0.0001). Tree mortality rates increased among the main species having larger biomass stocks in East Texas, indicating tree mortality was not influenced by lifehistory traits, such as shade tolerance. This finding suggests that successional dynamics cannot be the primary drivers of increased mortality, which is in agreement with the results of van Mantgem et al. (2009) for temperate forests in the western U.S. and Zhang et al. (2014) for the sub-humid and semi-arid zone of China. However, some researchers pointed out mortality at the stand, and forest levels are dependent on life-history traits and tolerances by species (Chao et al. 2008;Phillips et al. 2009; Prado-Junior et al. 2016).
Pine, a low-density pioneer species, suffered from a higher mortality rate (Pataki et al. 1998; Fridley and Wright 2012). Low-density pioneer species are often the first victims during prolonged periods of drought (Slik 2004). Moore et al. (2016) observed that the 2011 drought related mortality among four dominant genera was in the increasing order of sweetgum (Third), oak (Second), and pine (First), which is in agreement with the results from this study. Moreover, Klos et al. (2009) from the Forest Health and Monitoring data (1991-2005) reported that the pine and mesophytic species showed elevated mortality as drought severity increased. However, field observations carried out in 2014 and 2015 revealed, at small scales, the pattern of drought-induced tree mortality was patchy and often highest in heavy soils in East Texas (Subedi 2016).
The annual proportion of biomass lost to mortality(apbm) increased across both stand origins and ecoregions.An overall decline in biomass due to drought-induced tree mortality can be attributed to declining tree growth, reduction in net primary production, and an increase in tree mortality. Growth decline or mortality in general and mortality due to drought have been demonstrated by dendrochronological studies and modeling long-term data (Bigler and Bugmann 2004; Bigler et al. 2007; Free et al. 2014; Grogan et al. 2014; Mou et al. 2018; Saud et al. 2019). Drought has led to a significant decrease in NPP (e.g., Zhao and Running 2010; Huang et al. 2016). Similar results were reported forPicea abies, Fagus sylvatica, and Deschampsia flexuosa(Grote et al. 2011). Regional climate warming and drought have accelerated tree mortality in western North American pine forests (van Mantgem et al. 2009; Adams et al. 2009;Stone et al. 2012).
Increased tree mortality rates due to drought and physiological stress have been reported to be caused by (1) hydraulic failure (McDowell et al. 2008), (2) carbon starvation(McDowell and Sevanto 2010; Sala et al. 2010), (3) carbon metabolism limitation (Adams et al. 2013). The results from observational studies may fall within these categories but cannot be directly attributed to them. An observational study by van Mantgem et al. (2009) from the Pacific Northwest of the U.S., observed that background tree mortality doubled in 17 years. Our results accord with other observational studies from other areas of the U.S. (Ganey and Vojta 2011; Van Gunst et al. 2016), as well as from Texas(Moore et al. 2016; Klockow et al. 2018). Caution must beapplied to results from this study as models did not account for past life history strategies or drought resistance of the major species, utilized re-measured plots which experienced several droughts.

Table 4 Fixed effects of the linear mixed models describing the annual proportion of biomass lost to mortality (apbm). The negative sign of coefficient indicates the negative relationship between biomass loss to mortality and data
Minimizing the impacts of increasing drought frequency and intensity is one of the pressing questions to be addressed through forest management in the context of climate change. Forest resource managers deal directly with changes in forest conditions and disturbance regimes(Vose et al. 2016). Drought strongly affects tree growth and mortality, and changes in drought frequency and intensity are expected to elevate mortality rates, shift species composition, and reduce carbon sinks over broad geographic regions (Frolking et al. 2009; Klos et al. 2009;Allen et al. 2010; Clark et al. 2016). The past and future effects of environmental changes, climate change, wildf ires, insect infestations, and hurricanes on stand development processes (e.g., competition or succession) need to be quantified (Keller et al. 2002; Xi 2005; Desprez-Loustau et al. 2006; Xi et al. 2008; Hurteau et al. 2013). In a forest ecosystem, a multitude of disturbance agents interplays in nonlinear fashions at different spatial and temporal scales(Seidl et al. 2012; Gustafson and Shinneman douglas 2015). Currently, forests are experiencing unprecedented effects of climate change and resulting disturbances, thus understanding and predicting the dynamics of biotic and abiotic agents at different spatial-temporal scales is crucial for sustainable management of forest ecosystems (Seidl et al. 2012). Analyzing these interactions was beyond the scope of this study. However, future research can be directed to quantify the interacting effects of fire, drought,insects, and pathogens along with competition factors so as to reduce uncertainty in the mortality estimation process(Zens and Peart 2003).
In this study, we used drought severity as a major factor in the data filtering process for the drought-affected forest plots. Future mortality and biomass loss analyses along with drought severity gradient combined with drought length and other interplaying biotic factors may detect more tree morality for less severe but longer drought periods and could improve overall study results. When other long-term datasets for forests, both plantation and naturally regenerated, are not available: our approach using a set of selected FIA plots across a large region can be utilized in forest management to account for drought-induced tree mortality and biomass loss.
Conclusion
From our study, we can conclude that (for East Texas and in the Southeastern United States) when long-term Forest Inventory and Analysis (FIA) data were carefully filtered and examined, a widespread directional increase in droughtinduced tree mortality and biomass loss can be detected. A widespread, increasing tree mortality trend was noted over the 18-year period because oflong-lasting droughts, particularly the 2011 drought. At the plot level, the average tree mortality rate was about 5.89% year −1 during the study period. The annual plot-level recruitment rate decreased by 0.89%. The highest decline in recruitment rates was observed among pine (0.95%). The recruitment rate significantly declined in the South Central Plains by an annual rate of 0.92%.
Smaller sized (both diameter and height) trees were affected more than larger trees in East Texas. The result indicated that taller trees capable of reaching available soil water through their deeper root system during the severe drought have greater chances of survival. Pine trees were affected proportionally more (mortality rate = 6.2% year−1) than oak species (mortality rate = 5.7% year −1 ). The annual proportion of biomass lost to mortality (apbm) has increased in the Northeast and Southeast Units, East Central Texas Plains,and South Central Plains. Both drought (an exogenous factor) and density and basal areas (endogenous factors) were related to tree-mortality in East Texas.
AcknowledgementsWe are thankful to the Department of Physics and Geosciences, Texas A&M University-Kingsville, for providing access to the Geospatial Research Laboratory. We would like to thank the stafffrom Sam Houston, Sabine, Angelina, and Davy Crocket National Forests and Big Thicket National Preserve, especially Mr.Daniel P. Jauregui from Sam Houston National Forest, for his guidance during our field trip in 2014 and 2015. We would like to thank Drs. Eric Gustafson and Xiongqing Zhang for sharing their scripts.
杂志排行
Journal of Forestry Research的其它文章
- A commentary review on the use of normalized difference vegetation index (NDVI) in the era of popular remote sensing
- Reconciliation of research on forest carbon sequestration and water conservation
- A theory to link relationships of stand volume, density, mean diameter and height in forestry data
- A new model for predicting the total tree height for stems cut-to-length by harvesters in Pinus radiata plantations
- Comparative performances of new and existing indices of crown asymmetry: an evaluation using tall trees of Eucalyptus pilularis(Smith)
- A video camera recording method for measuring terminal velocity of seed dispersal by wind
