Range-wide genetic diversity in natural populations of Larix principis- rupprechtii Mayr.
2021-01-11XiaoyanDiXiangxiangMengMengbenWang
Xiaoyan Di · Xiangxiang Meng · Mengben Wang
Abstract Prince Rupprecht’s larch (Larix principis- rupprechtii Mayr.), a deciduous conifer, widely grows in middle and high elevations of Northern China. Its natural distribution has sharply decreased and has become fragmented,which may have resulted in the loss of genetic variation. In this study, ten natural populations across the entire range of this species were analyzed using amplified fragment length polymorphism markers. A total of 309 loci were detected from 225 individuals of these populations, of which 261(84.5%) were polymorphic. At the species level, the genetic diversity was high (average of the Nei’s genetic diversity H e = 0.2602, and Shannon’s information index I = 0.3967).The results of molecular variance analysis showed that 90.71% of the genetic diversity occurred within populations.The genetic differentiation among populations was moderate as a whole (F ST = 0.0929, G ST = 0.1510), which is consistent with the moderate level of gene flow among populations(N m = 2.8116). Based on the unweighted pair group method with arithmetic mean and STRU CTU RE analysis, these populations were grouped into three genetically distinct clusters.The degree of inter-population differentiation (G ST = 0.1338)for the south group was larger than that for the north group(G ST = 0.0915). There was a significant correlation between genetic distance and geographic distance across the species range (r = 0.316, P < 0.05). Genetic diversity was significantly associated with longitude but not elevation or climatic factors. The populations with high genetic diversity from each cluster are therefore recommended for future conservation and management of this species.
Keywords Larix principis- rupprechtii · Amplified fragment length polymorphism markers (AFLP) · Genetic diversity · Environmental factors
Introduction
Prince Rupprecht’s larch (Larix principis- rupprechtii Mayr.)is a dominant tree species of cold-temperate coniferous forests in Northern China. Its natural populations are mainly distributed at middle and high elevations (1400-2800 m)in Shanxi and Hebei Provinces, and in the Inner Mongolia Autonomous Region, with about 1400 km 2 extending from 36° 30′ to 43° 40′ N and from 111° 30′ to 118° 30′ E (Li et al. 1992; Wang et al. 1992; Editing Group of Tree Species in Northern China 1994). The species is also planted both within and outside its natural range. Therefore, it plays an important role in reforestation programs and in commerce due to its wide ecological plasticity, fast-growth rates, valuable timber and desirable wood production.
From the earliest fossil records, Larix speices may have had an extensive distribution in northern regions during the Last Glacial Maximum (LGM), and a subsequent reduction could have happened during the past 5000 years(Binney et al. 2009). Based on historical records, human disturbances caused a sharp decline of forest stands of L.principis- rupprechtii during the Liao dynasty (907-1125),during the Ming dynasty (1368-1644) and in the last century (Li et al. 1992; Editing Group of Tree Species in Northern China 1994). These changes have led to the fragmented habitats and possible loss of genetic diversity.
Genetic diversity represents the evolutionary potential of a species and is essential for a species to adapt to environmental changes (Barrett and Kohn 1991; Huang et al. 2018). Although such knowledge is fundamental to effective conservation and sustainable utilization, limited information is available about genetic diversity of L. principis - rupprechtii. Most previous studies have focused on morphological characteristics (Li et al. 1992), eco-physiology (Zhang et al. 2001; Zhao et al. 2018), biochemistry(Zhang et al. 1998), parentage analysis (Sun et al. 2017),and genetic relationships with other larch species (Lewandowski 1997; Hu et al. 1999; Qu et al. 2007; Peng et al.2014). These studies show that L. principis- rupprechlii possibly differentiated from L. olgensis or L. gmelinii in the Late Tertiary during the process of species formation(Hu et al. 1999), and each of these species had a distinctive niche (Peng et al. 2014). In recent years, DNA markers have been used to study the genetic diversity of L.principis- rupprechlii (Di et al. 2014; Fan et al. 2014; Dong et al. 2018) by analyzing natural populations over a limited area or clonal populations in seed orchards. It was found that the genetic diversity of populations from Hebei (Fan et al. 2014) and Shanxi Provinces (Di et al. 2014), and from seed orchards (Dong et al. 2018) was higher than most other species of Larix. The populations from Shanxi showed a higher level of genetic differentiation than those from Hebei (Di et al. 2014; Fan et al. 2014). More work on genetic structure across its entire range, especially in terms of the northern and southern populations, is essential for the efficient conservation of these genetic resources.
With around 13.2 Gb per haploid genome for the genus Larix, whole genome sequencing is difficult over short timescales (Dong et al. 2018). The amplified fragment length polymorphism (AFLP) technique has the advantage of reproducibility, reliability, stringency, and large numbers of polymorphisms. It provides a powerful fingerprinting technique for DNAs of any origin or complexity (Vos et al. 1995).In recent decades, AFLP has been used in the analysis of population genetic variation oflarch species (Semerikov and Lascoux 2003). As such, this technology is suitable for assessing genome-wide marker distributions and estimating genetic diversity (Aichi-Yousf i et al. 2016). The purposes of this study are to assess genetic diversity in populations of L. principis- rupprechtii across its native range,to analyze genetic relationships amongst the populations,and to identify associations between genetic diversity and environmental factors. The results should have important implications for the protection of natural larch resources in Northern China.
Materials and methods
Plant materials
A total of 225 individuals were made from 10 natural populations of L. principis- rupprechtii (Table 1, Fig. 1) across its entire range from Shanxi (populations LL, HM, WT, GD and TY) and Hebei provinces (populations WC, FN, WL and XWT), and from the Inner Mongolia Autonomous Region(population WYD). For each population, needles were collected from 15 to 30 individuals which were at least 50 m apart from each other to avoid sampling from the sameparents. The samples were immediately dried by silica-gel for later use. Climate data (1981-2010) for the sampling sites were obtained from the nearest meteorological stations(https://data.cma.cn/).

Table 1 Population codes, sampling sites, and genetic diversity of L. principis- rupprechtii
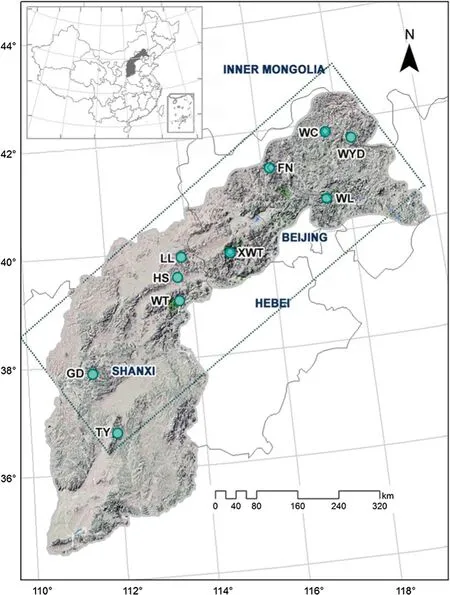
Fig. 1 Solid circles indicate the geographic locations of the ten populations of L. principisrupprechtii in northern China.The dotted line represents the boundary of the natural range of this species
AFLP analysis
Genomic DNA was extracted using a modified cetyltrimethyl ammonium bromide (CTAB) method (Wang and Hao 2010), and checked for purity and quality by 1.0% (w/v)agarose gel electrophoresis and a 6131 Biophotometer plus(Eppendorf, Germany).
The AFLP protocols were performed according to Vos et al. (1995) and Di et al. (2014) using six primer combinations (Table 2). Genomic DNA (20-30 ng) was digested with EcoR I and Mse I. DNA digestion, ligation of adaptors, pre-selective and selective amplifications were performed according to standard AFLP procedures, with 1:50 dilution factor of pre-selective productions and 5 μL of dilution to perform the selective amplification in a final volume of 20 μL. The final PCR products were checked by the AFLP silver-staining protocol.

Table 2 The number of polymorphic loci, and percentage of polymorphism loci obtained from six AFLP primer pairs
Data analysis
AFLP fragments were scored manually as either present(1) or absent (0) in a binary data matrix. We calculated the number (P) and percentage of polymorphic loci (PPL),Nei’s genetic diversity (He), Shannon’s information index(I), total genetic variation (H T ), genetic variation within and among populations (H S and D S ), the coefficient of genetic differentiation (G ST ), and gene flow (N m) with POPGENE 1.31, by assuming that the populations were in Hardy-Weinberg equilibrium.
To examine the genetic relationship among populations,a dendrogram was constructed using the unweighted pair group method with arithmetic mean (UPGMA) based on Nei’s genetic distance with NTSYS-pc 2.1 (Rohlf 2000).A Bayesian model-based cluster analysis was performed to identify the groups of these populations based on fragment data with the STRU CTU RE 2.3. The process was conducted based on Evanno et al. (2005). To test the genetic differentiation among populations, an analysis of molecular variance (AMOVA) was conducted with ARLEQUIN 3.0 (Excoffier et al. 2005). The Mantel test was carried out to examine the possible association between genetic and geographical distances among the ten populations using NTSYS-pc 2.1. The Pearson correlation coefficients between genetic diversity and environmental factors were computed using the SPSS 18.0.
Results
Genetic diversity
A total of 309 putative loci were detected by the six primer combinations, 261 of which were polymorphic (84.5%).The number of bands scored per primer combination varied from 43 to 61 and the percentage of polymorphic band per combination ranged from 75.5 to 87.5% (Table 2). The percentage of polymorphic loci (PPL) of the ten populations ranged from 44.0% for population TY to 66.0% for population WL, with an average value of 50.6%. The total Nei’s genetic diversity (He) and Shannon’s information index (I)were 0.2602 and 0.3967, respectively. For individual populations, the values of He ranged from 0.1956 to 0.2738 with an average of 0.2166, and those of I ranged from 0.2798 to 0.3973 with an average of 0.3123, respectively. The level of genetic diversity was highest for population WL, while lowest for population TY (Table 1).
Genetic structure
The total genetic variation (H T) of this species was 0.2551,primarily distributed within populations (H S = 0.2166).Based on the value of the coefficient of genetic differentiation (G ST = 0.1510), the estimated number of migrations per generation (N m) was 2.8116 (Table 3). The AMOVA analysis showed that 90.71% of the genetic variations occurred within populations, and there was a significant difference among populations (P< 0.001). The population differentiation (F ST) was estimated to be 0.0929.
When all populations were divided into three groups(Fig. 2), genetic diversity of the central group (population WL) was the highest among all groups (Table 3). Levels of H T and H S for the northern group were more or less the same as the southern group (Table 2). However, the degree of interpopulation differentiation (D S = 0.0318, G ST = 0.1338)for the southern group was larger than that for the northern group (D S = 0.0221, G ST = 0.0915). Accordingly, the values of gene flow (N m) were 4.9622 and 3.2368 for the northern and southern groups, respectively (Table 3). AMOVA results revealed limited but significantly variation (4.91%) among the three groups, and the remaining 5.81% and 89.28% variations were found among and within populations, respectively(P< 0.001, Table 4).

Table 3 Estimates of genetic diversity for three groups of L. principis- rupprechtii populations
Genetic relationships
The pairwise Nei’s genetic distances varied from 0.0640 to 0.1051, with an average of 0.0680 (Table 5). The minimum value (0.0640) occurred between populations GD and WT,while the maximum (0.1051) occurred between populations WL and LL. Overall, higher genetic distances existed between population WL and each of other populations(Table 5). The Mantel test showed a significant correlation between genetic and geographic distances for these populations (r = 0.316,P< 0.05).
The UPGMA analysis revealed that all populations could be grouped into three clusters (Fig. 2). Populations WYD,WC and FN were first grouped into a cluster, then gathered together with populations XWT, LL, HS, WT, GD, TY,and finally gathered with population WL. This result was highly consistent with the result of STRU CTU RE analysis,although with considerable overlap among individuals of populations (Fig. 3).
Correlation between genetic diversity and environmental factors
Correlation analysis showed that each of the three diversity indices (PPL, He and I) was significantly correlated with longitude (P< 0.05), while only PPL was significantly correlated with latitude (P< 0.05) (Table 6). As a whole, the genetic diversity of L. principis- rupprechtii showed a close relationship with longitude, but not with elevation or with climatic factors.
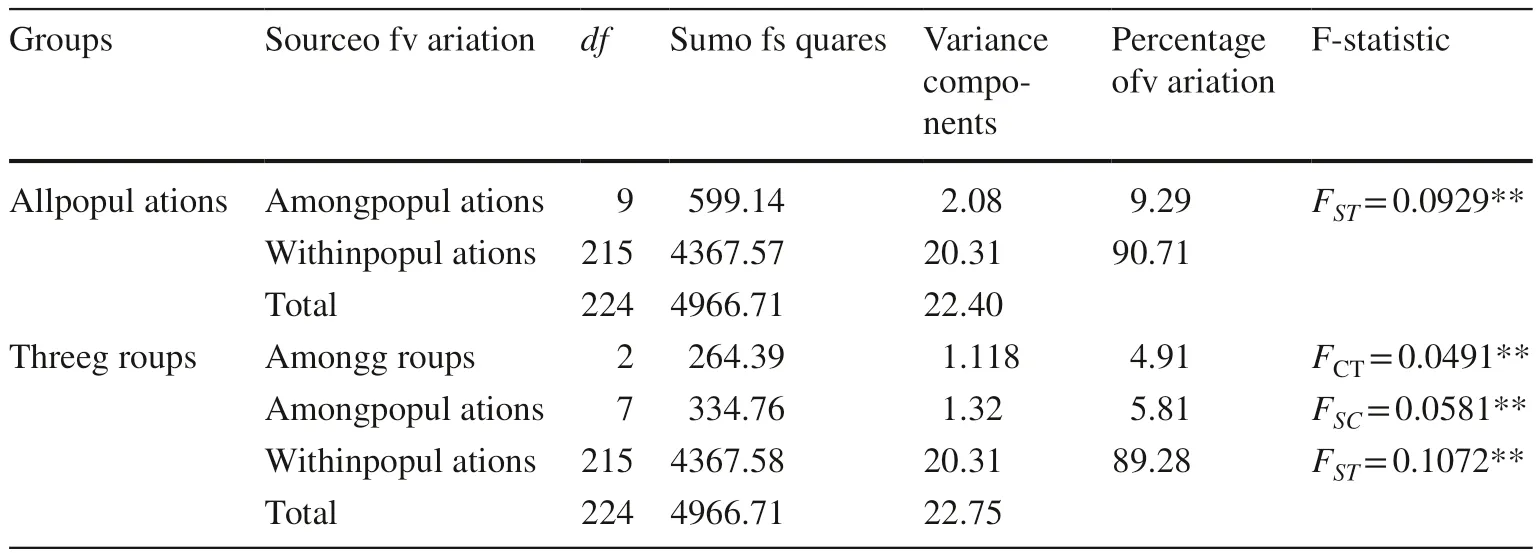
Table 4 Analysis of molecular variance (AMOVA) for the ten populations and three groups of L. principis- rupprechtii
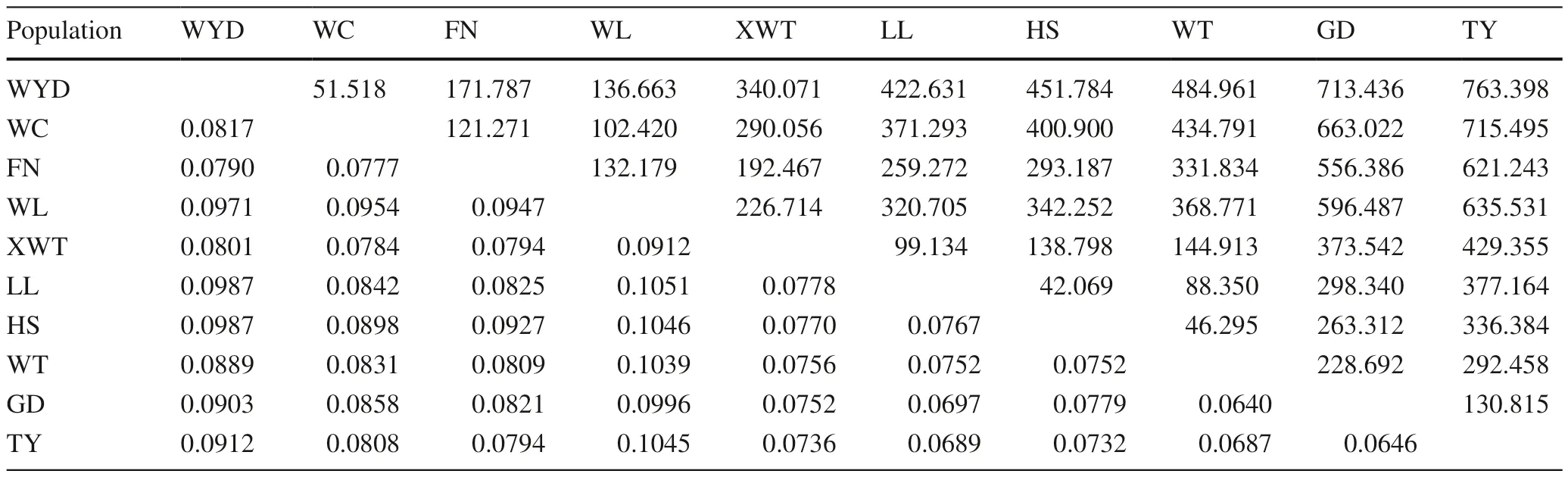
Table 5 Nei’s genetic distances and geographical distances among the ten populations of L. principis- rupprechtii

Fig. 3 Pattern of genetic relationship among the ten populations of L. principis- rupprechtii based on STRU CTU RE analysis with K = 3

Table 6 Pearson correlation coefficients between genetic diversity and environmental factors
Discussion
Ten populations of L. principis- rupprechtii were investigated across the natural range using AFLP markers in this study. It was found that the genetic diversity of this species in Northern China (He = 0.2602, I = 0.3967) was greater than the populations from Shanxi Province (He = 0.225, I = 0.341)(Di et al. 2014). The genetic diversity of L. principis- rupprechtii was higher than for the average of 13 other Larix species (He = 0.142), but similar to L. gmelinii (He = 0.211,I = 0.346) (Kozyrenko et al. 2007). The high level of genetic diversity of L. principis- rupprechtii was likely associated with its high habitat heterogeneity (Li et al. 1995; Semerikov and Polezhaeva 2007), its wide historical distribution (Wang et al. 1992; Leng et al. 2008a), and relatively short periods of fragmentation (Hu et al. 1999). The natural habitats of L. principis- rupprechtii are limited to mountainous areas and are fragmented into discontinuous alpine regions in Northern China. The complex topographical and climatic environments could provide abundant refugia for this species to adapt to the cold conditions during glacial periods (Zhang et al. 2017). Diverse habitats have also likely prompted the accumulation of genetic diversity in some isolated populations (Binney et al. 2009; Zhang et al. 2017).Thus, genetic variations among populations are expected because of random genetic drift in the fragmented habits as well as divergent natural selection in heterogeneous environments across the entire range. According to the earliest fossil records, the genus Larix occurred in the Tertiary period, and their distribution may have contracted to higher altitudes and retracted to fragmented alpine zones during the Last Glacial Maximum (LGM) period, in comparison with their present distributions (Binney et al. 2009; Zhang et al. 2014). The wide historical distribution of L. principis- rupprechtii might have played a role in its high genetic diversity. However, the relatively short periods of fragmentation possibly have not led to extensive loss of variation in this species (Hu et al.1999).
The genetic differentiation for all the populations of L.principis- rupprechtii (GST= 0.1510) was higher than that for the northern (G ST = 0.0915) or southern populations(G ST = 0.1338). Similar results have been reported for populations of Chinese pine (Pinus tabulaeformis Carr.) (Wang and Hao 2010) and red oak (Quercus rubra L.) (Borkowski et al. 2017). This could be attributed to the more divergent habitats across a wide range, the more diverse populations sampled over a wide range, and the higher differentiation for isolated populations than for contiguous populations (Noël et al. 2007; Di et al. 2014; Zhang et al. 2017).Moreover, genetic diversity generally decreased from the central population WL to the periphery of the range of L.principis- rupprechtii (the north or south populations). The reduced pattern has also been documented in Gmelin larch(Larix gmelinii (Rupr.) Rupr.) (Barchenkov 2011), and other pinaceae species, including Chinese pine (Wang and Hao 2010), black spruce (Picea mariana (Mill.) BSP.) (Gamache et al. 2003) and Douglas-f ir (Pseudotsuga menziesii Mirb.)Franco. (Li and Adams 1989).
Genetic diversity serves as a way for populations to adapt to changing environments (Huang et al. 2018). Geographical and climatic characteristics have significant effects on levels and partitioning of genetic diversity in plants (Mosca et al. 2012). In this study, there was a significant correlation (r = 0.316,P< 0.05) between genetic and geographical distances of L. principis- rupprechtii populations at the range-wide scale. However, no significant correlation was found for the six populations from Shanxi Province (Di et al.2014). The different results were to some extent related to not only the size of the study areas but also to the complex geographical and climatic factors (Huang et al. 2018). A similar finding was observed in Larix gmelinii populations from Northern China (Na 2006; Zhang et al. 2013). Correlation analysis showed a significantly positive association between the genetic diversity indices of L. principis- rupprechtii and longitude, consistent with those for L. gmelinii (Na 2006) and Siberian larch (L. sibirica Ledeb.) (Semerikov and Lascoux 1999). The changes oflongitude usually were related to the changes of precipitation (Wellenreuther et al.2011), which may influence the genetic diversity of species.On the other hand, no significant correlation was found between genetic diversity and temperature, precipitation or elevation in our study. Nevertheless, previous studies also showed that there were no significant correlations between genetic diversity and temperature or precipitation for rangewide populations of Pinus tabulaeformis (Wang and Hao 2010), and between genetic diversity and elevation for the L.principis- rupprechtii populations in the Guandi Mountains of north China (Wang and Wang 2012).
In this study, the genetic diversities of populations WL,WC and XWT were high, whereas those of WT, GD and TY populations were low. Zhao et al. (2012) and Zhang et al. (2014) suggested that populations with high genetic diversity should be selected first for in situ conservation,i.e., populations WL, WC and XWT. Furthermore, these populations should be a priority for germbank establishment for ex situ conservation. Previous studies have predicted that L. principis- rupprechtii will retreat northeastward 200 km by the year 2050, and 250-400 km by 2100 due to climatic changes, and suitable habitats will shrink significantly (Leng et al. 2008a, 2008b). Human disturbances could also make the distribution of southwestern populations more scattered and smaller (Di et al. 2014). Therefore, considering the level of genetic diversity, population size and complex habitat characteristics, populations WT, GD and TY should be chosen for in/ ex situ conservation to prevent the loss of genetic diversity. In situ conservation might also promote the migration of these populations to predicted suitable habitats(Leng et al. 2008b). More attention should also be paid to protect the habitats of these populations and minimize the impact from human activities. The results of this study can have important implications for the effective conservation of L. principis- rupprechtii.
Conclusions
Range-wide genetic diversity of natural populations of L.principis- rupprechtii has been analyzed using AFLP marker technology. The genetic structure of this species can be characterized as possessing relatively high levels of total diversity, with moderate and significant differentiation among populations. The degree of inter-population differentiation(G ST = 0.1338) for the southern group was larger than for the northern group (G ST = 0.0915). The UPGMA and STRU CTU RE analyses revealed that these populations may be grouped into three genetically distinct clusters. The mantel test showed that there was a significant correlation between the genetic distances and the corresponding geographic distances for L. principis- rupprechtii populations as a whole(r = 0.316,P< 0.05). The genetic diversity of the populations was associated primarily with longitude, but not with elevation or climatic factors.
AcknowledgementsWe would like to thank Xiaohui Fan and Wenchun Liu for their assistance in the field and Suqing Li for valuable comments on the manuscript.
杂志排行
Journal of Forestry Research的其它文章
- A commentary review on the use of normalized difference vegetation index (NDVI) in the era of popular remote sensing
- Reconciliation of research on forest carbon sequestration and water conservation
- A theory to link relationships of stand volume, density, mean diameter and height in forestry data
- A new model for predicting the total tree height for stems cut-to-length by harvesters in Pinus radiata plantations
- Comparative performances of new and existing indices of crown asymmetry: an evaluation using tall trees of Eucalyptus pilularis(Smith)
- Tree mortality and biomass loss in drought-affected forests of East Texas, USA
